The Fetal Type of Posterior Cerebral Artery
Abstract
1. Introduction
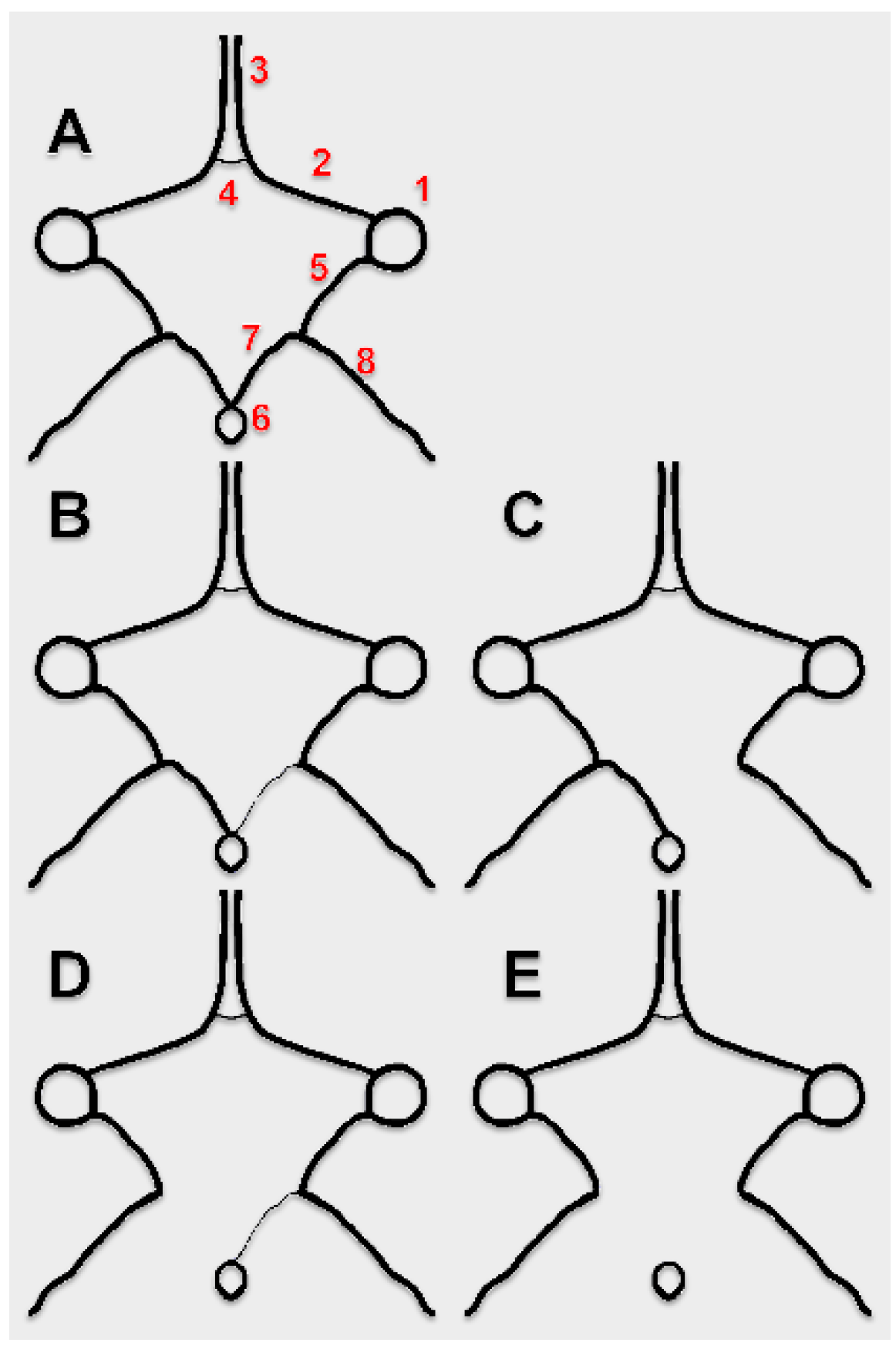
2. Materials and Methods
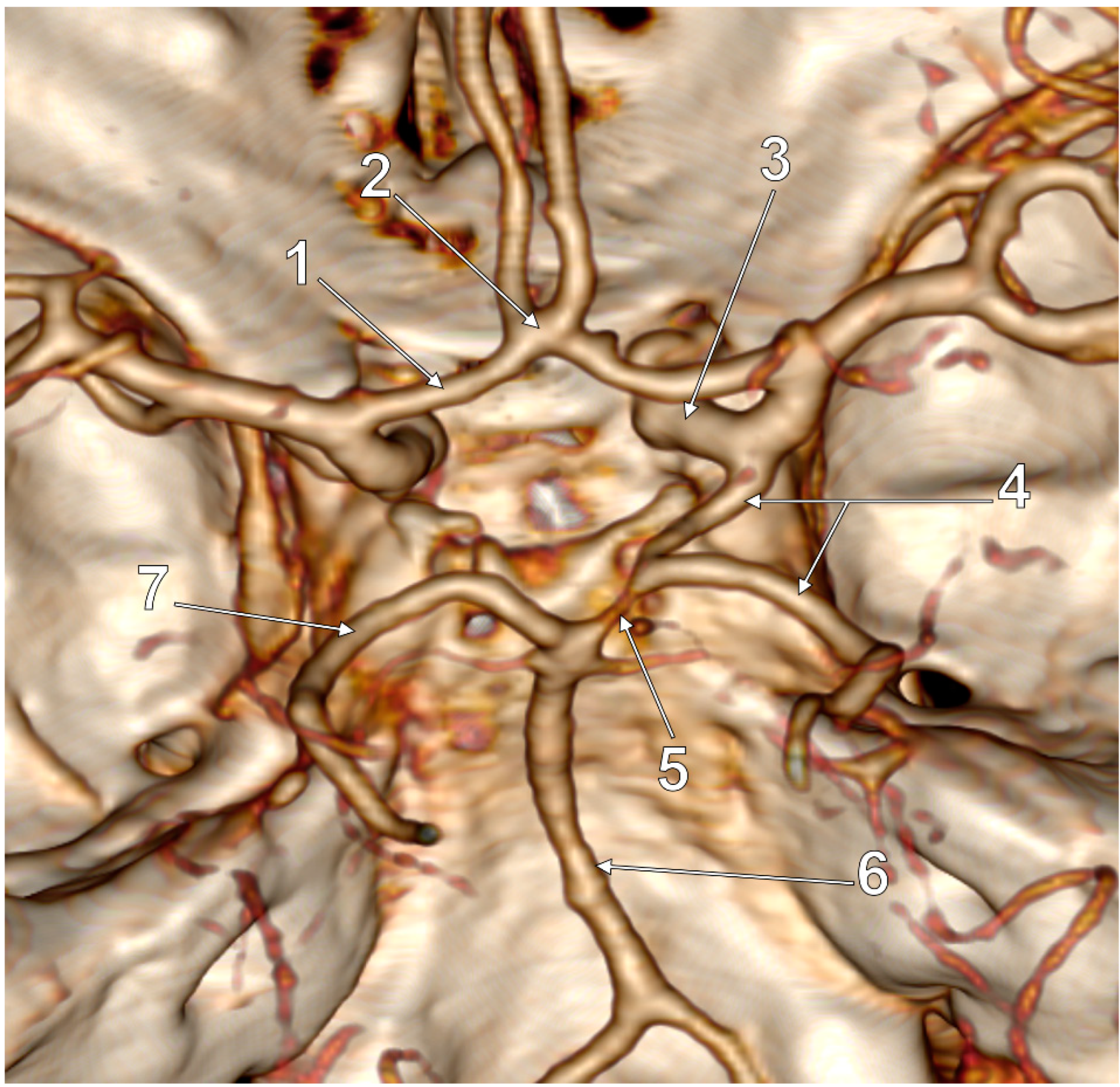
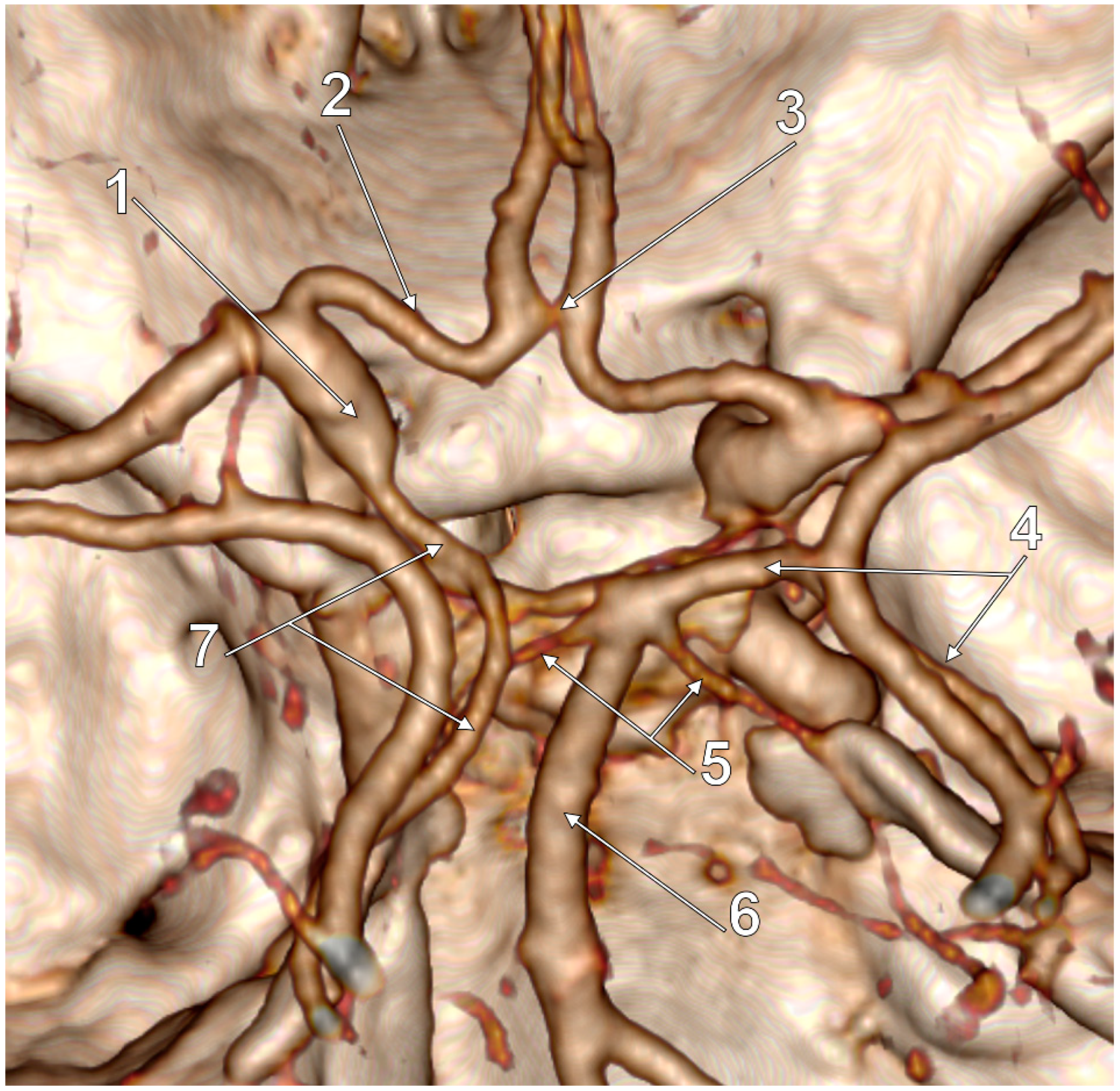
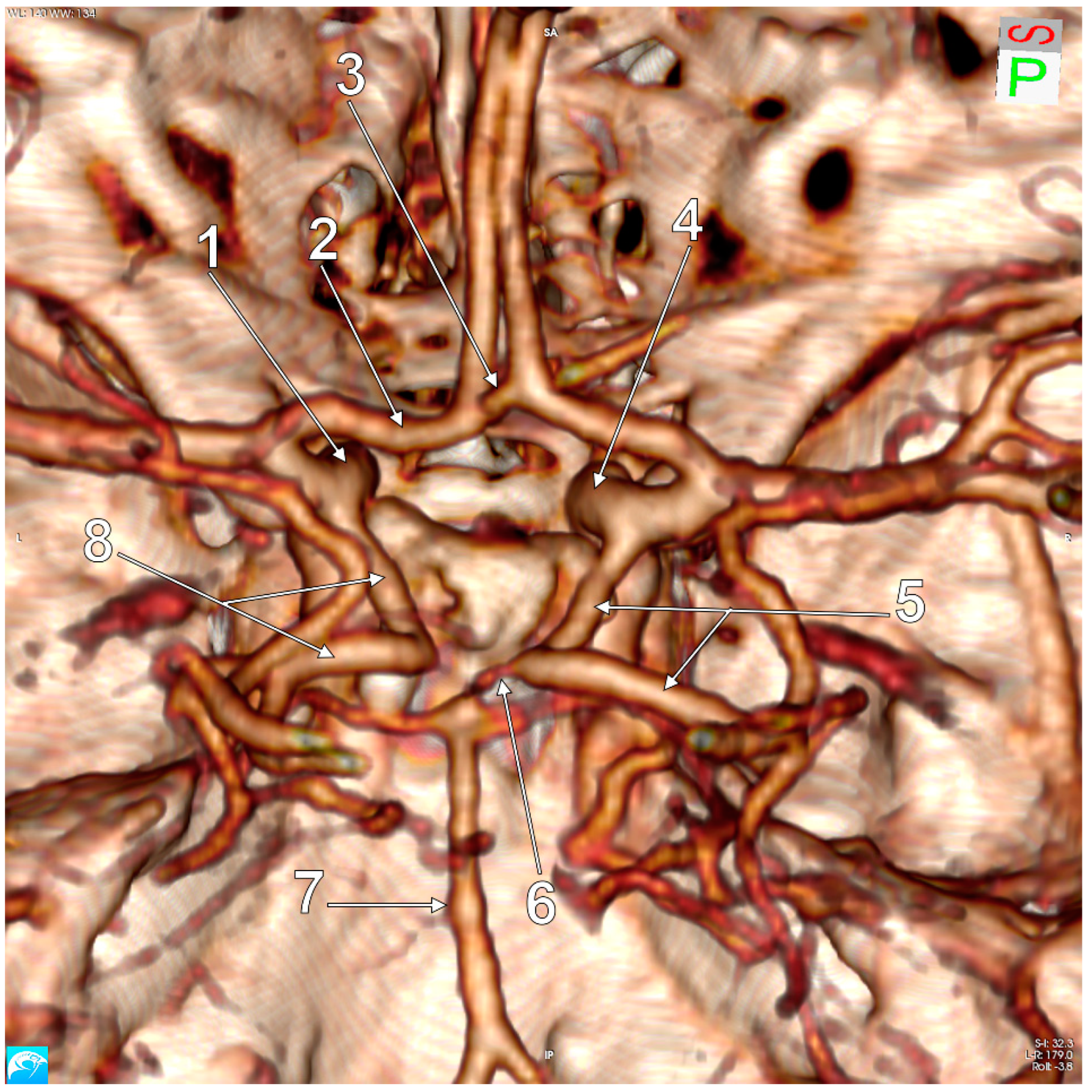
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dumitrescu, A.M.; Costea, C.F.; Cucu, A.I.; Dumitrescu, G.F.; Turliuc, M.D.; Scripcariu, D.V.; Ciocoiu, M.; Tanase, D.M.; Turliuc, S.; Bogdanici, C.M.; et al. The discovery of the circle of Willis as a result of using the scientific method in anatomical dissection. Rom. J. Morphol. Embryol. 2020, 61, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Osborn, A.G. Diagnostic Cerebral Angiography; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999. [Google Scholar]
- Jones, J.D.; Castanho, P.; Bazira, P.; Sanders, K. Anatomical variations of the circle of Willis and their prevalence, with a focus on the posterior communicating artery: A literature review and meta-analysis. Clin. Anat. 2021, 34, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Eftekhar, B.; Dadmehr, M.; Ansari, S.; Ghodsi, M.; Nazparvar, B.; Ketabchi, E. Are the distributions of variations of circle of Willis different in different populations?—Results of an anatomical study and review of literature. BMC Neurol. 2006, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rangus, I.; Milles, L.S.; Galinovic, I.; Villringer, K.; Audebert, H.J.; Fiebach, J.B.; Erdur, H. Reclassifications of ischemic stroke patterns due to variants of the Circle of Willis. Int. J. Stroke 