Bio-Humoral and Non-Invasive Haemodynamic Correlates of Renal Venous Flow Patterns across the Heart Failure Spectrum
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e876–e894. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Guazzi, M.; Testani, J.M.; Borlaug, B.A. Altered Hemodynamics and End-Organ Damage in Heart Failure: Impact on the Lung and Kidney. Circulation 2020, 142, 998–1012. [Google Scholar] [CrossRef]
- Harjola, V.-P.; Mullens, W.; Banaszewski, M.; Bauersachs, J.; Brunner-La Rocca, H.-P.; Chioncel, O.; Collins, S.P.; Doehner, W.; Filippatos, G.S.; Flammer, A.J.; et al. Organ dysfunction, injury and failure in acute heart failure: From pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2017, 19, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, N.R.; Pellicori, P.; Filidei, F.; Del Punta, L.; De Biase, N.; Balletti, A.; Di Fiore, V.; Mengozzi, A.; Taddei, S.; Gargani, L.; et al. The incremental value of multi-organ assessment of congestion using ultrasound in outpatients with heart failure. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 961–971. [Google Scholar] [CrossRef]
- Mullens, W.; Abrahams, Z.; Francis, G.S.; Sokos, G.; Taylor, D.O.; Starling, R.C.; Young, J.B.; Tang, W.H.W. Importance of Venous Congestion for Worsening of Renal Function in Advanced Decompensated Heart Failure. J. Am. Coll. Cardiol. 2009, 53, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Deursen VM van Navis, G.; Voors, A.A.; Veldhuisen DJ van Hillege, H.L. Increased Central Venous Pressure Is Associated with Impaired Renal Function and Mortality in a Broad Spectrum of Patients with Cardiovascular Disease. J. Am. Coll. Cardiol. 2009, 53, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Pellicori, P.; Platz, E.; Dauw, J.; Ter Maaten, J.M.; Martens, P.; Pivetta, E.; Cleland, J.G.F.; McMurray, J.J.V.; Mullens, W.; Solomon, S.D.; et al. Ultrasound imaging of congestion in heart failure: Examinations beyond the heart. Eur. J. Heart Fail. 2021, 23, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Seo, Y.; Sai, S.; Machino-Ohtsuka, T.; Yamamoto, M.; Ishizu, T.; Kawakami, Y.; Aonuma, K. Clinical Implications of Intrarenal Hemodynamic Evaluation by Doppler Ultrasonography in Heart Failure. JACC Heart Fail. 2016, 4, 674–682. [Google Scholar] [CrossRef]
- Nijst, P.; Martens, P.; Dupont, M.; Tang, W.H.W.; Mullens, W. Intrarenal Flow Alterations During Transition from Euvolemia to Intravascular Volume Expansion in Heart Failure Patients. JACC Heart Fail. 2017, 5, 672–681. [Google Scholar] [CrossRef]
