Abdominal Lymphadenopathies: Lymphoma, Brucellosis or Tuberculosis? Multidisciplinary Approach—Case Report and Review of the Literature
Abstract
1. Introduction
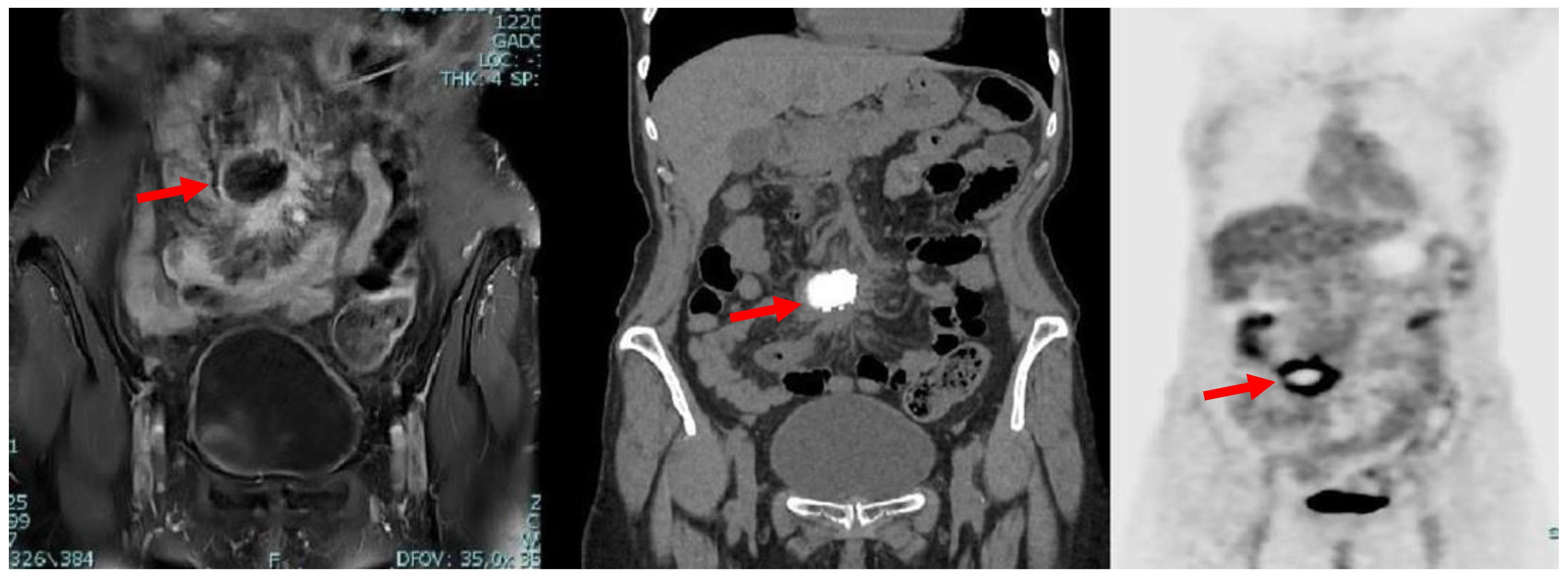
2. Case Description
“Pneumoperitoneum with open technique in the supraumbilical site. Introduction of 2 trocars. Exploration of the abdominal cavity reveals a voluminous nodule at the level of the meso-ileum; multiple ileal loops and a section of the transverse colon are attracted to the nodule. Considering the anatomical-surgical picture, laparotomic conversion is necessary. Supra-sub-umbilical midline incision. Release of the afore-mentioned intestinal loops from the neoformation and removal of the nodule which is sent for extemporaneous histological examination (non-neoplastic process, possibly infectious)…”.
