Ambiental Factors in Parkinson’s Disease Progression: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction and Data Measurement
2.5. Assessment of the Methodological Quality of the Included Studies
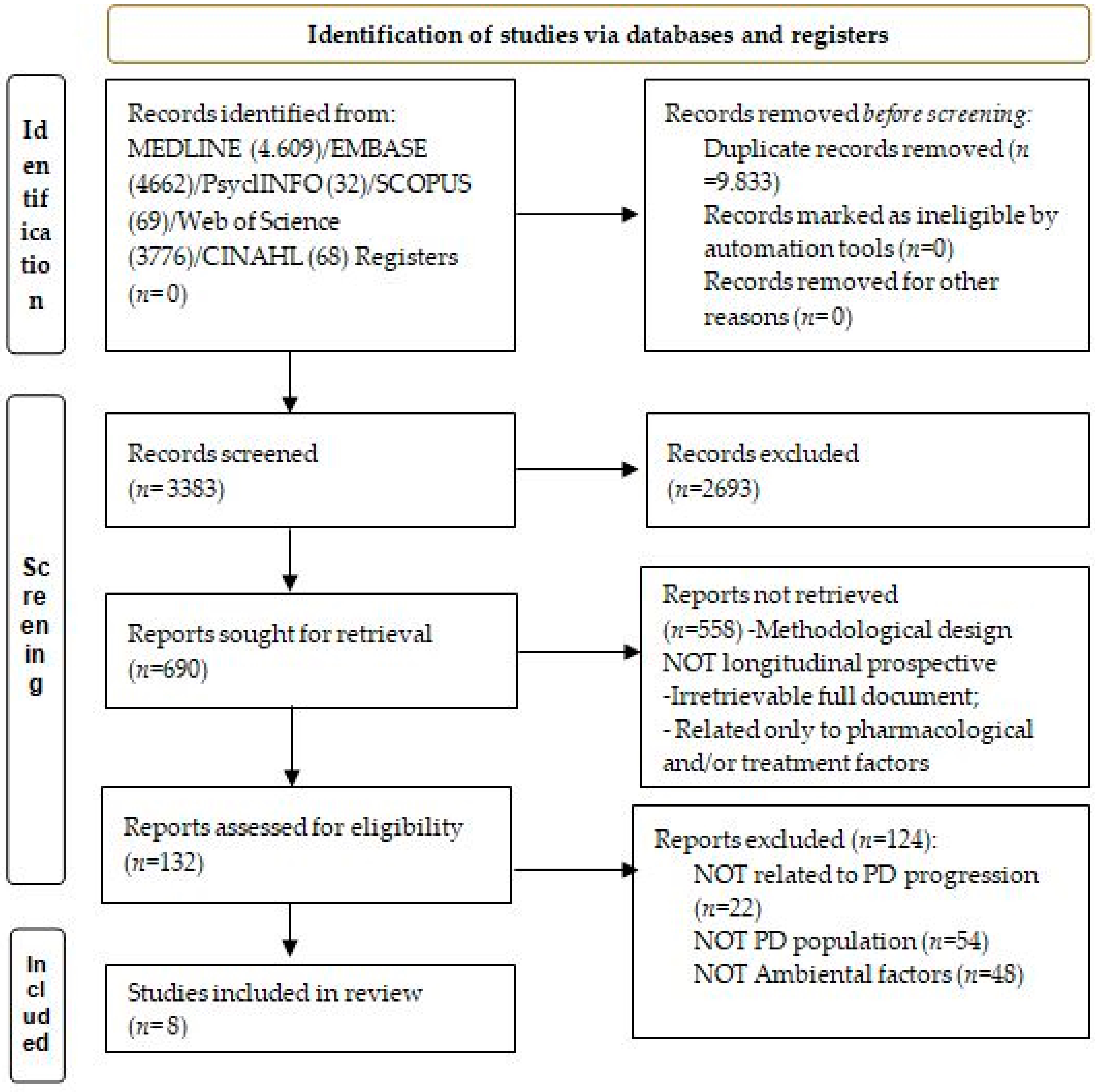
3. Results
3.1. Description of Selected Studies
3.2. Air Pollution Exposure
3.3. Temperature, Humidity, Solar Exposure and Seasonal Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pang, S.Y.-Y.; Ho, P.W.-L.; Liu, H.-F.; Leung, C.-T.; Li, L.; Chang, E.E.S.; Ramsden, D.B.; Ho, S.-L. The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson’s disease. Transl. Neurodegener. 2019, 8, 23. [Google Scholar] [CrossRef]
- Billingsley, K.J.; Bandres-Ciga, S.; Saez-Atienzar, S.; Singleton, A.B. Genetic risk factors in Parkinson’s disease. Cell Tissue Res. 2018, 373, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Shermon, S.; Goldfinger, M.; Morris, A.; Harper, B.; Leder, A.; Santella, A.J.; Krishnamachari, B. Effect of modifiable risk factors in Parkinson’s disease: A case-control study looking at common dietary factors, toxicants, and anti-inflammatory medications. Chronic Illn. 2021, 18, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Power, M.C.; Adar, S.D.; Yanosky, J.D.; Weuve, J. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: A systematic review of epidemiologic research. Neurotoxicology 2016, 56, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Brauer, M.; Avila-Casado, C.; Fortoul, T.; Vedal, S.; Stevens, B.; Churg, A. Air pollution and retained particles in the lung. Environ. Health Perspect. 2001, 109, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Solt, A.C.; Henríquez-Roldán, C.; Torres-Jardón, R.; Nuse, B.; Herritt, L.; Villarreal-Calderón, R.; Osnaya, N.; Stone, I.; García, R.; et al. Long-term Air Pollution Exposure Is Associated with Neuroinflammation, an Altered Innate Immune Response, Disruption of the Blood-Brain Barrier, Ultrafine Particulate Deposition, and Accumulation of Amyloid β-42 and α-Synuclein in Children and Young Adults. Toxicol. Pathol. 2008, 36, 289–310. [Google Scholar] [CrossRef]
- Block, M.L.; Calderón-Garcidueñas, L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef]
- Hajipour, S.; Farbood, Y.; Gharib-Naseri, M.K.; Goudarzi, G.; Rashno, M.; Maleki, H.; Bakhtiari, N.; Nesari, A.; Khoshnam, S.E.; Dianat, M.; et al. Exposure to ambient dusty particulate matter impairs spatial memory and hippocampal LTP by increasing brain inflammation and oxidative stress in rats. Life Sci. 2020, 242, 117210. [Google Scholar] [CrossRef]
- Costa, L.G.; Cole, T.B.; Dao, K.; Chang, Y.-C.; Coburn, J.; Garrick, J.M. Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol. Ther. 2020, 210, 107523. [Google Scholar] [CrossRef]
