Simple and Safe: Inverse Plication of the Posterior Mitral Leaflet in Everyday Mitral Valve Reconstruction with and without Concomitant Procedures
Abstract
1. Introduction
2. Patients and Methods
2.1. Ethical Statement
2.2. Statistical Analysis
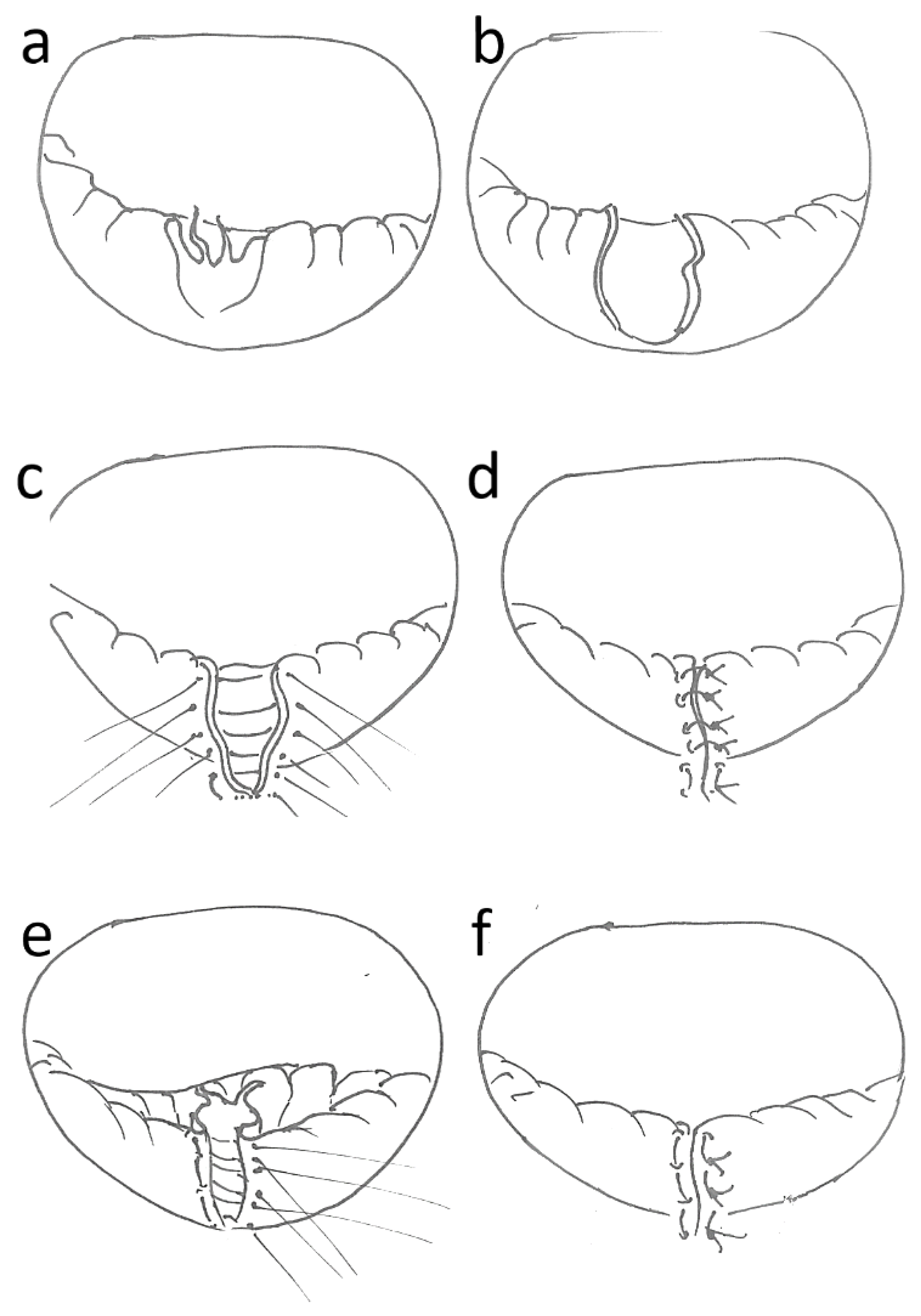
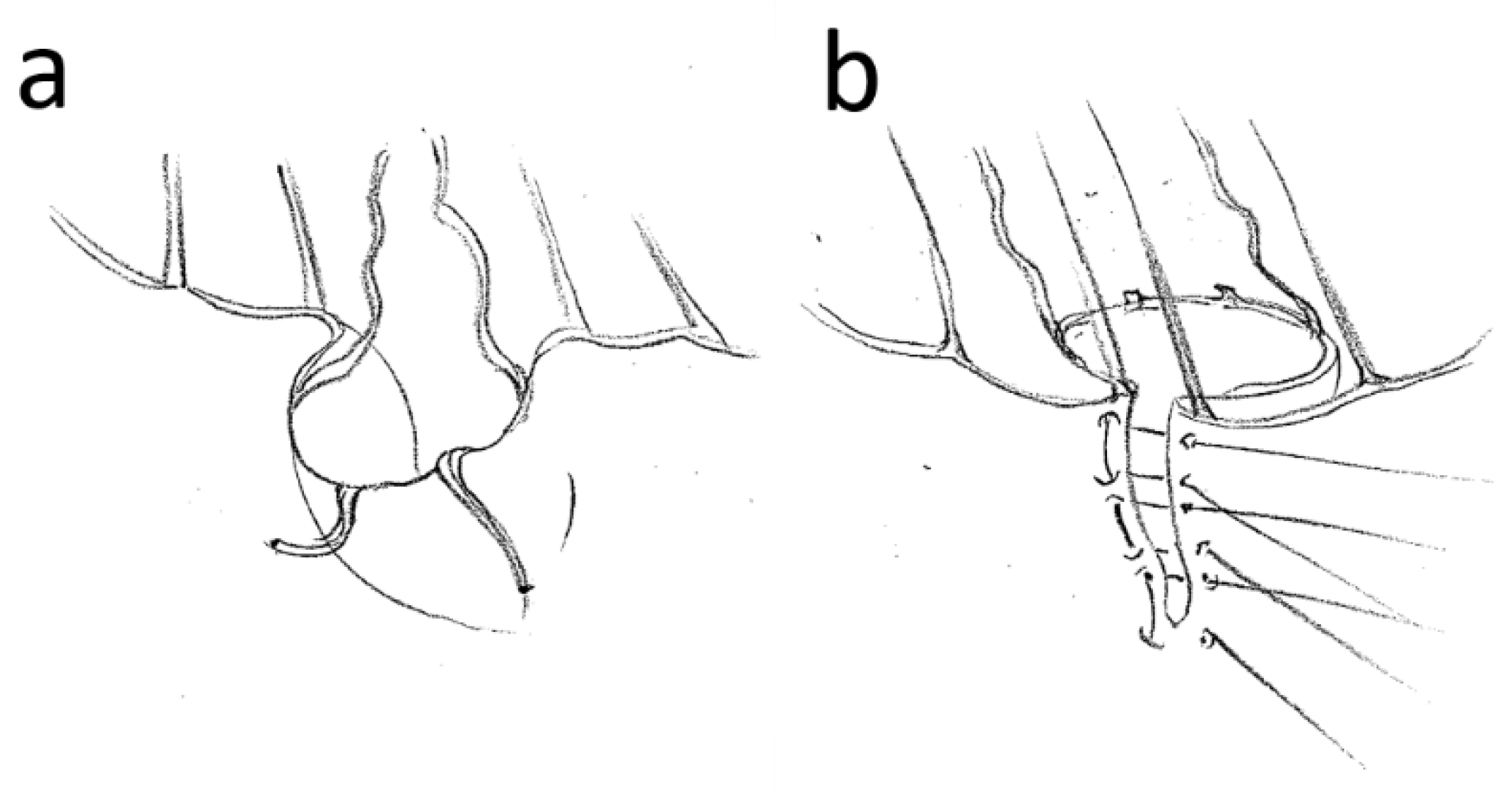
2.3. Surgical Procedure
3. Results
3.1. Baseline and Comorbidities
3.2. Echocardiographic Finding
3.3. Surgical Procedure
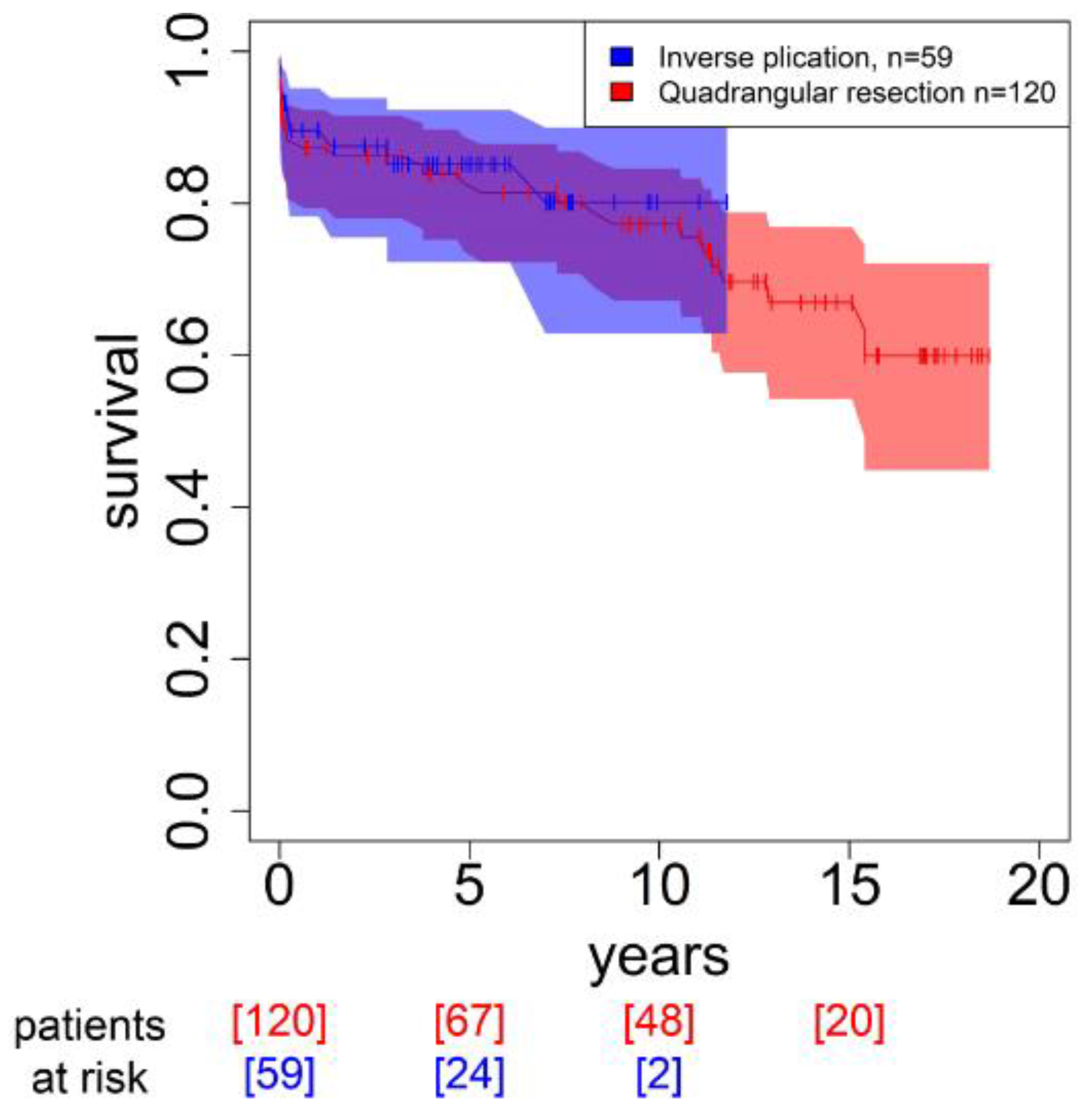
3.4. Outcome and Mortality
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Del Forno, B.; Ascione, G.; De Bonis, M. Advances in Mitral Valve Repair for Degenerative Mitral Regurgitation: Philosophy, Technical Details, and Long-Term Results. Cardiol. Clin. 2021, 39, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A. Reconstructive Valvuloplasty. A New Technique of Mitral Valvuloplasty. Presse Med. 1969, 77, 251–253. (In French) [Google Scholar]
- Carpentier, A.; Deloche, A.; Dauptain, J.; Soyer, R.; Blondeau, P.; Piwnica, A.; Dubost, C.; McGoon, D. A new reconstructive operation for correction of mitral and tricuspid insufficiency. J. Thorac. Cardiovasc. Surg. 1971, 61, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hanania, G.; Deloche, A.; Marcantoni, J.P.; Gerbaux, A. Midterm results of Carpentier’s reconstructive mitral annuloplasty. Arch. Mal. Coeur Vaiss. 1973, 66, 1297–1309. [Google Scholar] [PubMed]
- Beckmann, A.; Meyer, R.; Lewandowski, J.; Markewitz, A.; Gummert, J. German Heart Surgery Report 2019: The Annual Updated Registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac. Cardiovasc. Surg. 2020, 68, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, J.; Wei, P.; Yao, X.; Zhang, Y.; Fang, L.; Chen, Z.; Liu, Y.; Tan, T.; Wu, H.; et al. Quadrangular resection versus chordal replacement for degenerative posterior mitral leaflet prolapse. Ann. Transl. Med. 2021, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Motazedian, F.; Ostovar, R.; Hartrumpf, M.; Schröter, F.; Albes, J.M. Every day mitral valve reconstruction: What has changed over the last 15 years? PLoS ONE 2022, 17, e0269537. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardiothorac. Surg. 2022. Erratum in Eur. J. Cardiothorac. Surg. 2021, 60, 727–800. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 10 August 2021).
