Influence of Three Different Surgical Techniques on Microscopic Damage of Saphenous Vein Grafts—A Randomized Study
Abstract
1. Introduction
2. Methods
2.1. Study Design
Participants and Randomization
2.2. Surgical Procedure
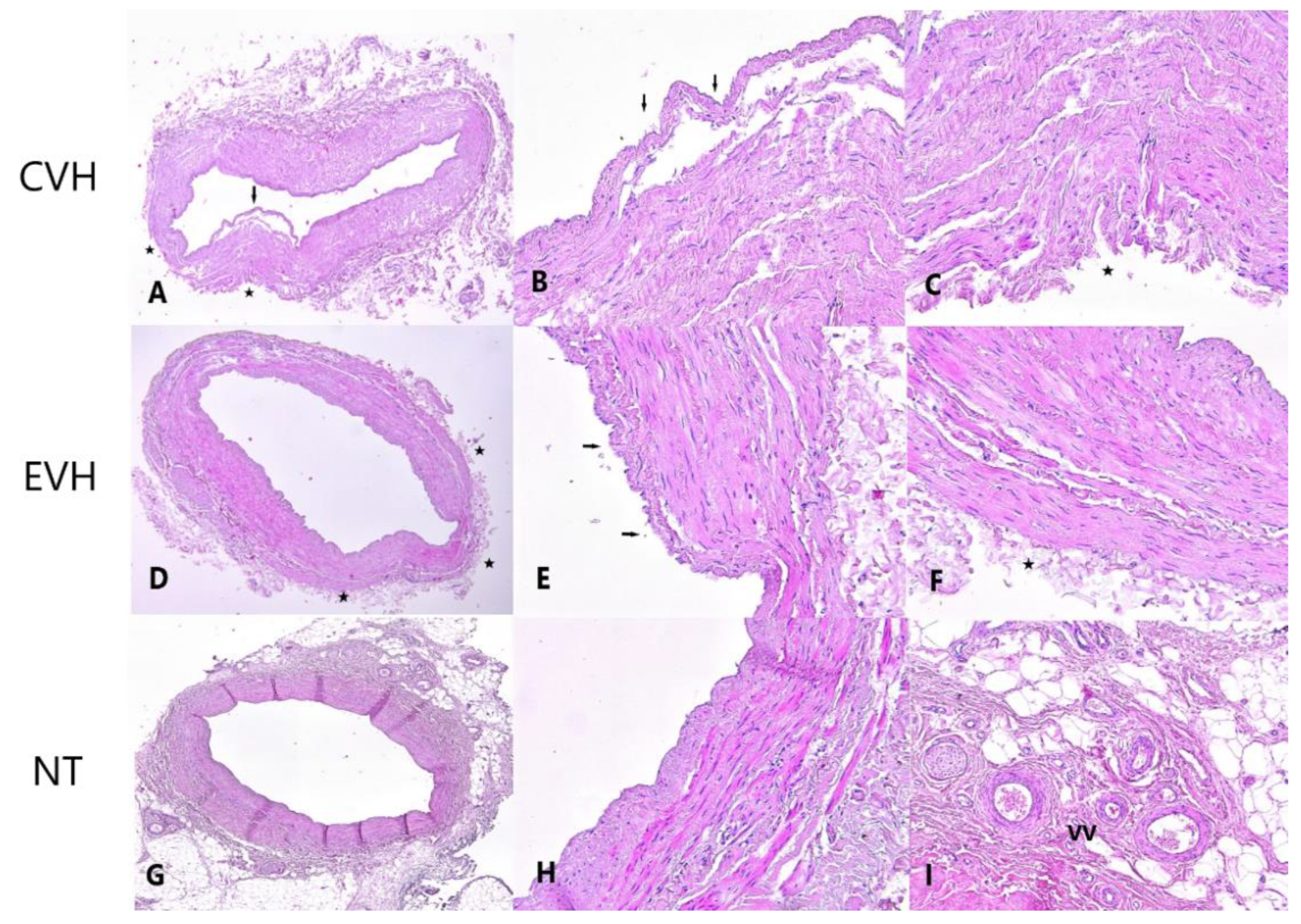
2.3. Pathohistological Protocol
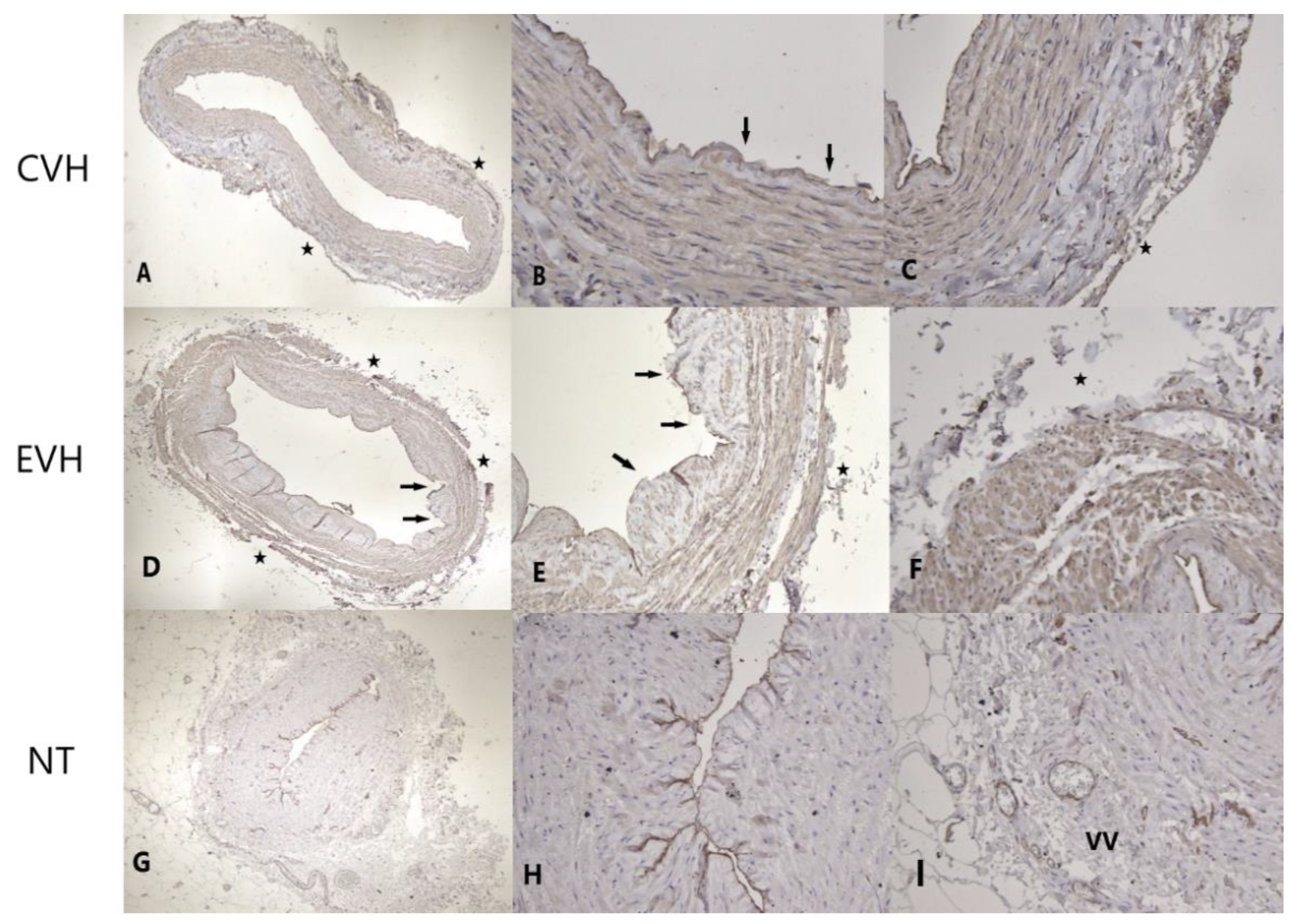
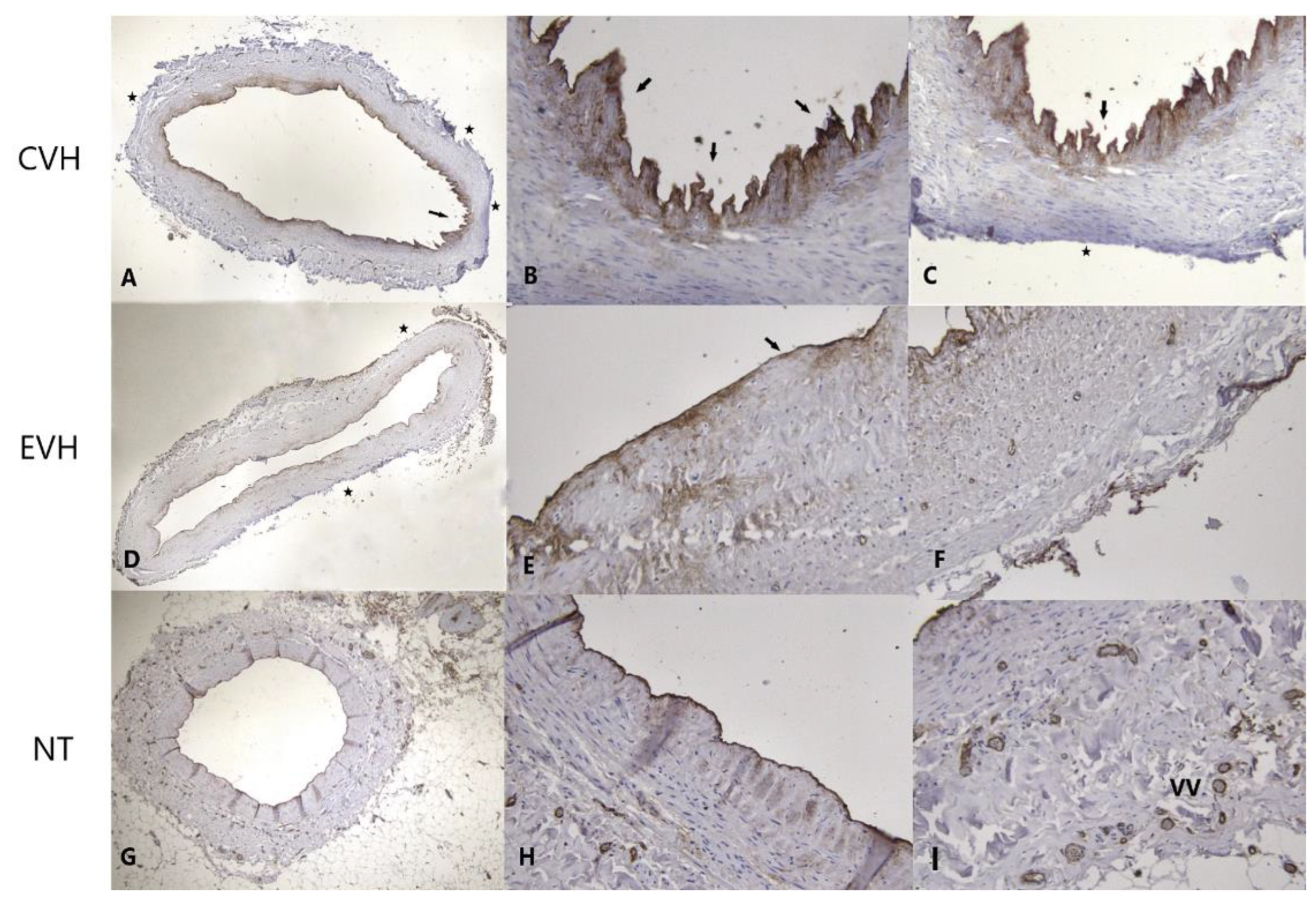
2.4. Immunohistochemistry Protocol
2.5. Statistical Protocol
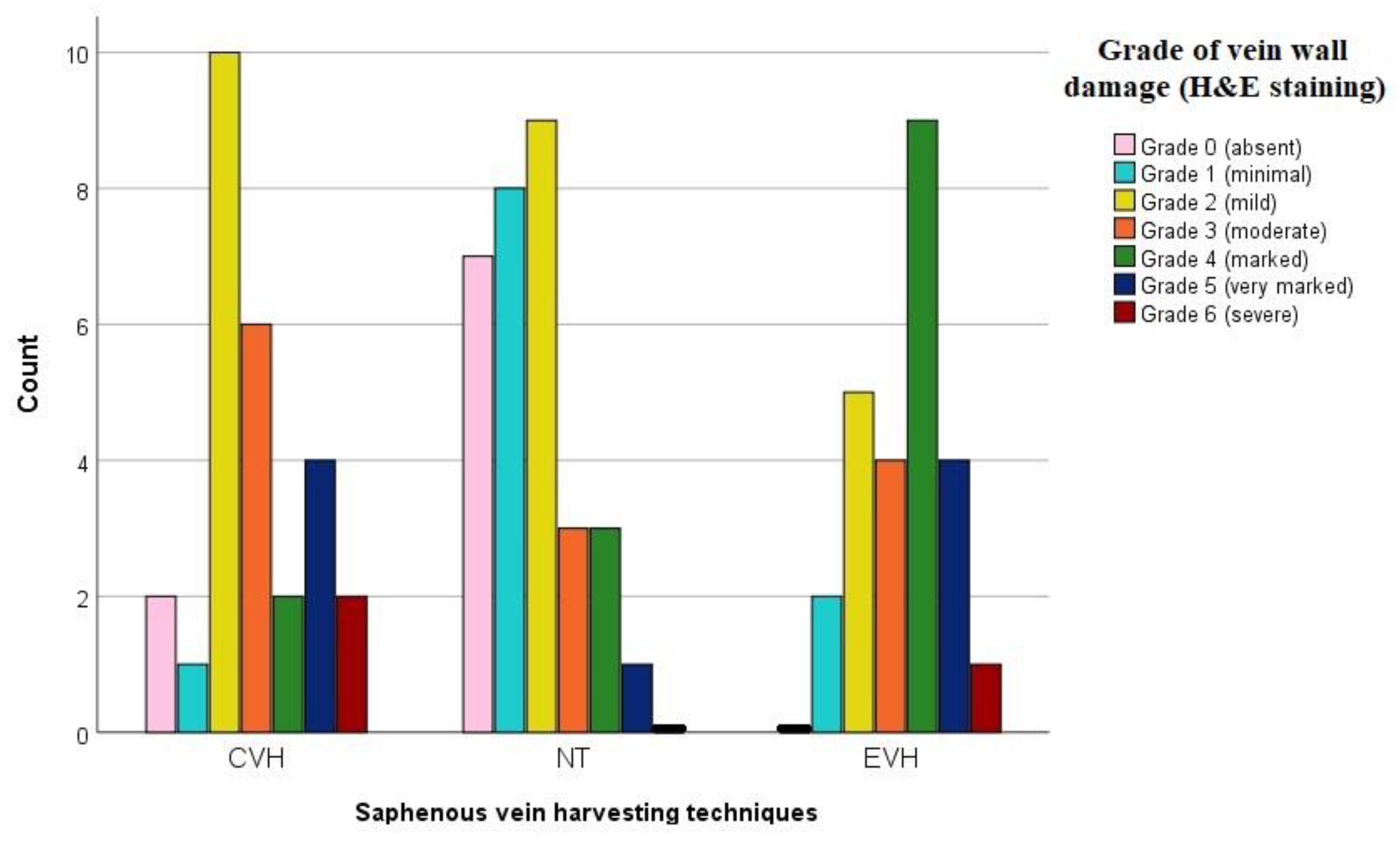
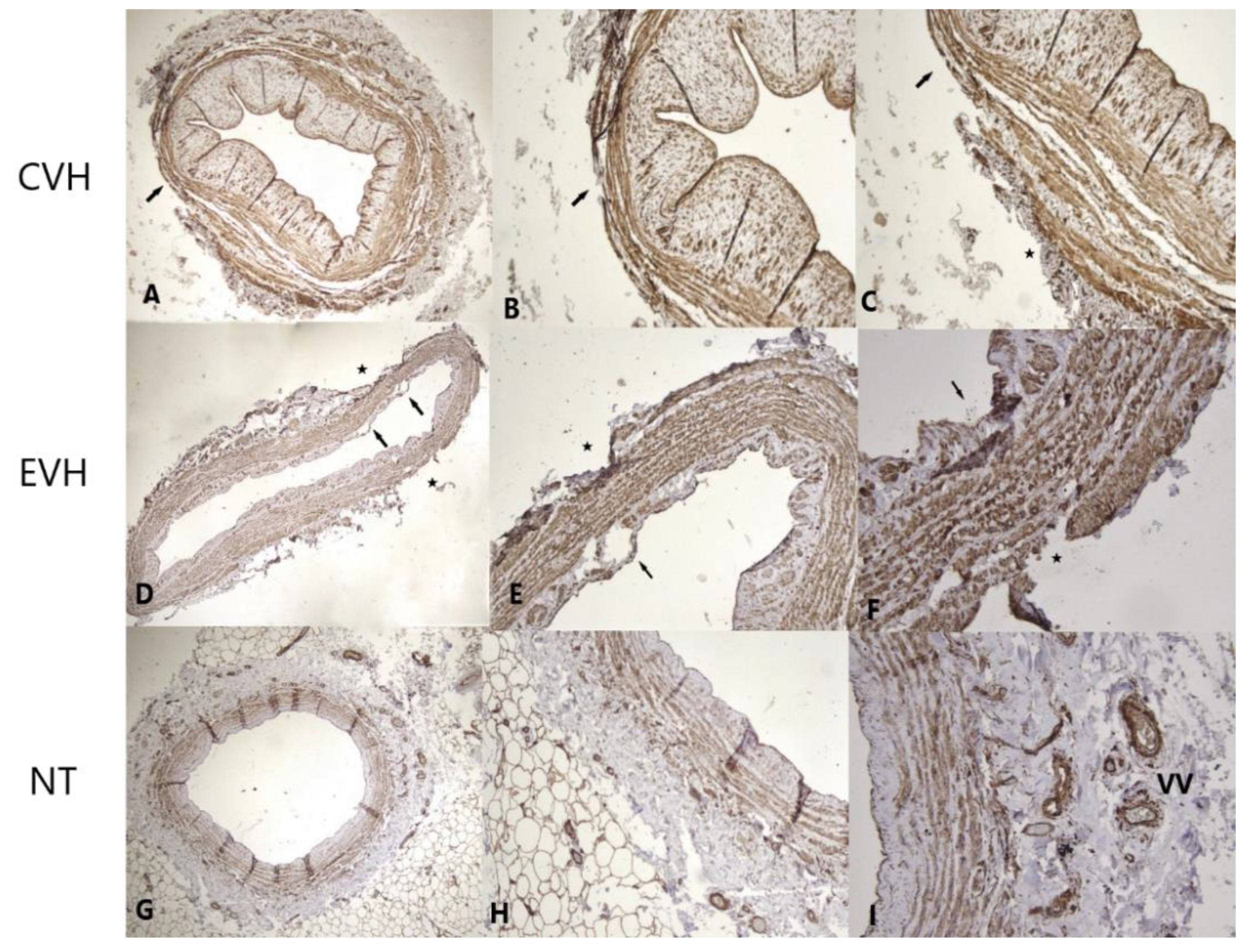
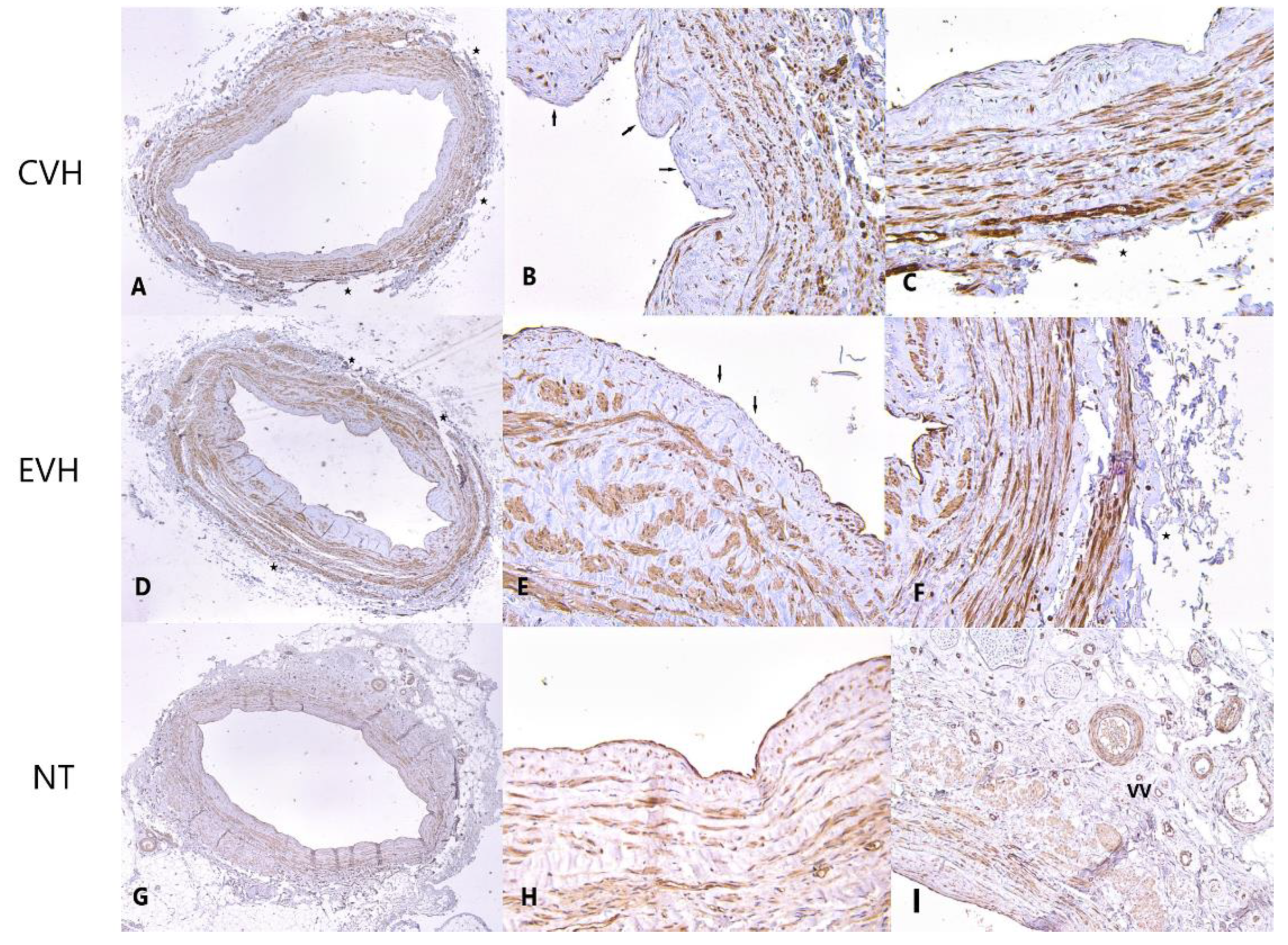
3. Results
Pathophysiological Examination
4. Discussion
4.1. Study Limitation
4.2. Futured Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CABG—coronary artery bypass grafting |
| CAD—coronary artery disease |
| CVH—conventional vein harvesting |
| eNOS—endothelial nitric oxide synthase |
| EVH—endoscopic vein harvesting |
| H&E—haematoxylin eosin |
| IQR—interquartile range |
| LITA—left internal thoracic artery |
| LAD—left anterior descending artery |
| LW—leg wound |
| MI—myocardial infarction |
| NT—no-touch harvesting |
| PCI—percutaneous coronary intervention |
| SVG—saphenous vein graft |
References
- Alexander, J.H.; Smith, P.K. Coronary-Artery Bypass Grafting. N. Engl. J. Med. 2016, 374, 1954–1964. [Google Scholar] [CrossRef]
