The Left Atrial Area Derived Cardiovascular Magnetic Resonance Left Ventricular Filling Pressure Equation Shows Superiority over Integrated Echocardiography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cardiac and Pulmonary Function Assessment
2.3. Follow-Up and Cohort Stratification
2.4. Statistical Analysis
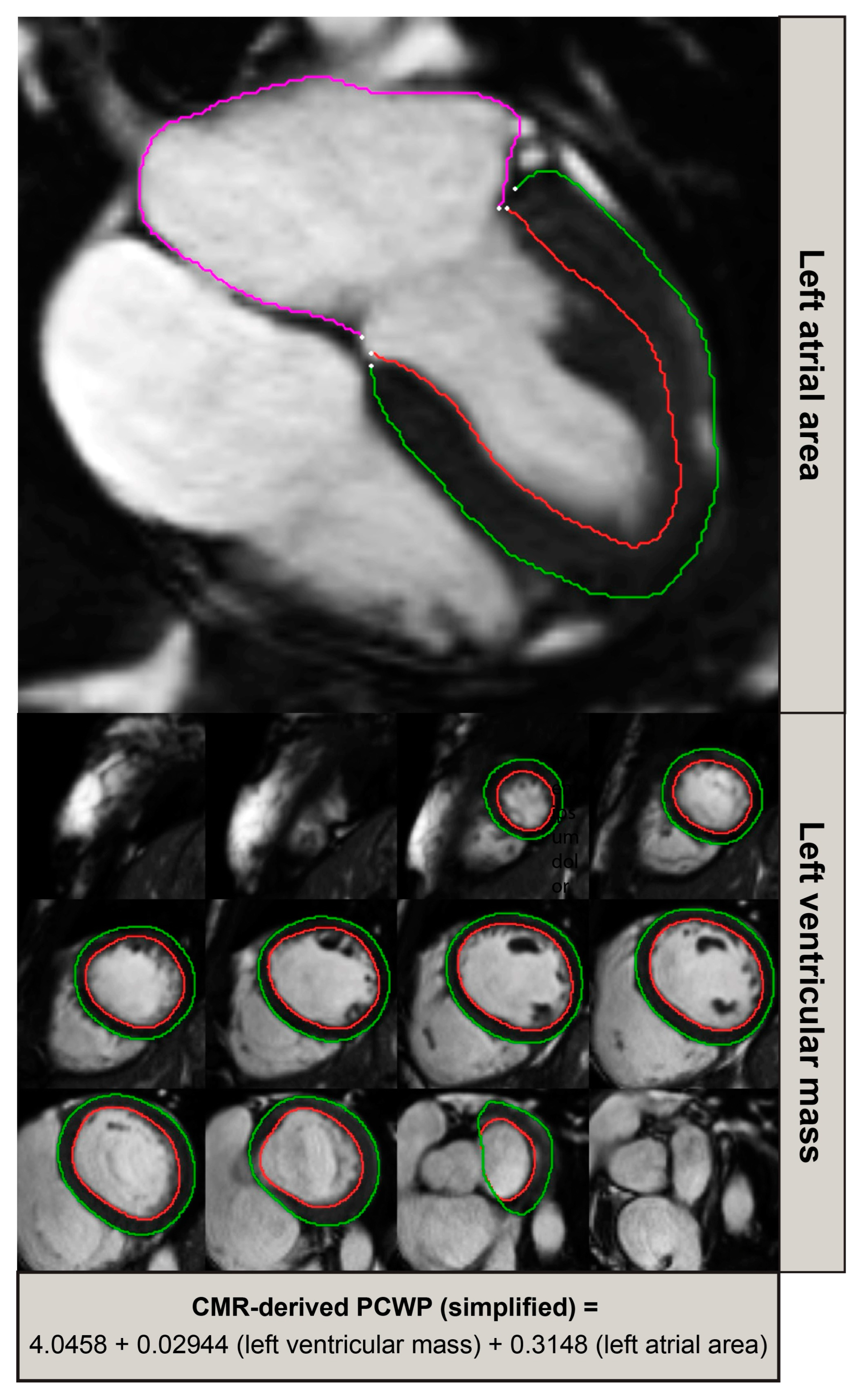
2.5. Model Development and Evaluation
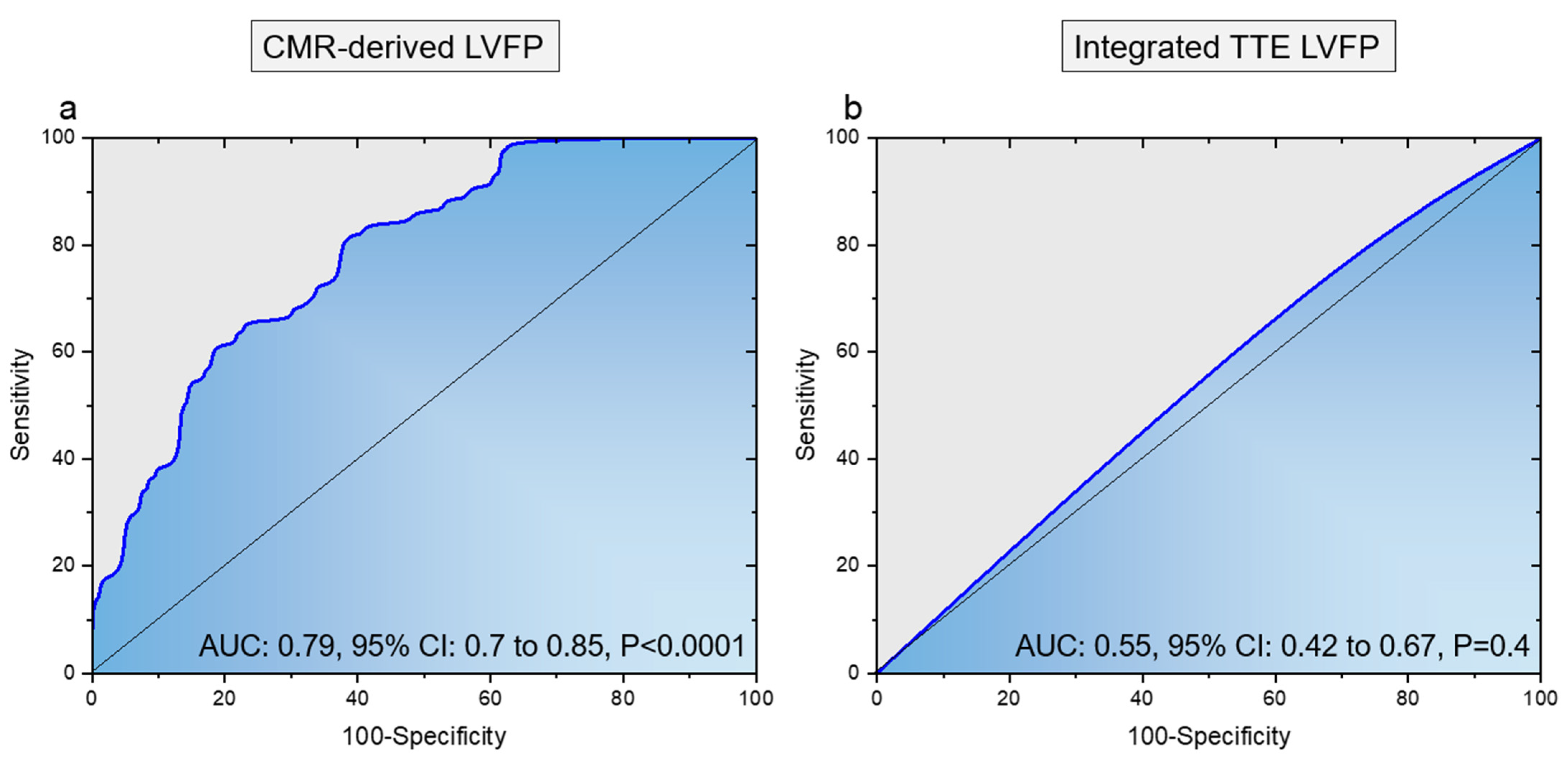
2.6. Validation and Diagnostic Performance Assessment
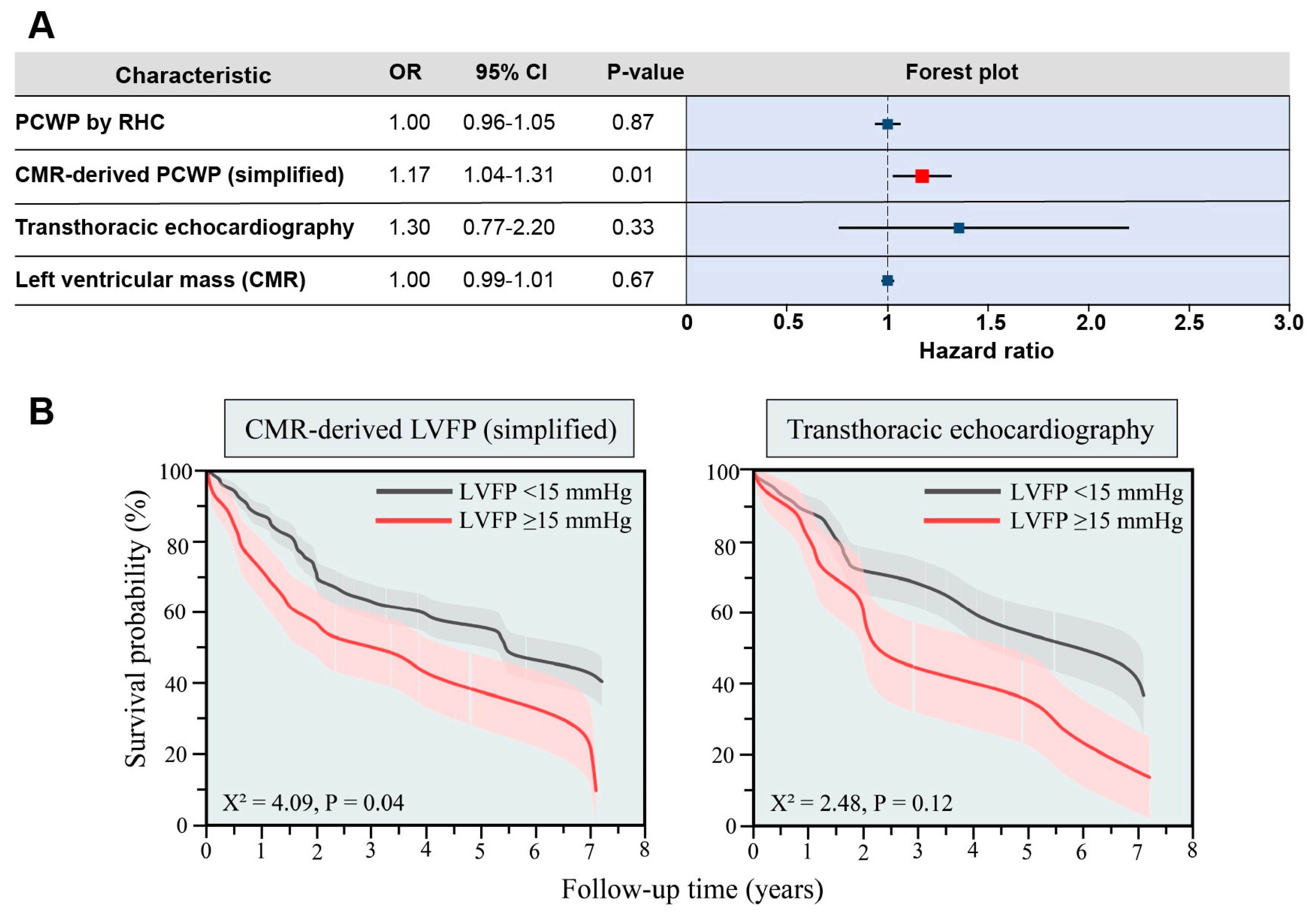
2.7. Prognostic Analysis
2.8. Software and Significance Threshold
2.9. Statistical Significance
2.10. Primary Objectives
3. Results
3.1. Derivation Cohort
3.2. Validation Cohort
4. Discussion
4.1. Main Findings
4.2. Mechanism of Findings
4.3. Clinical Translation
4.4. What This Study Adds
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA–PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Nkomo, V.T.; Badano, L.P.; Bergler-Klein, J.; Bogaert, J.; Davin, L.; Cosyns, B.; Coucke, P.; Dulgheru, R.; Edvardsen, T.; et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: A report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 721–740. [Google Scholar] [CrossRef] [PubMed]
- Nagesh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Sado, D.M.; Hasleton, J.M.; Herrey, A.S.; Moon, J.C. CMR in Heart Failure. Cardiol. Res. Pr. 2011, 2011, 739157. [Google Scholar] [CrossRef] [PubMed]