2022, 17, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Fujita, N.; Enoki, T.; Matsumoto, K.; Watanabe, Y.; Murase, K.; Nakamura, H. Relationship between variations in the circle of Willis and flow rates in internal carotid and basilar arteries determined by means of magnetic resonance imaging with semiautomated lumen segmentation: Reference data from 125 healthy volunteers. AJNR Am. J. Neuroradiol. 2006, 27, 1770–1775. [Google Scholar] [PubMed]
- Wolpert, S.M. The circle of Willis. AJNR Am. J. Neuroradiol. 1997, 18, 1033–1034. [Google Scholar]
- Fisher, C.M. The posterior cerebral artery syndrome. Can. J. Neurol. Sci. 1986, 13, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Van Overbeeke, J.J.; Hillen, B.; Tulleken, C.A. A comparative study of the circle of Willis in fetal and adult life. The configuration of the posterior bifurcation of the posterior communicating artery. J. Anat. 1991, 176, 45–54. [Google Scholar] [PubMed]
- van Raamt, A.F.; Mali, W.P.; van Laar, P.J.; van der Graaf, Y. The fetal variant of the circle of Willis and its influence on the cerebral collateral circulation. Cerebrovasc. Dis. 2006, 22, 217–224. [Google Scholar] [CrossRef]
- Arjal, R.K.; Zhu, T.; Zhou, Y. The study of fetal-type posterior cerebral circulation on multislice CT angiography and its influence on cerebral ischemic strokes. Clin. Imaging 2014, 38, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Gunnal, S.A.; Farooqui, M.S.; Wabale, R.N. Study of Posterior Cerebral Artery in Human Cadaveric Brain. Anat. Res. Int. 2015, 2015, 681903. [Google Scholar] [CrossRef] [PubMed]