- Kopjar, T.; Dashwood, M.R. Endoscopic Versus “No-Touch” Saphenous Vein Harvesting for Coronary Artery Bypass Grafting: A Trade-Off Between Wound Healing and Graft Patency. Angiology 2016, 67, 121–132. [Google Scholar] [CrossRef]
- Schwann, T.A.; Tatoulis, J.; Puskas, J.; Bonnell, M.; Taggart, D.; Kurlansky, P.; Jacobs, J.P.; Thourani, V.H.; O’Brien, S.; Wallace, A.; et al. Worldwide Trends in Multi-arterial Coronary Artery Bypass Grafting Surgery 2004–2014: A Tale of 2 Continents. Semin. Thorac Cardiovasc. Surg. 2017, 29, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Bakaeen, F.G.; Benedetto, U.; Di Franco, A.; Fremes, S.; Glineur, D.; Girardi, L.N.; Grau, J.; Puskas, J.D.; Ruel, M.; et al. ATLANTIC (Arterial Grafting International Consortium) Alliance members. Arterial Grafts for Coronary Bypass: A Critical Review After the Publication of ART and RADIAL. Circulation 2019, 140, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, B.; Critchley, W.R.; Venkateswaran, R.V.; Barnard, J.; Caress, A.; Fildes, J.E.; Yonan, N. A comprehensive review on learning curve associated problems in endoscopic vein harvesting and the requirement for a standardised training programme. J. Cardiothorac