- Chamsi-Pasha, M.A.; Zhan, Y.; Debs, D.; Shah, D.J. CMR in the evaluation of diastolic dysfunction and phenotyping of HFpEF: Current role and future perspectives. JACC Cardiovasc. Imaging 2020, 13, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Gosling, R.; Swoboda, P.; Jones, R.; Rothman, A.; Wild, J.M.; Kiely, D.G.; Condliffe, R.; Alabed, S.; Swift, A.J. Cardiac magnetic resonance identifies raised left ventricular filling pressure: Prognostic implications. Eur. Heart J. 2022, 43, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Schwinger, R.H.G. Pathophysiology of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Baritussio, A.; Muthurangu, V. Cardiovascular magnetic resonance for the assessment of left ventricular filling pressure in heart failure. Eur. Heart J. 2022, 43, 2523–2525. [Google Scholar] [CrossRef] [PubMed]
- Kobirumaki-Shimozawa, F.; Inoue, T.; Shintani, S.A.; Oyama, K.; Terui, T.; Minamisawa, S.; Ishiwata, S.; Fukuda, N. Cardiac thin filament regulation and the Frank–Starling mechanism. J. Physiol. Sci. 2014, 64, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Gradman, A.H.; Alfayoumi, F. From Left Ventricular Hypertrophy to Congestive Heart Failure: Management of Hypertensive Heart Disease. Prog. Cardiovasc. Dis. 2006, 48, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Prognostic Significance of Left Ventricular Mass Change during Treatment of Hypertension|Cardiology|JAMA|JAMA Network. Available online: https://jamanetwork.com/journals/jama/fullarticle/199809 (accessed on 6 October 2023).