- Lee, M.K.; Park, H.J.; Zhao, T.T.; Kim, S.H.; Kil Lee, C.; Hwang, B.Y.; Lee, K.E. Ethanol extract from Gynostemmapentaphyllum ameliorates dopaminergic neuronal cell death in transgenic mice expressing mutant A53T human alpha-synuclein. Neural Regen. Res. 2020, 15, 361–368. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, Y.; Liu, C.; Tian, Y.; Xia, D.; Liu, Z.; Pan, L.; Xiong, M.; Xiong, J.; Meng, L.; et al. Fine Particulate Matter Triggers α-Synuclein Fibrillization and Parkinson-like Neurodegeneration. Mov. Disord. 2022, 37, 1817–1830. [Google Scholar] [CrossRef]
- Rowell, D.; Nghiem, S.; Ramagopalan, S.; Meier, U.-C. Seasonal temperature is associated with Parkinson’s disease prescriptions: An ecological study. Int. J. Biometeorol. 2017, 61, 2205–2211. [Google Scholar] [CrossRef]
- Swinn, L.; Schrag, A.; Viswanathan, R.; Bloem, B.R.; Lees, A.; Quinn, N. Sweating dysfunction in Parkinson’s disease. Mov. Disord. 2003, 18, 1459–1463. [Google Scholar] [CrossRef]
- Meigal, A.; Lupandin, Y. “Thermoregulation-dependent component” in pathophysiology of motor disorders in Parkinson’s disease? Pathophysiology 2005, 11, 187–196. [Google Scholar] [CrossRef]
- Guatteo, E.; Chung, K.K.H.; Bowala, T.K.; Bernardi, G.; Mercuri, N.B.; Lipski, J. Temperature Sensitivity of Dopaminergic Neurons of the Substantia Nigra Pars Compacta: Involvement of Transient Receptor Potential Channels. J. Neurophysiol. 2005, 94, 3069–3080. [Google Scholar] [CrossRef]
- Kravietz, A.; Kab, S.; Wald, L.; Dugravot, A.; Singh-Manoux, A.; Moisan, F.; Elbaz, A. Association of UV radiation with Parkinson disease incidence: A nationwide French ecologic study. Environ. Res. 2017, 154, 50–56. [Google Scholar] [CrossRef]
- Hu, C.-Y.; Fang, Y.; Li, F.-L.; Dong, B.; Hua, X.-G.; Jiang, W.; Zhang, H.; Lyu, Y.; Zhang, X.-J. Association between ambient air pollution and Parkinson’s disease: Systematic review and meta-analysis. Environ. Res. 2019, 168, 448–459. [Google Scholar] [CrossRef]
- Bellou, V.; Belbasis, L.; Tzoulaki, I.; Evangelou, E.; Ioannidis, J.P.A. Environmental risk factors and Parkinson’s disease: An umbrella review of meta-analyses. Parkinsonism Relat. Disord. 2015, 23, 1–9. [Google Scholar] [CrossRef]
- Weed, D.L. Does paraquat cause Parkinson’s disease? A review of reviews. Neurotoxicology 2021, 86, 180–184. [Google Scholar] [CrossRef]
- González-Aramburu, I.; Sánchez-Juan, P.; Jesús, S.; Gorostidi, A.; Fernández-Juan, E.; Carrillo, F.; Sierra, M.; Gómez-Garre, P.; Cáceres-Redondo, M.T.; Berciano, J.; et al. Genetic variability related to serum uric acid concentration and risk of Parkinson’s disease. Mov. Disord. 2013, 28, 1737–1740. [Google Scholar] [CrossRef]
- Ullah, I.; Zhao, L.; Hai, Y.; Fahim, M.; Alwayli, D.; Wang, X.; Li, H. Metal elements and pesticides as risk factors for Parkinson’s disease—A review. Toxicol. Rep. 2021, 8, 607–616. [Google Scholar] [CrossRef]
- Raj, K.; Kaur, P.; Gupta, G.; Singh, S. Metals associated neurodegeneration in Parkinson’s disease: Insight to physiological, pathological mechanisms and management. Neurosci. Lett. 2021, 753, 135873. [Google Scholar] [CrossRef]