- Guazzi, M.; Gatto, P.; Giusti, G.; Pizzamiglio, F.; Previtali, I.; Vignati, C.; Arena, R. Pathophysiology of cardiorenal syndrome in decompensated heart failure: Role of lung-right heart-kidney interaction. Int. J. Cardiol. 2013, 169, 379–384. [Google Scholar] [CrossRef]
- Mullens, W.; Damman, K.; Harjola, V.P.; Mebazaa, A.; Brunner-La Rocca, H.P.; Martens, P.; Testani, J.M.; Tang, W.H.W.; Orso, F.; Rossignol, P.; et al. The use of diuretics in heart failure with congestion—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 137–155. [Google Scholar] [CrossRef]
- Kobayashi, M.; Huttin, O.; Donal, E.; Duarte, K.; Hubert, A.; Breton HLe Galli, E.; Fournet, M.; Mabo, P.; Schnell, F.; Leclercq, C.; et al. Association of estimated plasma volume status with hemodynamic and echocardiographic parameters. Clin. Res. Cardiol. 2020, 109, 1060–1069. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Chubuchny, V.; Pugliese, N.R.; Taddei, C.; Poggianti, E.; Spini, V.; Barison, A.; Formichi, B.; Airò, E.; Bauleo, C.; Prediletto, R.; et al. A novel echocardiographic method for estimation of pulmonary artery wedge pressure and pulmonary vascular resistance. ESC Heart Fail. 2021, 8, 1216–1229. [Google Scholar] [CrossRef] [PubMed]
- Reeves, J.T.; Groves, B.M.; Cymerman, A.; Sutton, J.R.; Wagner, P.D.; Turkevich, D.; Houston, C.S. Operation Everest II: Cardiac filling pressures during cycle exercise at sea level. Respir. Physiol. 1990, 80, 147–154. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Nishimura, R.A.; Sorajja, P.; Lam, C.S.P.; Redfield, M.M. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ. Heart Fail. 2010, 3, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Borgdorff, M.A.; Dickinson, M.G.; Berger, R.M.; Bartelds, B. Right ventricular failure due to chronic pressure load: What have we learned in animal models since the NIH working group statement? Heart Fail. Rev. 2015, 20, 475–491. [Google Scholar] [CrossRef][Green Version]
- Pagnamenta, A.; Dewachter, C.; Mcentee, K.; Fesler, P.; Brimioulle, S.; Naeije, R. Early right ventriculo-arterial uncoupling in borderline pulmonary hypertension on experimental heart failure. J. Appl. Physiol. 2010, 109, 1080–1085. [Google Scholar] [CrossRef]
- Guazzi, M.; Bandera, F.; Pelissero, G.; Castelvecchio, S.; Menicanti, L.; Ghio, S.; Temporelli, P.L.; Arena, R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: An index of right ventricular contractile function and prognosis. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1373–H1381. [Google Scholar] [CrossRef]