- Hohendanner, F.; Messroghli, D.; Bode, D.; Blaschke, F.; Parwani, A.; Boldt, L.; Heinzel, F.R. Atrial remodelling in heart failure: Recent developments and relevance for heart failure with preserved ejection fraction. ESC Heart Fail 2018, 5, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Marwick, T.H.; Popescu, B.A.; Donal, E.; Badano, L.P. Left Atrial Structure and Function, and Left Ventricular Diastolic Dysfunction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1961–1977. [Google Scholar] [CrossRef] [PubMed]

| Derivation Cohort (n = 708) | Validation Cohort (n = 127) | p-Value | |
|---|---|---|---|
| Age (years) | 66.3 ± 13.2 | 66.0 ± 12.7 | 0.82 |
| Male sex | 295 (42%) | 43 (34%) | 0.10 |
| Body surface area (m2) | 1.91 ± 0.25 | 1.87 ± 0.22 | 0.22 |
| HFpEF | 371 (52%) | 71 (56%) | 0.47 |
| HFmrEF | 29 (4.1%) | 3 (2.4%) | 0.34 |
| HFrEF | 15 (2.1%) | 8 (6.3%) | 0.008 |
| Other | 293 (41%) | 45 (35%) | 0.20 |
| Heart rate (bpm) | 76.0 ± 14.2 | 75.2 ± 14.7 | 0.5670 |
| Systolic blood pressure (mmHg) | 143.0 ± 26.2 | 145.6 ± 30.3 | 0.3174 |
| Diastolic blood pressure (mmHg) | 77.5 ± 12.6 | 78.9 ± 14.0 | 0.2644 |
| Mean arterial pressure (mmHg) | 102.0 ± 17.5 | 103.9 ± 18.9 | 0.2854 |
| Mean PCWP (mmHg) | 14.0 ± 6.2 | 13.4 ± 6.3 | 0.2866 |