- Ostovar, R.; Kuehnel, R.U.; Hartrumpf, M.; Zytowski, M.; Erb, M.; Albes, J.M. MitraClip: A Word of Caution Regarding an all too Liberal Indication and Delayed Referral to Surgery in Case of Failure. Eur. J. Cardiothorac. Surg. 2021, 59, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Chee, T.; Haston, R.; Togo, A.; Raja, S.G. Is a flexible mitral annuloplasty ring superior to a semi-rigid or rigid ring in terms of improvement in symptoms and survival? Interact. Cardiovasc. Thorac. Surg. 2008, 7, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Chotivatanapong, T. Mitral annuloplasty ring design and selection: Complete semi-rigid is best. JTCVS Tech. 2021, 22, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Hata, H.; Fujita, T.; Shimahara, Y.; Sato, S.; Ishibashi-Ueda, H.; Kobayashi, J. A 25-year study of chordal replacement with expanded polytetrafluoroethylene in mitral valve repair. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 463–468; discussion 468. [Google Scholar] [CrossRef] [PubMed]
- Bouchez, S.; Timmermans, F.; Philipsen, T.; François, K.; Bové, T. Comparison of the sustainability of mitral annular dynamics between two semi-rigid annuloplasty devices. Eur. J. Cardiothorac. Surg. 2019, 56, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Doll, N.; Sheytanov, V.; Labrousse, L.; A Chu, M.W.; Stefàno, P.; Mokráček, A.; Baron, O.; Li, S.; Günzinger, R. Clinical performance of a three-dimensional saddle-shaped, rigid ring for mitral valve repair. Eur. J. Cardiothorac. Surg. 2019, 55, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Sideris, K.; Boehm, J.; Voss, B.; Guenther, T.; Lange, R.S.; Guenzinger, R. Functional and Degenerative Mitral Regurgitation: One Ring Fits All? Thorac. Cardiovasc. Surg. 2020, 68, 470–477. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, G.; Noirhomme, P.; Verhelst, R.; Rubay, J.; Dion, R. Surgical repair of the prolapsing anterior leaflet in degenerative mitral valve disease. J. Heart Valve Dis. 2000, 9, 75–80; discussion 81. [Google Scholar]
- Maisano, F.; Torracca, L.; Oppizzi, M.; Stefano, P.; D’Addario, G.; La Canna, G.; Zogno, M.; Alfieri, O. The edge-to-edge technique: A simplified method to correct mitral insufficiency. Eur. J. Cardiothorac. Surg. 1998, 13, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, G.F.; Carvalho, L.; Antunes, M.J. Acute mitral regurgitation due to ruptured ePTFE neo-chordae. J. Heart Valve Dis. 2007, 16, 278–281. [Google Scholar] [PubMed]
| InvPlic (n = 60) | QuadRes (n = 120) | p | |
|---|---|---|---|
| Age (years) | 65.27 ± 13.21 | 63.1 ± 12.52 | 0.179 |
| Female sex (%) | 28.81 | 37.5 | 0.327 |
| EuroSCORE | 6.46 ± 2.75 | 5.68 ± 3.11 | 0.039 |
| Logistic EuroSCORE (%) | 8.71 ± 9.55 | 9.05 ± 12.82 | 0.225 |
| EuroSCORE II | 4.2 ± 5.24 | 1.96 ± 1.92 | 0.142 |
| Coronary heart disease (%) | 40.54% | 31.25% | 0.509 |
| Peripheral arterial disease | 2.7% | 0% | 0.435 |
| Kidney failure | 13.51% | 12.5% | 1 |
| COPD | 5.41% | 4.35% | 1 |
| Pulmonary hypertension | 16.22% | 10.87% | 0.698 |
| Urgency status | 0.438 | ||