- Chambers-Richards, T.; Su, Y.; Chireh, B.; D’Arcy, C. Exposure to toxic occupations and their association with Parkinson’s disease: A systematic review with meta-analysis. Rev. Environ. Health 2021. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp (accessed on 19 September 2022).
- Zanobetti, A.; Dominici, F.; Wang, Y.; Schwartz, J.D. A national case-crossover analysis of the short-term effect of PM2.5 on hospitalizations and mortality in subjects with diabetes and neurological disorders. Environ. Health 2014, 13, 38. [Google Scholar] [CrossRef]
- Kioumourtzoglou, M.-A.; Schwartz, J.D.; Weisskopf, M.G.; Melly, S.J.; Wang, Y.; Dominici, F.; Zanobetti, A. Long-term PM 2.5 Exposure and Neurological HospitalAdmissions in the Northeastern United States. Environ. Health Perspect. 2016, 124, 23–29. [Google Scholar] [CrossRef]
- Lee, H.; Myung, W.; Kim, D.K.; Kim, S.E.; Kim, C.T.; Kim, H. Short-term air pollution exposure aggravates Parkinson’s disease in a population-based cohort. Sci. Rep. 2017, 7, 44741. [Google Scholar] [CrossRef]
- Shi, L.; Wu, X.; Danesh Yazdi, M.; Braun, D.; Abu Awad, Y.; Wei, Y.; Liu, P.; Di, Q.; Wang, Y.; Schwartz, J.; et al. Long-term effects of PM2·5 on neurological disorders in the American Medicare population: A longitudinal cohort study. Lancet Planet. Health 2020, 4, e557–e565. [Google Scholar] [CrossRef]
- Nunez, Y.; Boehme, A.K.; Li, M.; Goldsmith, J.; Weisskopf, M.G.; Re, D.B.; Navas-Acien, A.; van Donkelaar, A.; Martin, R.V.; Kioumourtzoglou, M.-A. Parkinson’s disease aggravation in association with fine particle components in New York State. Environ. Res. 2021, 201, 111554. [Google Scholar] [CrossRef]
- Postuma, R.B.; Arenovich, T.; Lang, A.E. Does severity of Parkinson’s disease vary according to season? Mov. Disord. 2005, 20, 476–479. [Google Scholar] [CrossRef]
- Goetz, C.G.; Li, J.M.; Wuu, J.; Leurgans, S. Does seasonal variation affect hallucinations in PD? a longitudinal study. Mov. Disord. 2006, 21, 863–865. [Google Scholar] [CrossRef]
- Jankowska-Kieltyka, M.; Roman, A.; Nalepa, I. The Air We Breathe: Air Pollution as a Prevalent Proinflammatory Stimulus Contributing to Neurodegeneration. Front. Cell. Neurosci. 2021, 15, 647643. [Google Scholar] [CrossRef]
- Haghani, A.; Morgan, T.E.; Forman, H.J.; Finch, C.E. Air Pollution Neurotoxicity in the Adult Brain: Emerging Concepts from Experimental Findings. J. Alzheimer Dis. 2020, 76, 773–797. [Google Scholar] [CrossRef]
- Segalowitz, S.J. Public health, brain health, and the dangers of air pollution for neural development. Brain Cogn. 2008, 68, 115–116. [Google Scholar] [CrossRef]