- Bhanu, S.P.; Pentyala, S.; Sankar, D.K. Incidence of hypoplastic posterior communicating artery and fetal posterior cerebral artery in Andhra population of India: A retrospective 3-Tesla magnetic resonance angiographic study. Anat. Cell Biol. 2020, 53, 272–278. [Google Scholar] [CrossRef]
- Moraru, L.; Rusu, M.C.; Popescu, S.A. True terminal pentafurcation of the external carotid artery and terminal trifurcation of the contralateral one, occipitoauricular trunk, retropharyngeal internal carotid artery. Surg. Radiol. Anat. 2021, 43, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Puchades-Orts, A.; Nombela-Gomez, M.; Ortuno-Pacheco, G. Variation in form of circle of Willis: Some anatomical and embryological considerations. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 1976, 185, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Okahara, M.; Kiyosue, H.; Mori, H.; Tanoue, S.; Sainou, M.; Nagatomi, H. Anatomic variations of the cerebral arteries and their embryology: A pictorial review. Eur. Radiol. 2002, 12, 2548–2561. [Google Scholar] [CrossRef] [PubMed]
- Gregg, L.; Gailloud, P. The Role of the Primitive Lateral Basilovertebral Anastomosis of Padget in Variations of the Vertebrobasilar Arterial System. Anat. Rec. 2017, 300, 2025–2038. [Google Scholar] [CrossRef]
- Padget, D.H. The development of the cranial arteries in the human embryo. Contrib Embryol 1948, 32, 205–261. [Google Scholar]