Surg. 2016, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.; Kiani, S.; Thiruvanthan, N.; Henkin, S.; Kurian, D.; Ziu, P.; Brown, A.; Patel, N.; Poston, R. Impact of the learning curve for endoscopic vein harvest on conduit quality and early graft patency. Ann. Thorac Surg. 2011, 91, 1385–1391. [Google Scholar] [CrossRef]
- Caliskan, E.; De Souza, D.R.; Boening, A.; Liakopoulos, O.J.; Choi, Y.H.; Pepper, J.; Gibson, C.M.; Perrault, L.P.; Wolf, R.K.; Kim, K.B.; et al. Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat. Rev. Cardiol. 2020, 17, 155–169. [Google Scholar] [CrossRef]
- Parang, P.; Arora, R. Coronary vein graft disease: Pathogenesis and prevention. Can. J. Cardiol. 2009, 25, e57–e62. [Google Scholar] [CrossRef]
- Guida, G.; Ward, A.O.; Bruno, V.D.; George, S.J.; Caputo, M.; Angelini, G.D.; Zakkar, M. Saphenous vein graft disease, pathophysiology, prevention, and treatment. A review of the literature. J. Card Surg. 2020, 35, 1314–1321. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Wu, Z.; Liu, Z.; Zheng, J. Mid-term and long-term outcomes of endoscopic versus open vein harvesting for coronary artery bypass: A systematic review and meta-analysis. Int. J. Surg. 2019, 72, 167–173. [Google Scholar] [CrossRef]