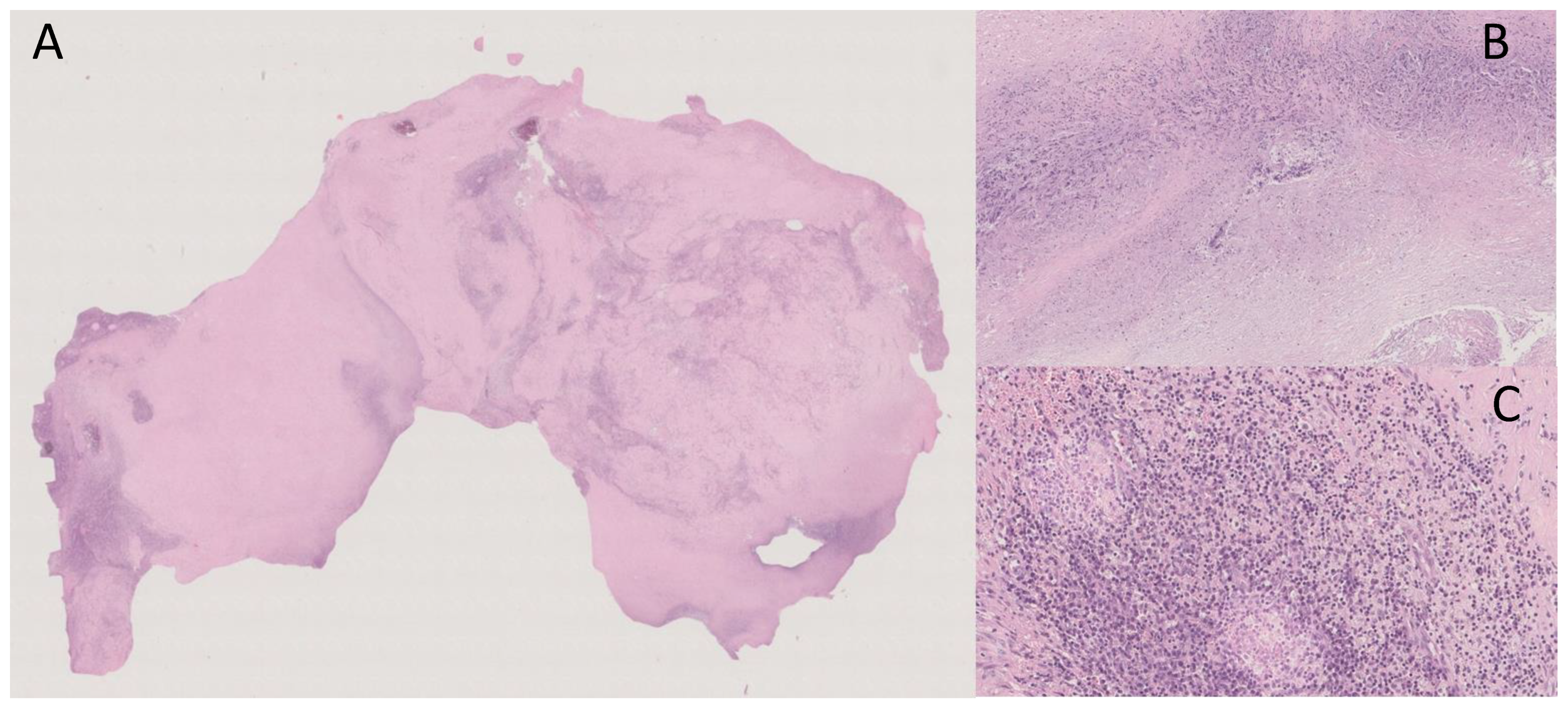
“Macroscopically, the surgical specimen consisted of solid nodular fragment, whitish in color, 4 × 3.5 × 3 cm in size, homogeneous in cut surface, partially calcified. Microscopically, Hematoxylin and Eosin stained sections documented extensive fibrosis with dystrophic calcifications and foci of lymphomonocytic, plasma cell and granulocytic infiltrate, mixed with areas of necrosis. To investigate the presence of fungi and/or mycobacteria, histochemistry was performed with Periodic Acid-Schiff (PAS), PAS-D (Periodic Acid-Schiff with diastase), Grocott’s and Ziehl- Neelsen stains in seriate sections. No fungi and mycobacteria were detected. A descriptive final diagnosis of fibro-hyaline nodule with mixed inflammatory infiltrate and suppurative necrosis was made, advising to carry out culture tests and integration with clinical-serological data” (Figure 3).