- Bergman, T.J.; Saporito, R.C.; Hope, T. Bilateral Superior Cerebellar Artery Embolic Occlusion with a Fetal-Type Posterior Cerebral Artery Providing Collateral Circulation. Case Rep. Neurol. 2016, 8, 258–263. [Google Scholar] [CrossRef]
- Uchino, A.; Saito, N.; Takahashi, M.; Okano, N.; Tanisaka, M. Variations of the posterior cerebral artery diagnosed by MR angiography at 3 tesla. Neuroradiology 2016, 58, 141–146. [Google Scholar] [CrossRef]
- Uchino, A.; Kamide, T.; Kurita, H. Replaced posterior cerebral artery (PCA): Origin of all branches of the PCA from the anterior choroidal artery diagnosed by MR angiography. Surg. Radiol. Anat. 2019, 41, 703–705. [Google Scholar] [CrossRef]
- Horikoshi, T.; Akiyama, I.; Yamagata, Z.; Sugita, M.; Nukui, H. Magnetic resonance angiographic evidence of sex-linked variations in the circle of willis and the occurrence of cerebral aneurysms. J. Neurosurg. 2002, 96, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Frid, P.; Wasselius, J.; Drake, M.; Wu, O.; Petersson, J.; Rost, N.S.; Lindgren, A.; MRI-GENIE Study, The ISGC. Fetal posterior cerebral artery configurations in an ischemic stroke versus an unselected hospital population. Acta Neurol. Scand. 2022, 145, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Diogo, M.C.; Fragata, I.; Dias, S.P.; Nunes, J.; Pamplona, J.; Reis, J. Low prevalence of fetal-type posterior cerebral artery in patients with basilar tip aneurysms. J. Neurointerv. Surg. 2017, 9, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Shaban, A.; Albright, K.C.; Boehme, A.K.; Martin-Schild, S. Circle of Willis Variants: Fetal PCA. Stroke Res. Treat 2013, 2013, 105937. [Google Scholar] [CrossRef] [PubMed]