- Verma, S.; Lovren, F.; Pan, Y.; Yanagawa, B.; Deb, S.; Karkhanis, R.; Quan, A.; Teoh, H.; Feder-Elituv, R.; Moussa, F.; et al. Pedicled no-touch saphenous vein graft harvest limits vascular smooth muscle cell activation: The PATENT saphenous vein graft study. Eur. J. Cardiothorac. Surg. 2014, 45, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Shimamura, J.; Takagi, H.; Kuno, T. Harvesting techniques of the saphenous vein graft for coronary artery bypass: Insights from a network meta-analysis. J. Card Surg. 2021, 36, 4369–4375. [Google Scholar] [CrossRef] [PubMed]
- Loesch, A.; Dashwood, M.R. Vasa vasorum inside out/outside in communication: A potential role in the patency of saphenous vein coronary artery bypass grafts. J. Cell Commun. Signal. 2018, 12, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Dreifaldt, M.; Souza, D.S.; Loesch, A.; Muddle, J.R.; Karlsson, M.G.; Filbey, D.; Bodin, L.; Norgren, L.; Dashwood, M.R. The “no-touch” harvesting technique for vein grafts in coronary artery bypass surgery preserves an intact vasa vasorum. J. Thorac Cardiovasc. Surg. 2011, 141, 145–150. [Google Scholar] [CrossRef]
- Vasilakis, V.; Dashwood, M.R.; Souza, D.S.; Loesch, A. Human saphenous vein and coronary bypass surgery: Scanning electron microscopy of conventional and ‘no-touch’ vein grafts. Vasc. Dis. Prevent. 2004, 1, 133–139. [Google Scholar] [CrossRef]