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Natesan, S.; Lee, J.; Volkamer, H.; Thoureen, T. Evidence-Based Medicine Approach to Abdominal Pain. Emerg. Med. Clin. N. Am. 2016, 34, 165–190. [Google Scholar] [CrossRef] [PubMed]
- Addolorato, G.; Mirijello, A.; D’Angelo, C.; Leggio, L.; Ferrulli, A.; Abenavoli, L.; Vonghia, L.; Cardone, S.; Leso, V.; Cossari, A.; et al. State and trait anxiety and depression in patients affected by gastrointestinal diseases: Psychometric evaluation of 1641 patients referred to an internal medicine outpatient setting. Int. J. Clin. Pract. 2008, 62, 1063–1069. [Google Scholar] [CrossRef]
- Gaddey, H.L.; Riegel, A.M. Unexplained Lymphadenopathy: Evaluation and Differential Diagnosis. Am. Fam. Physician 2016, 94, 896–903. [Google Scholar]
- Mirijello, A.; D’Errico, M.M.; Notarangelo, S.; De Cosmo, S. The role of medical history in the diagnostic process of unexplainable weight loss. BMJ. Case. Rep. 2019, 12, e231182. [Google Scholar] [CrossRef] [PubMed]
- Shakir, R. Brucellosis. J. Neurol. Sci. 2021, 15, 117280. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.K.; Sehrawat, A.; Tiwari, R.; Prasad, M.; Gulati, B.; Shabbir, M.Z.; Chhabra, R.; Karthik, K.; Patel, S.K.; Pathak, M.; et al. Bovine brucellosis—A comprehensive review. Vet. Q. 2021, 41, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Köse, Ş.; Serin Senger, S.; Akkoçlu, G.; Kuzucu, L.; Ulu, Y.; Ersan, G.; Oğuz, F. Clinical manifestations, complications, and treatment of brucellosis: Evaluation of 72 cases. Turk. J. Med. Sci. 2014, 44, 220–223. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report; WHO: Geneva, Switzerland, 2018; Available online: https://www.who.int/tb/publications/global_report/en/ (accessed on 30 January 2023).
- Lewinsohn, D.M.; Leonard, M.K.; LoBue, P.A.; Cohn, D.L.; Daley, C.L.; Desmond, E.; Keane, J.; Lewinsohn, D.A.; Loeffler, A.M.; Mazurek, G.H.; et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin. Infect. Dis. 2017, 64, 111–115. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, M. Disseminated tuberculosis mimicking abdominal metastatic carcinoma: A case report. Medicine 2021, 100, e27886. [Google Scholar] [CrossRef]
- Lucey, B.C.; Stuhlfaut, J.W.; Soto, J.A. Mesenteric lymph nodes seen at imaging: Causes and significance. Radiographics 2005, 25, 351–365. [Google Scholar] [CrossRef]
- Massoud, M.; Kerbage, F.; Antoun, L.; Merhi, R.; Hallit, S.; Hallit, R. Brucellosis: A lymphoma-like presentation. Asian Pac. J. Trop. Med. 2017, 10, 833–834. [Google Scholar] [CrossRef]
- Hayoun, M.A.; Muco, E.; Shorman, M. Brucellosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ulu-Kilic, A.; Metan, G.; Alp, E. Clinical presentations and diagnosis of brucellosis. Recent Pat. Antiinfect. Drug Discov. 2013, 8, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Buzgan, T.; Karahocagil, M.K.; Irmak, H.; Baran, A.I.; Karsen, H.; Evirgen, O.; Akdeniz, H. Clinical manifestations and complications in 1028 cases of brucellosis: A retrospective evaluation and review of the literature. Int. J. Infect. Dis. 2010, 14, e469–e478. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ma, J.; Chen, X.; Dong, L. A 10-year retrospective comparative analysis of the clinical features of brucellosis in children and adults. J. Infect. Dev. Ctries. 