- Coulier, B. Morphologic variants of the Cerebral Arterial Circle on computed tomographic angiography (CTA): A large retrospective study. Surg. Radiol. Anat. 2021, 43, 417–426. [Google Scholar] [CrossRef]
- Iqbal, S. A comprehensive study of the anatomical variations of the circle of willis in adult human brains. J. Clin. Diagn. Res. 2013, 7, 2423–2427. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Piotrowska, W.; Rybicka, M.; Wojnarska, A.; Wojtowicz, A.; Koziej, M.; Holda, M.K. A multitude of variations in the configuration of the circle of Willis: An autopsy study. Anat. Sci. Int. 2016, 91, 325–333. [Google Scholar] [CrossRef]
- Jinkins, J.R. Atlas of Neuroradiologic Embryology, Anatomy, and Variants; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- Kovac, J.D.; Stankovic, A.; Stankovic, D.; Kovac, B.; Saranovic, D. Intracranial arterial variations: A comprehensive evaluation using CT angiography. Med. Sci. Monit. 2014, 20, 420–427. [Google Scholar] [PubMed]
- Lambert, S.L.; Williams, F.J.; Oganisyan, Z.Z.; Branch, L.A.; Mader, E.C., Jr. Fetal-Type Variants of the Posterior Cerebral Artery and Concurrent Infarction in the Major Arterial Territories of the Cerebral Hemisphere. J. Investig. Med. High Impact Case Rep. 2016, 4, 2324709616665409. [Google Scholar] [CrossRef]
- De Caro, J.; Ciacciarelli, A.; Tessitore, A.; Buonomo, O.; Calzoni, A.; Francalanza, I.; Dell’Aera, C.; Cosenza, D.; Curro, C.T.; Granata, F.; et al. Variants of the circle of Willis in ischemic stroke patients. J. Neurol. 2021, 268, 3799–3807. [Google Scholar] [CrossRef] [PubMed]