- Pugliese, N.R.; De Biase, N.; Conte, L.; Gargani, L.; Mazzola, M.; Fabiani, I.; Natali, A.; Dini, F.L.; Frumento, P.; Rosada, J.; et al. Cardiac Reserve and Exercise Capacity: Insights from Combined Cardiopulmonary and Exercise Echocardiography Stress Testing. J. Am. Soc. Echocardiogr. 2021, 34, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Paré, P.D.; Brooks, L.A.; Baile, E.M. Effect of systemic venous hypertension on pulmonary function and lung water. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981, 51, 592–597. [Google Scholar] [CrossRef]
- Laine, G.A.; Allen, S.J.; Katz, J.; Gabel, J.C.; Drake, R.E. Effect of systemic venous pressure elevation on lymph flow and lung edema formation. J. Appl. Physiol. (1985) 1986, 61, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- Fudim, M.; Salah, H.M.; Sathananthan, J.; Bernier, M.; Pabon-Ramos, W.; Schwartz, R.S.; Rodés-Cabau, J.; Côté, F.; Khalifa, A.; Virani, S.A.; et al. Lymphatic Dysregulation in Patients with Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 78, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Obokata, M.; Wiley, B.; Koepp, K.E.; Jorgenson, C.C.; Egbe, A.; Melenovsky, V.; Carter, R.E.; Borlaug, B.A. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur. Heart J. 2019, 40, 3721–3730. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Reddy, Y.N.V. The Role of the Pericardium in Heart Failure: Implications for Pathophysiology and Treatment. JACC Heart Fail. 2019, 7, 574–585. [Google Scholar] [CrossRef]
- Fallick, C.; Sobotka, P.A.; Dunlap, M.E. Sympathetically mediated changes in capacitance: Redistribution of the venous reservoir as a cause of decompensation. Circ. Heart Fail. 2011, 4, 669–675. [Google Scholar] [CrossRef]
- Miller, W.L. Fluid Volume Overload and Congestion in Heart Failure: Time to Reconsider Pathophysiology and How Volume Is Assessed. Circ. Heart Fail. 2016, 9, e002922. [Google Scholar] [CrossRef]
- Pugliese, N.R.; Pellicori, P.; Filidei, F.; De Biase, N.; Maffia, P.; Guzik, T.J.; Masi, S.; Taddei, S.; Cleland, J.G. Inflammatory pathways in heart failure with preserved left ventricular ejection fraction: Implications for future interventions. Cardiovasc. Res. 2022, 118, 3536–3555. [Google Scholar] [CrossRef]
- Januzzi, J.L.J.; Chen-Tournoux, A.A.; Christenson, R.H.; Doros, G.; Hollander, J.E.; Levy, P.D.; Nagurney, J.T.; Nowak, R.M.; Pang, P.S.; Patel, D.; et al. N-Terminal Pro-B-Type Natriuretic Peptide in the Emergency Department: The ICON-RELOADED Study. J. Am. Coll. Cardiol. 2018, 71, 1191–1200. [Google Scholar] [CrossRef]