- Kiani, S.; Desai, P.H.; Thirumvalavan, N.; Kurian, D.J.; Flynn, M.M.; Zhao, X.; Poston, R.S. Endoscopic venous harvesting by inexperienced operators compromises venous graft remodeling. Ann. Thorac Surg. 2012, 93, 11–17, discussion 17–18. [Google Scholar] [CrossRef]
- Samano, N.; Dashwood, M.; Souza, D. No-touch vein grafts and the destiny of venous revascularization in coronary artery bypass grafting—A 25th anniversary perspective. Ann. Cardiothorac. Surg. 2018, 7, 681–685. [Google Scholar] [CrossRef]
- Zaborska, K.E.; Wareing, M.; Austin, C. Comparisons between perivascular adipose tissue and the endothelium in their modulation of vascular tone. Br. J. Pharmacol. 2017, 174, 3388–3397. [Google Scholar] [CrossRef]
- Samano, N.; Geijer, H.; Liden, M.; Fremes, S.; Bodin, L.; Souza, D. The no-touch saphenous vein for coronary artery bypass grafting maintains a patency, after 16 years, comparable to the left internal thoracic artery: A randomized trial. J. Thorac Cardiovasc. Surg. 2015, 150, 880–888. [Google Scholar] [CrossRef]
- Kopjar, T.; Gasparovic, H.; Biocina, B. The no-touch saphenous vein should be considered in a risk score of vein graft failure. J. Thorac Cardiovasc. Surg. 2020, 160, e1–e2. [Google Scholar] [CrossRef]