2021, 15, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Xie, S.; Lu, X.; Sun, L.; Zhou, Y.; Zhang, Y.; Wang, K. A Systematic Review and Meta-Analysis of Epidemiology and Clinical Manifestations of Human Brucellosis in China. Biomed. Res. Int. 2018, 2018, 5712920. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.V.; Al-Aska, A.K.; Subesinghe, N.; Wright, S.G. Unusual presentation of culture positive brucellosis. Postgrad. Med. J. 1988, 64, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Göke, M.; Neurath, M.; Braunstein, S.; Daniello, S.; Knolle, P.; Dippold, W.; Meyer zum Büschenfelde, K.H. Brucellosis: Differential diagnosis of acute abdominal pain. Z. Gastroenterol. 1993, 31, 671–674. [Google Scholar]
- Rodrigues Dos Santos, J.; Silva, R.; Nejo, P.; Vassalo, T.; Coimbra, A.; Peixoto, L. A Case of Brucellosis with Possible Ileal Involvement. GE Port. J. Gastroenterol. 2020, 27, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Pourbagher, M.A.; Pourbagher, A.; Savas, L.; Turunc, T.; Demiroglu, Y.Z.; Erol, I.; Yalcintas, D. Clinical pattern and abdominal sonographic findings in 251 cases of brucellosis in southern Turkey. AJR. Am. J. Roentgenol. 2006, 187, W191–W194. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Yang, Y. Evasion of anti-infectious immunity by Brucella—A review. Wei Sheng Wu Xue Bao 2016, 56, 747–752. [Google Scholar] [PubMed]
- Billard, E.; Cazevieille, C.; Dornand, J.; Gross, A. High susceptibility of human dendritic cells to invasion by the intracellular pathogens Brucella suis, B. abortus, and B. melitensis. Infect. Immun. 2005, 73, 8418–8424. [Google Scholar] [CrossRef] [PubMed]
- Porte, F.; Naroeni, A.; Ouahrani-Bettache, S.; Liautard, J.P. Role of the Brucella suis lipopolysaccharide O antigen in phagosomal genesis and in inhibition of phagosome-lysosome fusion in murine macrophages. Infect. Immun. 2003, 71, 1481–1490. [Google Scholar] [CrossRef]
- Carranza, C.; Chavez-Galan, L. Several Routes to the Same Destination: Inhibition of Phagosome-Lysosome Fusion by Mycobacterium tuberculosis. Am. J. Med. Sci. 2019, 357, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Masoudian, M.; Derakhshandeh, A.; Ghahramani Seno, M.M. Brucella melitensis and Mycobacterium tuberculosis depict overlapping gene expression patterns induced in infected THP-1 macrophages. Iran J. Vet. Res. 2015, 16, 368–373. [Google Scholar] [PubMed]
- Dasari, S.; Naha, K.; Prabhu, M. Brucellosis and tuberculosis: Clinical overlap and pitfalls. Asian Pac. J. Trop. Med. 2013, 6, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Zhou, J.; Jiang, X. Diagnosis and management of spinal tuberculosis combined with brucellosis: A case report and literature review. Exp. Ther. Med. 2018, 15, 3455–3458. [Google Scholar] [CrossRef]
- Erdem, H.; Senbayrak, S.; Gencer, S.; Hasbun, R.; Karahocagil, M.K.; Sengoz, G.; Karsen, H.; Kaya, S.; Civljak, R.; Inal, A.S.; et al. Tuberculous and brucellosis meningitis differential diagnosis. Travel Med. Infect. Dis. 2015, 13, 185–191. [Google Scholar] [CrossRef]
- Esendagli-Yilmaz, G.; Uluoglu, O. Pathologic basis of pyogenic, nonpyogenic, and other spondylitis and discitis. Neuroimaging Clin. N. Am. 2015, 25, 159–161. [Google Scholar] [CrossRef]

| Results | Normal Values | |
|---|---|---|
| Hemoglobin (g/dL) | 10.8 | 14.00–18.00 |
| MCV (fl) | 85 | 77.00–98.00 |
| PLT (U/mm³) | 261.000 | 130.00–400.00 |
| WBC (U/mm³) | 4840 | 4.30–10.80 |
| ESR (mm/h) | 42 | 2.0–15.0 |