- Gargani, L.; Pang, P.S.; Frassi, F.; Miglioranza, M.H.; Dini, F.L.; Landi, P.; Picano, E. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: A lung ultrasound study. Cardiovasc. Ultrasound 2015, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Miglioranza, M.H.; Picano, E.; Badano, L.P.; Sant’Anna, R.; Rover, M.; Zaffaroni, F.; Sicari, R.; Kalil, R.K.; Leiria, T.L.; Gargani, L. Pulmonary congestion evaluated by lung ultrasound predicts decompensation in heart failure outpatients. Int. J. Cardiol. 2017, 240, 271–278. [Google Scholar] [CrossRef]
- Pugliese, N.R.; De Biase, N.; Balletti, A.; Filidei, F.; Pieroni, A.; D’Angelo, G.; Armenia, S.; Mazzola, M.; Gargani, L.; Del Punta, L.; et al. Characterization of hemodynamic and metabolic abnormalities in the heart failure spectrum: The role of combined cardiopulmonary and exercise echocardiography stress test. Minerva Cardiol. Angiol. 2021, 70, 370–384. [Google Scholar] [CrossRef]
- Del Punta, L.; De Biase, N.; Armenia, S.; Di Fiore, V.; Maremmani, D.; Gargani, L.; Mazzola, M.; Carlo MDe Mengozzi, A.; Lomonaco, T.; Galeotti, G.; et al. Combining cardiopulmonary exercise testing with echocardiography: A multiparametric approach to the cardiovascular and cardiopulmonary systems. Eur. Heart J.-Imaging Methods Pract. 2023, 1, qyad021. [Google Scholar] [CrossRef]
- Pellicori, P.; Carubelli, V.; Zhang, J.; Castiello, T.; Sherwi, N.; Clark, A.L.; Cleland, J.G.F. IVC diameter in patients with chronic heart failure: Relationships and prognostic significance. JACC Cardiovasc. Imaging 2013, 6, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Tello, K.; Wan, J.; Dalmer, A.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Mohajerani, E.; Seeger, W.; Herberg, U.; et al. Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio for the Assessment of Right Ventricular-Arterial Coupling in Severe Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2019, 12, 9047. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, N.R.; De Biase, N.; Del Punta, L.; Balletti, A.; Armenia, S.; Buralli, S.; Mengozzi, A.; Taddei, S.; Metra, M.; Pagnesi, M.; et al. Deep phenotype characterization of hypertensive response to exercise: Implications on functional capacity and prognosis across the heart failure spectrum. Eur. J. Heart Fail. 2023, 25, 497–509. [Google Scholar] [CrossRef]