- Block, M.L.; Elder, A.; Auten, R.L.; Bilbo, S.D.; Chen, H.; Chen, J.-C.; Cory-Slechta, D.A.; Costa, D.; Diaz-Sanchez, D.; Dorman, D.C.; et al. The outdoor air pollution and brain health workshop. NeuroToxicology 2012, 33, 972–984. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Ton, T.G.; Jain, S.; Biggs, M.L.; Thacker, E.L.; Strotmeyer, E.S.; Boudreau, R.; Newman, A.B.; Longstreth, W.T., Jr.; Checkoway, H. Markers of inflammation in prevalent and incident Parkinson’s disease in the Cardiovascular Health Study. Park. Relat. Disord. 2012, 18, 274–278. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, L.; Tang, M. Toxicity of inhaled particulate matter on the central nervous system: Neuroinflammation, neuropsychological effects and neurodegenerative disease. J. Appl. Toxicol. 2017, 37, 644–667. [Google Scholar] [CrossRef]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of Inhaled Ultrafine Particles to the Brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef]
- Kaasinen, V.; Jokinen, P.; Joutsa, J.; Eskola, O.; Rinne, J.O. Seasonality of striatal dopamine synthesis capacity in Parkinson’s disease. Neurosci. Lett. 2012, 530, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Dimakakou, E.; Johnston, H.J.; Streftaris, G.; Cherrie, J.W. Exposure to Environmental and Occupational Particulate Air Pollution as a Potential Contributor to Neurodegeneration and Diabetes: A Systematic Review of Epidemiological Research. Int. J. Environ. Res. Public Health 2018, 15, 1704. [Google Scholar] [CrossRef]
- Peters, R.; Ee, N.; Peters, J.; Booth, A.; Mudway, I.; Anstey, K.J. Air Pollution and Dementia: A Systematic Review. J. Alzheimer’s Dis. 2019, 70, S145–S163. [Google Scholar] [CrossRef] [PubMed]
- Davalos, A.D.; Luben, T.J.; Herring, A.H.; Sacks, J.D. Current approaches used in epidemiologic studies to examine short-term multipollutant air pollution exposures. Ann. Epidemiol. 2017, 27, 145–153.e1. [Google Scholar] [CrossRef] [PubMed]
| Article | Representativeness | Selection of Non-Exposed Cohort | Ascertainment of Data Collection | PD Present at Start | Comparability (Adjustments) | Outcome Assessment | Duration of Follow-Up | Adequacy of Follow-Up | Total Score |
|---|---|---|---|---|---|---|---|---|---|
| Zanobetti, 2014; [27] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Kioumourtzoglou, 2016; [28] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Lee, 2017; [29] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Shi, 2020; [30] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Nunez, 2021; [31] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Postuma, 2005; [32] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Goetz, 2006; [33] | 0 | 1 | 1 | 1 | 2 | 1 | 0 | 0 | 6 |
| Authors, Year | Country | Study Type | Ambient Factor | N° PD Cases | PD Progression Measure | Follow-Up, Year | RR or HR | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Zanobetti, 2014; [27] | 121 US communities (1999–2010) | case-crossover | short-term exposure to PM2.5 1 | NR | PDhospital admissions | 11 | 3.23 | 1.08, 5.43 |