| Mean right atrial pressure (mmHg) | 10.2 ± 5.8 | 8.5 ± 5.2 | 0.0016 |
| Mean pulmonary artery pressure (mmHg) | 38.4 ± 13.7 | 35.4 ± 14.0 | 0.0265 |
| Systolic pulmonary artery pressure (mmHg) | 63.3 ± 24.1 | 58.3 ± 23.2 | 0.0317 |
| Diastolic pulmonary artery pressure (mmHg) | 22.2 ± 9.6 | 20.1 ± 10.1 | 0.0230 |
| Arterial oxygen saturations (%) | 94.0 ± 4.3 | 95.4 ± 3.5 | 0.0006 |
| Venous oxygen saturations (%) | 65.7 ± 8.4 | 67.2 ± 9,1 | 0.0866 |
| Cardiac output (L) | 5.0 ± 2.0 | 4.9 ± 1.5 | 0.5523 |
| Cardiac index (L/min/m2) | 2.7 ± 1.0 | 2.7 ± 0.8 | 0.8798 |
| Left atrial volume (cm3) | 80.0 ± 43.8 | 72.5 ± 41.7 | 0.1239 |
| Left ventricular end-diastolic volume (mL) (indexed) | 58.2 ± 18.5 | 58.1 ± 19.1 | 0.9784 |
| Left ventricular end-systolic volume (mL) (indexed) | 19.6 ± 10.2 | 20.5 ± 14.9 | 0.3908 |
| Left ventricular stroke volume (mL) (indexed) | 38.6 ± 12.4 | 37.6 ± 12.4 | 0.3881 |
| Left ventricular ejection fraction (%) (indexed) | 50.6 ± 14.4 | 56.4 ± 21.4 | 0.0001 |
| Left ventricular mass (g) (indexed) | 78.8 ± 31.6 | 73.7 ± 29.1 | 0.0911 |
| Right ventricular end-diastolic volume (mL) (indexed) | 45.7 ± 25.6 | 40.5 ± 22.2 | 0.0319 |
| Right ventricular end-systolic volume (mL) (indexed) | 33.0 ± 13.7 | 33.2 ± 14.0 | 0.9438 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grafton-Clarke, C.; Matthews, G.; Gosling, R.; Swoboda, P.; Rothman, A.; Wild, J.M.; Kiely, D.G.; Condliffe, R.; Alabed, S.; Swift, A.J.; et