| PCR (mg/dL) | 2.39 | <0.30 |
| Glycemia (mg/dL) | 67 | 60–100 |
| Total blood proteins (g/dL) | 5.8 | 6.40–8.20 |
| Albumin/serum (g/dL) | 3.1 | 3.50–5.50 |
| Sideremia (mcg/dL) | 41 | 65.00–175.00 |
| Ferritin (ng/mL) | 36 | 26.0–388.0 |
| Transferrin (mg/dL) | 178 | 200–360 |
| Transferrin saturation levels (%) | 18.4 | 20–50 |
| Albumin (%) | 55.1 | 55.80–66.10 |
| Alfa-1-globulin (%) | 6.6% | 2.90–4.90 |
| Alfa-2-globulin (%) | 13.6% | 7.10–11.80 |
| Beta-1-globulin (%) | 6.3% | 4.70–7.20 |
| Beta-2-globulin (%) | 5.3% | 3.20–6.50 |
| Gamma-globulin (%) | 13.1% | 11.10–18.50 |
| (A) | ||
| IgM | IgG | |
| Toxoplasmosis | - | + |
| Rubella | - | + |
| CMV | - | + |
| EBV | - | + |
| Herpes Simplex | - | - |
| (B) | ||
| Result (t1) | Result (t2) | |
| Salmonella Typhi | - | - |
| Parathyfoid B | - | - |
| Brucella Melitensis | + | + |
| Quantiferon TB-Gold Plus | + | INDETERMINATE |
| Authors | Age | Gender | Symptoms/Signs | Lab Exams | Radiology/Biopsy | Other | |
|---|---|---|---|---|---|---|---|
| 1 | Jayakumar et al. [18] | 19 | M | Abdominal pain, fever, vomit, abdominal rigidity, tenderness in right iliac fossa | Slightly elevated WBC | Lymph node laparotomy | Blood culture + |
| 2 | Göke et al. [19] | 34 | F | Septic fever, sweats, abdominal pain, arthralgia, hepato-splenomegaly | Pancytopenia | n.a. | n.a. |
| 3 | Massoud et al. [12] | 52 | M | Fever, chills, night sweats, abdominal pain | Normal WBC | CT scan Lymph node biopsy | Wright test + (1/1280) Blood culture + |
| 4 | Rodrigues Dos Santos et al. [20] | 68 | F | Night sweats, anorexia, colicky abdominal pain, fever, diarrhea | Leukopenia, anemia, slightly elevated CRP and ESR | Abdominal US Chest X-ray CT scan Lymph node biopsy | Blood culture + Rose-Bengal test + ELISA IgM + IgG − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirijello, A.; Ritrovato, N.; D’Agruma, A.; de Matthaeis, A.; Pazienza, L.; Parente, P.; Cassano, D.P.; Biancofiore, A.; Ambrosio, A.; Carosi, I.; et al. Abdominal Lymphadenopathies: Lymphoma, Brucellosis or Tuberculosis? Multidisciplinary Approach—Case Report and Review of the Literature. Medicina 2023, 59, 293. https://doi.org/10.3390/medicina59020293
Mirijello A, Ritrovato N, D’Agruma A, de Matthaeis A, Pazienza L, Parente P, Cassano DP, Biancofiore A, Ambrosio A, Carosi I, et al. Abdominal Lymphadenopathies: Lymphoma, Brucellosis or Tuberculosis? Multidisciplinary Approach—Case Report and Review of the Literature. Medicina. 2023; 59(2):293. https://doi.org/10.3390/medicina59020293
Chicago/Turabian StyleMirijello, Antonio, Noemi Ritrovato, Angelo D’Agruma, Angela de Matthaeis, Luca Pazienza, Paola Parente, Dario Pio Cassano, Annalucia Biancofiore, Angelo Ambrosio, Illuminato Carosi, and et al. 2023. "Abdominal Lymphadenopathies: Lymphoma, Brucellosis or Tuberculosis? Multidisciplinary Approach—Case Report and Review of the Literature" Medicina 59, no. 2: 293. https://doi.org/10.3390/medicina59020293
APA StyleMirijello, A., Ritrovato, N., D’Agruma, A., de Matthaeis, A., Pazienza, L., Parente, P., Cassano, D. P., Biancofiore, A., Ambrosio, A., Carosi, I., Serricchio, E., Graziano, P., Bazzocchi, F., Piscitelli, P., & De Cosmo, S. (2023). Abdominal Lymphadenopathies: Lymphoma, Brucellosis or Tuberculosis? Multidisciplinary Approach—Case Report and Review of the Literature. Medicina, 59(2), 293. https://doi.org/10.3390/medicina59020293