| Elective (%) | 49.15% | 59.17% | 0.268 |
| Urgent (%) | 50.85% | 37.5% | 0.124 |
| Emergent (%) | 0% | 3.33% | 0.304 |
| Echocardiographic finding | |||
| Left ventricular ejection fraction (%) | 56.11 ± 8.95 | 57.9 ± 11.75 | 0.303 |
| TAPSE (mm) | 15.54 ± 9.55 | 18.35 ± 5.06 | 0.33 |
| Systolic left ventricular diameter (mm) | 33.58 ± 4.35 | 35.99 ± 9.17 | 0.663 |
| Diastolic left ventricular diameter (mm) | 49.98 ± 9.25 | 51.78 ± 11.71 | 0.302 |
| Left atrial diameter (mm) | 41.77 ± 10.55 | 43.15 ± 10.84 | 0.861 |
| Pulmonary arterial pressure (mmHg) | 28.86 ± 9.55 | 29.05 ± 14.19 | 0.814 |
| Vena contracta (mm) | 9.1 ± 2.18 | 9.2 ± 2.7 | 0.928 |
| EROA (cm2) | 41.25 ± 12.68 | 32.33 ± 15.76 | 0.261 |
| Regurgitation fraction (%) | 58 ± 5.87 | 54.33 ± 6.95 | 0.368 |
| InvPlic (n = 60) | QuadRes (n = 120) | p | |
|---|---|---|---|
| CPB time (min) | 136.35 ± 43.89 | 140.48 ± 48.15 | 0.669 |
| X-Clamp time (min) | 90.86 ± 30.75 | 89.5 ± 31.86 | 0.807 |
| Annuloplasty ring (%) | 100 | 100 | 1.000 |
| Hospitalization time (day) | 15.53 ± 8.7 | 16.1 ± 11.81 | 0.708 |
| Re-do procedure (%) | 5.08% | 0% (8) | 0.035 |
| Discharge status | 0.953 | ||
| Discharged (%) | 66.1% | 56.67 | 0.295 |
| Rehabilitation (%) | 10.17% | 13.33% | 0.716 |
| Transferred (%) | 16.95% | 21.67% | 0.588 |
| Isolated MV repair (%) | 40.68% | 60.83% | 0.017 |
| With CABG (%) | 15.25% | 24.17% | 0.239 |
| With AVR (%) | 16.95% | 9.17% | 0.203 |
| Postoperative complications | |||
| Stroke | 1.67% (1) | 0% | 0.863 |
| Low cardiac output syndrome | 0% | 0.83% (1) | 1 |
| Postoperative hemorrhage | 2.22% (1) | 2.04% (1) | 1 |
| Pneumonia | 5% (3) | 0.83% (1) | 0.313 |
| Wound healing disorders | 0% | 2,25% (2) | 0.244 |
| Early mortality | 5% | 8.33 % (10) | 0.55 |
| Long-term mortality | 15.25 (7) | 32.32 (32) | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostovar, R.; Motazedian, F.; Hartrumpf, M.; Schröter, F.; Albes, J.M. Simple and Safe: Inverse Plication of the Posterior Mitral Leaflet in Everyday Mitral Valve Reconstruction with and without Concomitant Procedures. Medicina 2023, 59, 218. https://doi.org/10.3390/medicina59020218
Ostovar R, Motazedian F, Hartrumpf M, Schröter F, Albes JM. Simple and Safe: Inverse Plication of the Posterior Mitral Leaflet in Everyday Mitral Valve Reconstruction with and without Concomitant Procedures. Medicina. 2023; 59(2):218. https://doi.org/10.3390/medicina59020218
Chicago/Turabian StyleOstovar, Roya, Farnoosh Motazedian, Martin Hartrumpf, Filip Schröter, and Johannes Maximilian Albes. 2023. "Simple and Safe: Inverse Plication of the Posterior Mitral Leaflet in Everyday Mitral Valve Reconstruction with and without Concomitant Procedures" Medicina 59, no. 2: 218. https://doi.org/10.3390/medicina59020218
APA StyleOstovar, R., Motazedian, F., Hartrumpf, M., Schröter, F., & Albes, J. M. (2023). Simple and Safe: Inverse Plication of the Posterior Mitral Leaflet in Everyday Mitral Valve Reconstruction with and without Concomitant Procedures. Medicina, 59(2), 218. https://doi.org/10.3390/medicina59020218