- Levy, W.C.; Mozaffarian, D.; Linker, D.T.; Sutradhar, S.C.; Anker, S.D.; Cropp, A.B.; Anand, I.; Maggioni, A.; Burton, P.; Sullivan, M.D.; et al. The Seattle Heart Failure Model: Prediction of survival in heart failure. Circulation 2006, 113, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Pocock, S.J.; Ariti, C.A.; McMurray, J.J.V.; Maggioni, A.; Køber, L.; Squire, I.B.; Swedberg, K.; Dobson, J.; Poppe, K.K.; Whalley, G.A.; et al. Predicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur. Heart J. 2013, 34, 1404–1413. [Google Scholar] [CrossRef]
- Agostoni, P.; Corrà, U.; Cattadori, G.; Veglia, F.; Gioia RLa Scardovi, A.B.; Emdin, M.; Metra, M.; Sinagra, G.; Limongelli, G.; Raimondo, R.; et al. Metabolic exercise test data combined with cardiac and kidney indexes, the MECKI score: A multiparametric approach to heart failure prognosis. Int. J. Cardiol. 2013, 167, 2710–2718. [Google Scholar] [CrossRef]
- Yamamoto, M.; Seo, Y.; Iida, N.; Ishizu, T.; Yamada, Y.; Nakatsukasa, T.; Nakagawa, D.; Kawamatsu, N.; Sato, K.; Machino-Ohtsuka, T.; et al. Prognostic Impact of Changes in Intrarenal Venous Flow Pattern in Patients with Heart Failure. J. Card. Fail. 2021, 27, 20–28. [Google Scholar] [CrossRef]
- Maaten JMTer Dauw, J.; Martens, P.; Somers, F.; Damman, K.; Metalidis, C.; Nijst, P.; Dupont, M.; Mullens, W. The Effect of Decongestion on Intrarenal Venous Flow Patterns in Patients with Acute Heart Failure. J. Card. Fail. 2021, 27, 29–34. [Google Scholar] [CrossRef]
- Boorsma, E.M.; Ter Maaten, J.M.; Voors, A.A.; van Veldhuisen, D.J. Renal Compression in Heart Failure: The Renal Tamponade Hypothesis. JACC Heart Fail. 2022, 10, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Cops, J.; Mullens, W.; Verbrugge, F.H.; Swennen, Q.; Moor, B.; De Reynders, C.; Penders, J.; Achten, R.; Driessen, A.; Dendooven, A.; et al. Selective abdominal venous congestion induces adverse renal and hepatic morphological and functional alterations despite a preserved cardiac function. Sci. Rep. 2018, 8, 17757. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.; Dupont, M.; Verbrugge, F.H.; Damman, K.; Degryse, N.; Nijst, P.; Reynders, C.; Penders, J.; Tang, W.H.W.; Testani, J.; et al. Urinary Sodium Profiling in Chronic Heart Failure to Detect Development of Acute Decompensated Heart Failure. JACC Heart Fail. 2019, 7, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; Canna, G.L.; Pepi, M.; Dulgheru, R.; Dweck, M.; Delgado, V.; Garbi, M.; Vannan, M.A.; et al. Multi–modality imaging assessment of native valvular regurgitation: An EACVI and ESC council of valvular heart disease position paper. Eur. Heart J. -Cardiovasc. Imaging 2022, 23, e171–e232. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; Bonis, M.D.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 76, 1–62. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Obokata, M.; Egbe, A.; Yang, J.H.; Pislaru, S.; Lin, G.; Carter, R.; Borlaug, B.A. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2019, 21, 891–900. [Google Scholar] [CrossRef]