- Saeki, N.; Rhoton, A.L., Jr. Microsurgical anatomy of the upper basilar artery and the posterior circle of Willis. J. Neurosurg. 1977, 46, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Alpers, B.J.; Berry, R.G.; Paddison, R.M. Anatomical studies of the circle of Willis in normal brain. AMA Arch. Neurol. Psychiatry 1959, 81, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Riggs, H.E.; Rupp, C. Variation in form of circle of Willis. The relation of the variations to collateral circulation: Anatomic analysis. Arch. Neurol. 1963, 8, 8–14. [Google Scholar] [CrossRef]
- Lippert, H.; Pabst, R. Arterial Variations in Man: Classification and Frequency; JF Bergmann Verlag: München, Germany, 1985. [Google Scholar]
- Krabbe-Hartkamp, M.J.; van der Grond, J.; de Leeuw, F.E.; de Groot, J.C.; Algra, A.; Hillen, B.; Breteler, M.M.; Mali, W.P. Circle of Willis: Morphologic variation on three-dimensional time-of-flight MR angiograms. Radiology 1998, 207, 103–111. [Google Scholar] [CrossRef]
- Li, Q.; Li, J.; Lv, F.; Li, K.; Luo, T.; Xie, P. A multidetector CT angiography study of variations in the circle of Willis in a Chinese population. J. Clin. Neurosci. 2011, 18, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Enyedi, M.; Scheau, C.; Baz, R.O.; Didilescu, A.C. Circle of Willis: Anatomical variations of configuration. A magnetic resonance angiography study. Folia Morphol. 2021. [Google Scholar] [CrossRef]