| Kioumourtzoglou, 2016; [28] | 50 northeastern U.S. cities (1999–2010) | cohort | long-term PM2.5 2 | NR | PDhospital admissions | 11 | 1.08 | 1.04, 1.12 |
| Lee, 2017; [29] | North Seoul | cohort | short-term exposure to PM2.5 1, NO2, SO2, CO | 77 | PDhospital admissions | 11 | PM2.5: 1.61 NO2: 2.35 SO2: 1.54 CO: 1.46 | PM2.5: 1.14–2.29 NO2: 1.39–3.97 SO2: 1.11–2.14 CO: 1.05–2.04 |
| Shi, 2020; [30] | USA(2000–2016) | Longitudinal-cohort | short-term exposure to PM2.5 3 | 106 | PDhospital admissions | 16 | 1.13 | 1.12–1.14 |
| Nunez, 2021; [31] | 62 NYS counties (2000–2014) | cohort | long-term PM2.5 4, Nitrate, OM, black carbon, sulfate, soil particles | 197, 545 | PDhospital admissions | 14 | Nitrate: 1.06 OM: 1.06 | PM2.5: Nitrate: 1.03–1.10 OM: 1.04–1.09 |
| Author, Year | Country | Study Type | Ambient Factor | N° PD Cases | PD Progression Measure | Follow-Up Time, Years | Main Findings |
|---|---|---|---|---|---|---|---|
| Rowell, 2017; [12] | Australia (eightstates *) | Ecological | Temperature, humidity and solar exposure | NR | Changes in the aggregate (LED) | 23 | The prescribed LED was 7.4% greater in January and 8% lower in July. Temperature but not UV lightand humiditywas associated with the prescription of PD medications. |
| Postuma, 2005; [32] | Canada (Toronto) | Longitudinal cohort | Fourseasons | 546 | UPDRS I-III off/on score in two evaluations | 3 | No significant seasonal variation in any UPDRS subscale |
| Goetz, 2006; [33] | USA (Chicago) | Longitudinal cohort | Two seasons | 51 PD with hallucination | Thought Disorder (TD) score for assessment of hallucinations | 1 | The level of wintertime exacerbation was no greater than summertime exacerbation. No seasonal change in hallucination severity of PD patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bougea, A.; Papagiannakis, N.; Simitsi, A.-M.; Panagiotounakou, E.; Chrysovitsanou, C.; Angelopoulou, E.; Koros, C.; Stefanis, L. Ambiental Factors in Parkinson’s Disease Progression: A Systematic Review. Medicina 2023, 59, 294. https://doi.org/10.3390/medicina59020294
Bougea A, Papagiannakis N, Simitsi A-M, Panagiotounakou E, Chrysovitsanou C, Angelopoulou E, Koros C, Stefanis L. Ambiental Factors in Parkinson’s Disease Progression: A Systematic Review. Medicina. 2023; 59(2):294. https://doi.org/10.3390/medicina59020294
Chicago/Turabian StyleBougea, Anastasia, Nikolas Papagiannakis, Athina-Maria Simitsi, Elpida Panagiotounakou, Chrysa Chrysovitsanou, Efthalia Angelopoulou, Christos Koros, and Leonidas Stefanis. 2023. "Ambiental Factors in Parkinson’s Disease Progression: A Systematic Review" Medicina 59, no. 2: 294. https://doi.org/10.3390/medicina59020294
APA StyleBougea, A., Papagiannakis, N., Simitsi, A.-M., Panagiotounakou, E., Chrysovitsanou, C., Angelopoulou, E., Koros, C., & Stefanis, L. (2023). Ambiental Factors in Parkinson’s Disease Progression: A Systematic Review. Medicina, 59(2), 294. https://doi.org/10.3390/medicina59020294