al. The Left Atrial Area Derived Cardiovascular Magnetic Resonance Left Ventricular Filling Pressure Equation Shows Superiority over Integrated Echocardiography. Medicina 2023, 59, 1952. https://doi.org/10.3390/medicina59111952
Grafton-Clarke C, Matthews G, Gosling R, Swoboda P, Rothman A, Wild JM, Kiely DG, Condliffe R, Alabed S, Swift AJ, et al. The Left Atrial Area Derived Cardiovascular Magnetic Resonance Left Ventricular Filling Pressure Equation Shows Superiority over Integrated Echocardiography. Medicina. 2023; 59(11):1952. https://doi.org/10.3390/medicina59111952
Chicago/Turabian StyleGrafton-Clarke, Ciaran, Gareth Matthews, Rebecca Gosling, Peter Swoboda, Alexander Rothman, Jim M. Wild, David G. Kiely, Robin Condliffe, Samer Alabed, Andrew J. Swift, and et al. 2023. "The Left Atrial Area Derived Cardiovascular Magnetic Resonance Left Ventricular Filling Pressure Equation Shows Superiority over Integrated Echocardiography" Medicina 59, no. 11: 1952. https://doi.org/10.3390/medicina59111952
APA StyleGrafton-Clarke, C., Matthews, G., Gosling, R., Swoboda, P., Rothman, A., Wild, J. M., Kiely, D. G., Condliffe, R., Alabed, S., Swift, A. J., & Garg, P. (2023). The Left Atrial Area Derived Cardiovascular Magnetic Resonance Left Ventricular Filling Pressure Equation Shows Superiority over Integrated Echocardiography. Medicina, 59(11), 1952. https://doi.org/10.3390/medicina59111952