- Kapoor, K.; Singh, B.; Dewan, L.I. Variations in the configuration of the circle of Willis. Anat. Sci. Int. 2008, 83, 96–106. [Google Scholar] [CrossRef]
- Rothberg, M.; Mattey, W.E.; Bastidas, J. Anomalous internal carotid-posterior cerebral artery circulation: One form of congenital incomplete circle of Willis. AJR Am. J. Roentgenol. 1977, 128, 153–155. [Google Scholar] [CrossRef]
- Bugnicourt, J.M.; Garcia, P.Y.; Peltier, J.; Bonnaire, B.; Picard, C.; Godefroy, O. Incomplete posterior circle of willis: A risk factor for migraine? Headache 2009, 49, 879–886. [Google Scholar] [CrossRef]
- Ikeda, K.; Iwamoto, K.; Murata, K.; Ito, H.; Kawase, Y.; Kano, O.; Kawabe, K.; Iguchi, H.; Iwasaki, Y. Incomplete Posterior Circle of Willis in Migraineurs with Aura. Headache 2017, 57, E19–E20. [Google Scholar] [CrossRef]
- Brandt, T.; Steinke, W.; Thie, A.; Pessin, M.S.; Caplan, L.R. Posterior cerebral artery territory infarcts: Clinical features, infarct topography, causes and outcome. Multicenter results and a review of the literature. Cerebrovasc. Dis. 2000, 10, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.; Di Stefano, V.; Cannella, R.; Di Blasio, F.; De Angelis, M.V. Fetal variant of posterior cerebral artery: Just a physiologic variant or a window for possible ischemic stroke? Neurol. Sci. 2021, 42, 2535–2538. [Google Scholar] [CrossRef] [PubMed]
- Wentland, A.L.; Rowley, H.A.; Vigen, K.K.; Field, A.S. Fetal origin of the posterior cerebral artery produces left-right asymmetry on perfusion imaging. AJNR Am. J. Neuroradiol. 2010, 31, 448–453. [Google Scholar] [CrossRef] [PubMed]
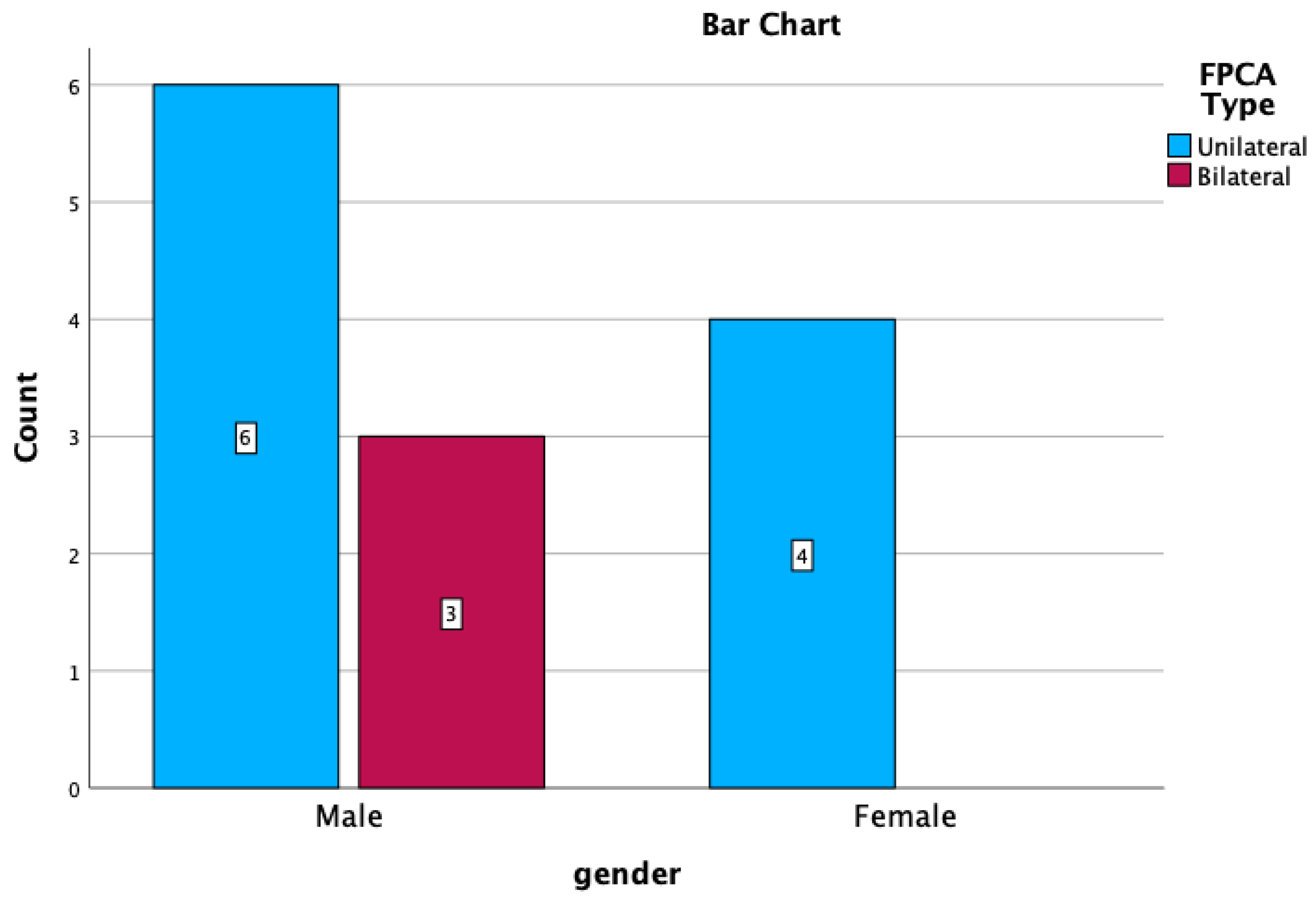