- Li, F.D.; Eagle, S.; Brophy, C.; Hocking, K.M.; Osgood, M.; Komalavilas, P.; Cheung-Flynn, J. Pressure control during preparation of saphenous veins. JAMA Surg. 2014, 149, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.D.; de Castro Miguel, E.; Pinheiro Paiva, S.K.; Silva Matos, J.I.; Mesquita Fernandes, M.A.; Fechine Jamacaru, F.V. Effect of preservation solution and distension pressure on saphenous vein’s endothelium. Interact. Cardiovasc. Thorac Surg. 2022, 16, ivac124. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Yamazaki, M.; Ohono, M.; Yamashita, K.; Izumida, H.; Hayashi, K.; Takahashi, T.; Kimura, N.; Ito, T.; Shimizu, H. No-touch saphenous vein graft harvesting technique for coronary artery bypass grafting. Gen. Thorac Cardiovasc. Surg. 2020, 68, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.Y.; Kim, M.A.; Seo, J.W.; Kim, K.B. Endothelial preservation of the minimally manipulated saphenous vein composite graft: Histologic and immunohistochemical study. J. Thorac Cardiovasc. Surg. 2012, 144, 690–696. [Google Scholar] [CrossRef]
- Saito, T.; Kurazumi, H.; Suzuki, R.; Matsuno, Y.; Mikamo, A.; Hamano, K. Preserving the endothelium in saphenous vein graft with both conventional and no-touch preparation. J. Cardiothorac. Surg. 2020, 15, 317. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Wang, X.; Sun, H.; Feng, W.; Song, Y.; Lu, F.; Wang, L.; Wang, Y.; Xu, B.; Wang, H.; et al. No-Touch Versus Conventional Vein Harvesting Techniques at 12 Months After Coronary Artery Bypass Grafting Surgery: Multicenter Randomized, Controlled Trial. Circulation 2021, 144, 1120–1129. [Google Scholar] [CrossRef]
- Lopes, R.D.; Hafley, G.E.; Allen, K.B.; Ferguson, T.B.; Peterson, E.D.; Harrington, R.A.; Mehta, R.H.; Gibson, C.M.; Mack, M.J.; Kouchoukos, N.T.; et al. Endoscopic versus open vein-graft harvesting in coronary-artery bypass surgery. N. Engl. J. Med. 2009, 361, 235–244. [Google Scholar] [CrossRef]
- Zenati, M.A.; Bhatt, D.L.; Bakaeen, F.G.; Stock, E.M.; Biswas, K.; Gaziano, J.M.; Kelly, R.F.; Tseng, E.E.; Bitondo, J.; Quin, J.A.; et al. REGROUP Trial Investigators. Randomized Trial of Endoscopic or Open Vein-Graft Harvesting for Coronary-Artery Bypass. N. Engl. J. Med. 2019, 380, 132–141. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zivkovic, I.; Krasic, S.; Stankovic, M.; Milacic, P.; Milutinovic, A.; Zdravkovic, D.; Tabakovic, Z.; Peric, M.; Krstic, M.; Bojic, M.; et al. Influence of Three Different Surgical Techniques on Microscopic Damage of Saphenous Vein Grafts—A Randomized Study. Medicina 2023, 59, 217. https://doi.org/10.3390/medicina59020217
Zivkovic I, Krasic S, Stankovic M, Milacic P, Milutinovic A, Zdravkovic D, Tabakovic Z, Peric M, Krstic M, Bojic M, et al. Influence of Three Different Surgical Techniques on Microscopic Damage of Saphenous Vein Grafts—A Randomized Study. Medicina. 2023; 59(2):217. https://doi.org/10.3390/medicina59020217
Chicago/Turabian StyleZivkovic, Igor, Stasa Krasic, Milica Stankovic, Petar Milacic, Aleksandar Milutinovic, Djordje Zdravkovic, Zoran Tabakovic, Miodrag Peric, Miljan Krstic, Milovan Bojic, and et al. 2023. "Influence of Three Different Surgical Techniques on Microscopic Damage of Saphenous Vein Grafts—A Randomized Study" Medicina 59, no. 2: 217. https://doi.org/10.3390/medicina59020217
APA StyleZivkovic, I., Krasic, S., Stankovic, M., Milacic, P., Milutinovic, A., Zdravkovic, D., Tabakovic, Z., Peric, M., Krstic, M., Bojic, M., Milic, D., & Micovic, S. (2023). Influence of Three Different Surgical Techniques on Microscopic Damage of Saphenous Vein Grafts—A Randomized Study. Medicina, 59(2), 217. https://doi.org/10.3390/medicina59020217