| GENDER | UNILATERAL | BILATERAL | ||
|---|---|---|---|---|
| R | L | p-FPCA + f-FPCA | f-FPCA + f-FPCA | |
| M | 4 (30.76%) | 2 (15.38%) | 1 1 (7.69%) | 2 (15.38%) |
| F | 3 (23.07%) | 1 (7.69%) | - | - |
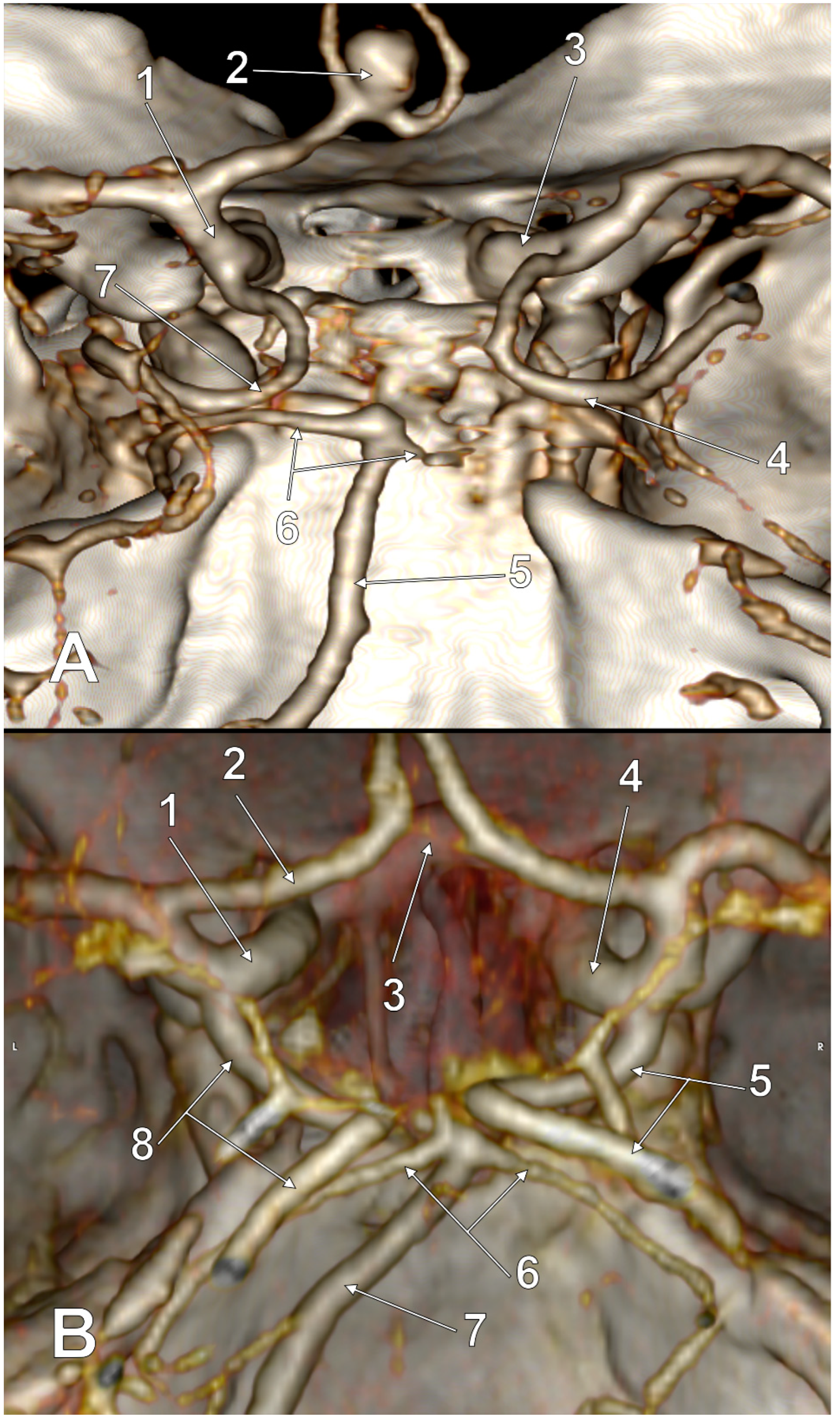
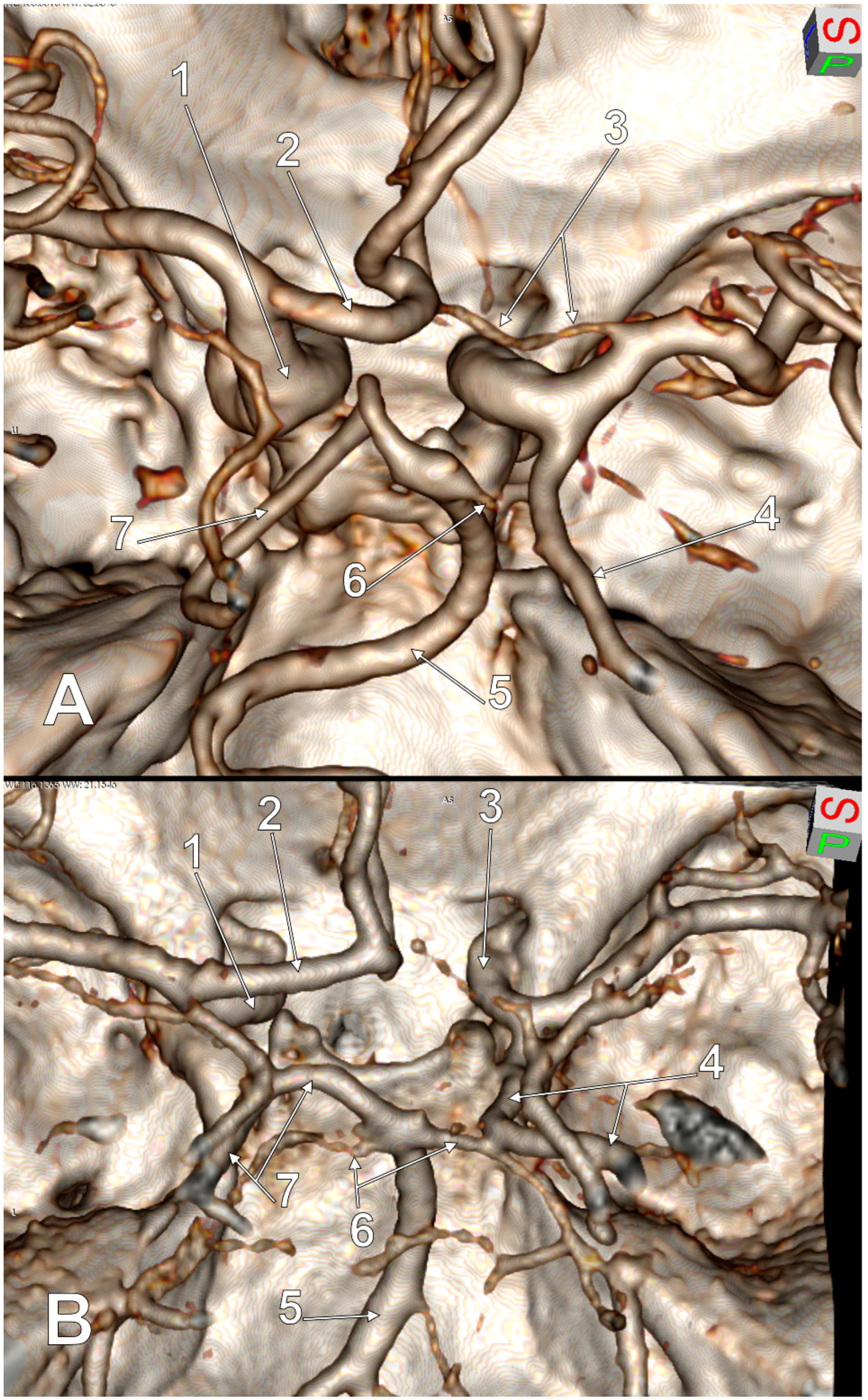
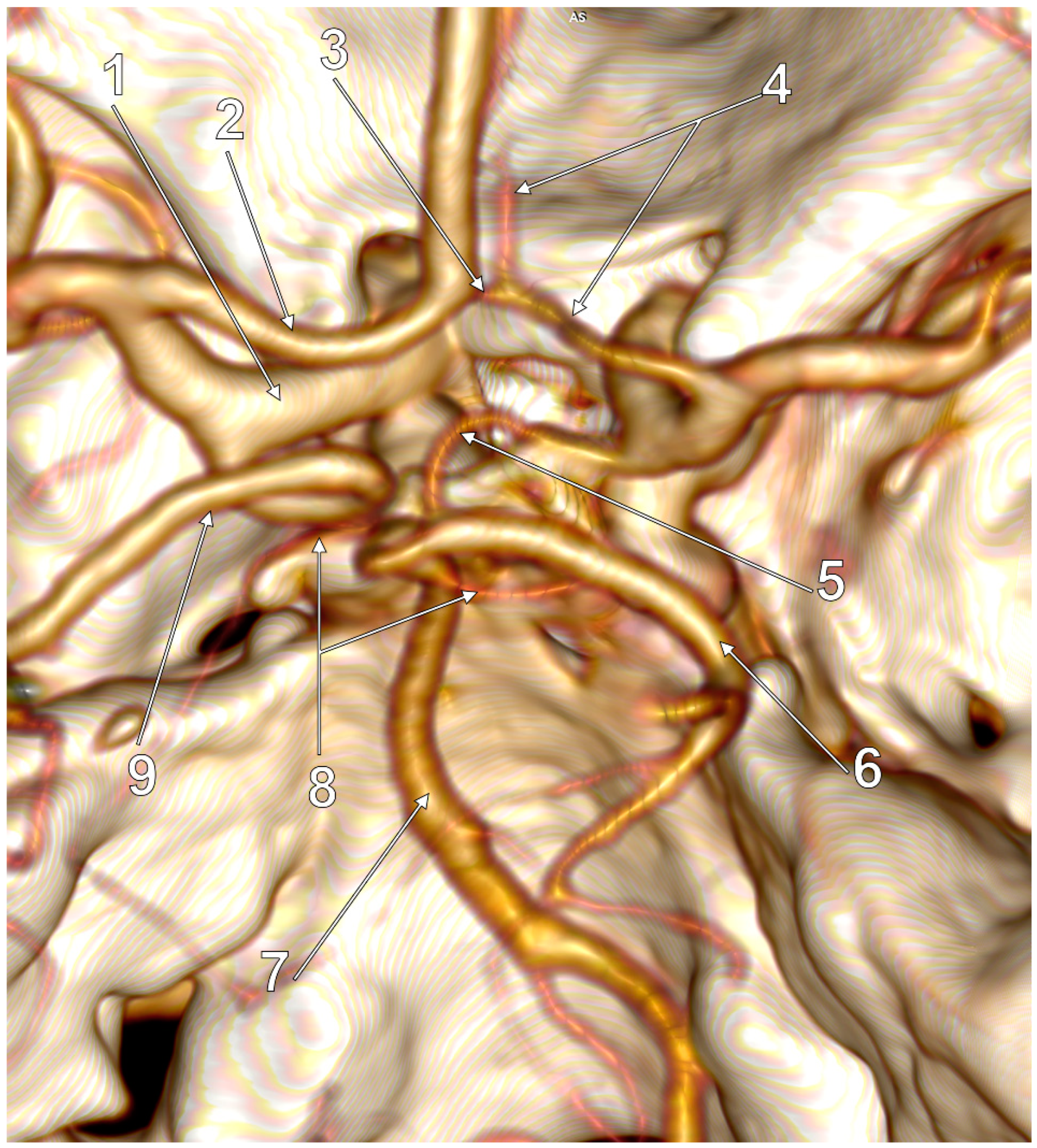
| No. of Cases, Sex | Number of Variant Sides of the cW | Morphology of the cW | Subvariant of FPCA | Figure |
|---|---|---|---|---|
| 2 M | 2 |
| p-FPCA | 2 |
| 1 M, 3 F | 2 |
| f-FPCA | 3 |
| 1 M | 2 |
| p-FPCA+f-FPCA | 4 |
| 1 M, 1 F | 3 |
| f-FPCA | 6A |
| 1 M | 3 |
| f-FPCA+f-FPCA | 5A |
| 1 M | 3 |
| f-FPCA+f-FPCA | 5B |
| 1 M | 3 |
| f-FPCA | 6B |
| 1 M | 4 |
| f-FPCA | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davidoiu, A.-M.; Mincă, D.I.; Rusu, M.C.; Hostiuc, S.; Toader, C. The Fetal Type of Posterior Cerebral Artery. Medicina 2023, 59, 231. https://doi.org/10.3390/medicina59020231
Davidoiu A-M, Mincă DI, Rusu MC, Hostiuc S, Toader C. The Fetal Type of Posterior Cerebral Artery. Medicina. 2023; 59(2):231. https://doi.org/10.3390/medicina59020231
Chicago/Turabian StyleDavidoiu, Ana-Maria, Dragoş Ionuţ Mincă, Mugurel Constantin Rusu, Sorin Hostiuc, and Corneliu Toader. 2023. "The Fetal Type of Posterior Cerebral Artery" Medicina 59, no. 2: 231. https://doi.org/10.3390/medicina59020231
APA StyleDavidoiu, A.-M., Mincă, D. I., Rusu, M. C., Hostiuc, S., & Toader, C. (2023). The Fetal Type of Posterior Cerebral Artery. Medicina, 59(2), 231. https://doi.org/10.3390/medicina59020231