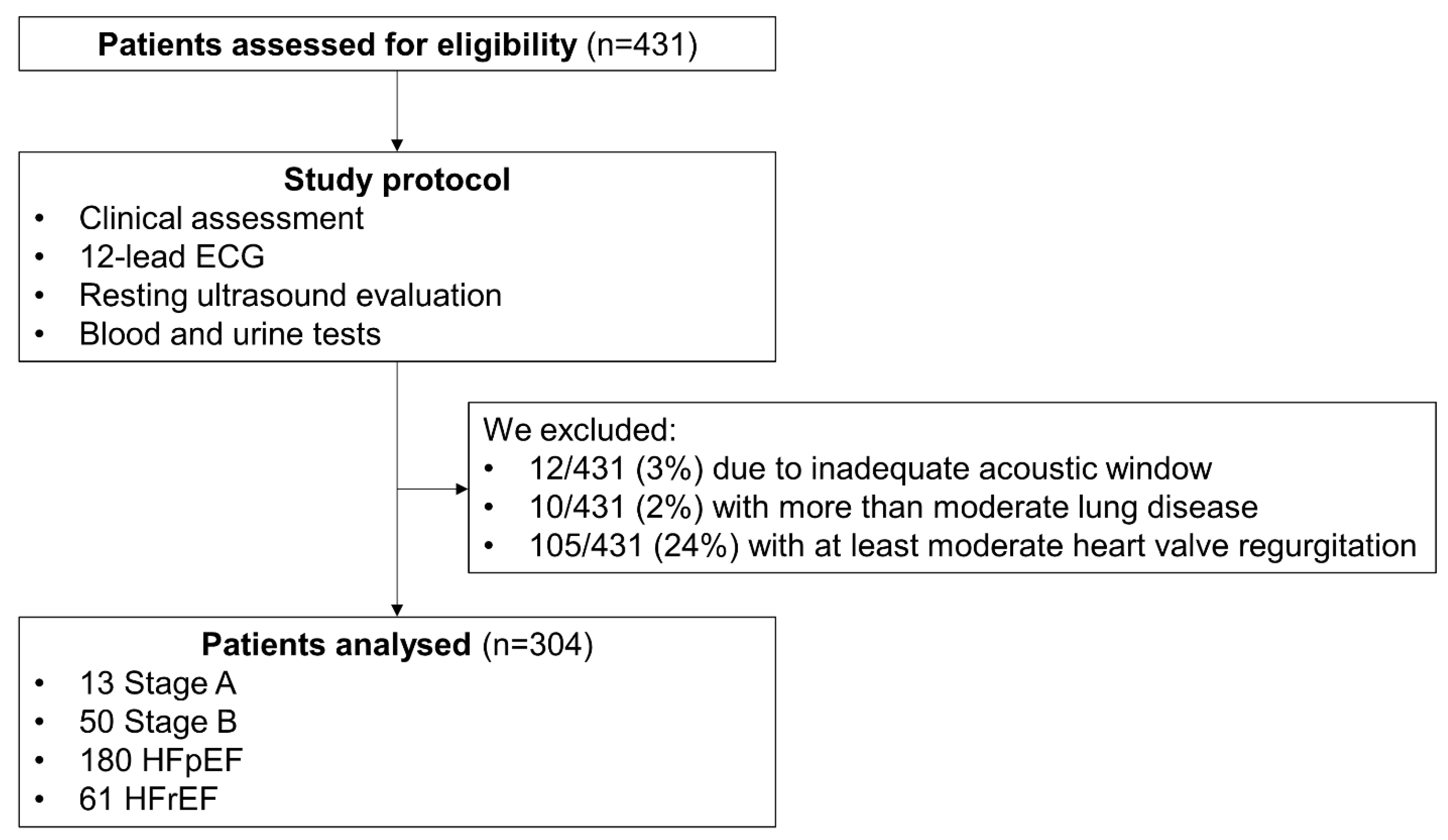
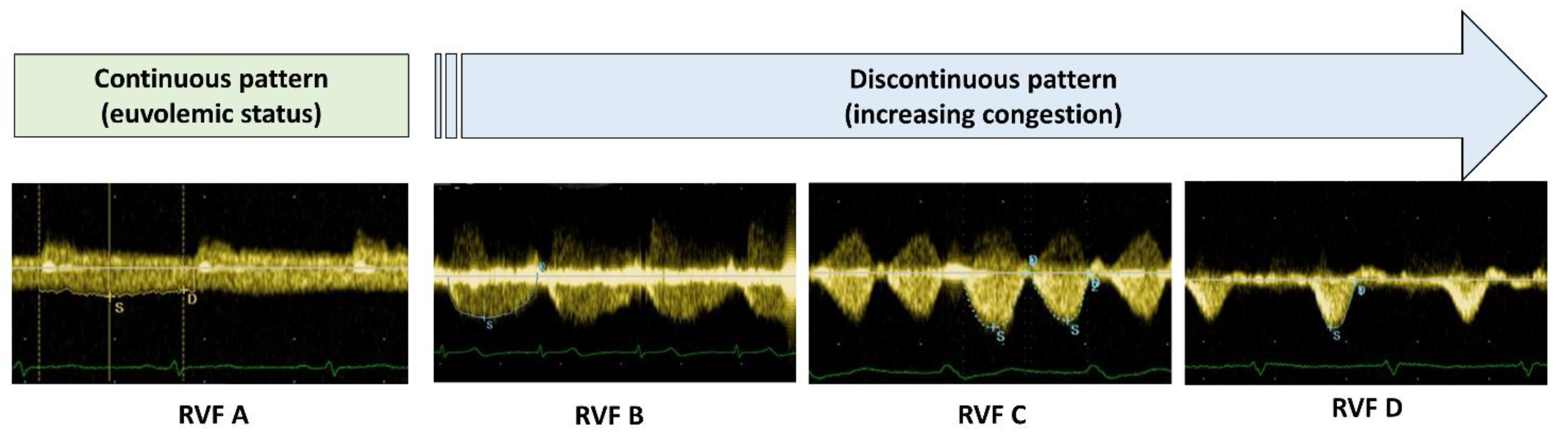
| Variable | Whole Population (n = 304) | Continuous RVF (n = 230) | Discontinuous RVF (n = 74) | p-Value |
|---|---|---|---|---|
| Demographics (0 missing) | ||||
| Age, years | 76 (66–82) | 76 (65–81) | 76 (68–83) | 0.35 |
| Men | 176 (58) | 130 (57) | 46 (62) | 0.39 |
| BMI, Kg/m2 | 26.5 ± 4.7 | 26.6 ± 4.6 | 26.4 ± 5.0 | 0.79 |
| BSA, m2 | 1.9 ± 0.2 | 1.9 ± 0.2 | 1.9 ± 0.2 | 0.23 |
| Smoker | 53 (17) | 43 (19) | 10 (14) | |
| HF Stages | <0.0001 | |||
| Stages A/B | 63 (21) | 59 (26) | 4 (5) | |
| Stage C-HFpEF | 180 (59) | 134 (58) | 46 (62) | |
| Stage C- HFrEF | 61 (20) | 37 (16) | 24 (33) | |
| NYHA class | 0.11 | |||
| I | 128 (42) | 104 (45) | 24 (32) | |
| II | 135 (44) | 97 (42) | 38 (51) | |
| III | 41 (14) | 29 (13) | 12 (16) | |
| KCCQ score | 71 (53–82) | 72 (55–84) | 62 (47–76) | 0.014 |
| Arterial hypertension | 212 (70) | 167 (73) | 45 (61) | 0.08 |
| Diabetes mellitus | 67 (22) | 49 (21) | 18 (24) | 0.55 |
| CAD | 70 (23) | 55 (24) | 15 (20) | 0.55 |
| Previous MI | 40 (13) | 28 (12) | 12 (16) | 0.35 |
| Pacemaker | 39 (13) | 28 (12) | 11 (15) | 0.52 |
| ICD/CRT | 36 (12) | 24 (10) | 12 (16) | 0.17 |
| AFib | 63 (21) | 30 (13) | 33 (44) | <0.0001 |
| Clinical evaluation (0 missing) | ||||
| Brachial systolic BP, mmHg | 130 ± 19 | 131 ± 20 | 127 ± 17 | 0.25 |
| Brachial diastolic BP, mmHg | 78 ± 13 | 77 ± 12 | 78 ± 13 | 0.90 |
| Heart rate, beats/min | 77 ± 11 | 77 ± 12 | 76 ± 10 | 0.56 |
| No clinical signs of congestion | 183 (67) | 163 (77) | 17 (23) | <0.0001 |
| Pitting oedema (any degree) | 74 (27) | 49 (21) | 30 (41) | 0.001 |
| Lung crackles (any degree) | 18 (7) | 4 (2) | 16 (22) | <0.0001 |
| Jugular vein distension (any degree) | 14 (5) | 1 (1) | 13 (20) | <0.0001 |
| Blood tests (0 missing) | ||||
| Haemoglobin, g/dL | 12.7 ± 2.0 | 12.7 ± 2.0 | 12.7 ± 1.8 | 0.97 |
| Creatinine, mg/dL | 0.97 (0.81–1.25) | 0.92 (0.79–1.20) | 1.08 (0.89–1.36) | 0.005 |
| eGFR, mL/min/1.73 m2 | 62 (52–81) | 69 (53–83) | 59 (46–76) | 0.038 |
| BUN, mg/dL | 20 (16–25) | 20 (16–24) | 24 (18–29) | 0.001 |
| ePVS, mL/g | 4.9 (4.2–5.5) | 4.8 (4.2–5.3) | 5.2 (4.5–5.9) | 0.01 |
| Plasma osmolality, mOsm/kg | 294 (291–300) | 294 (291–299) | 297 (293–303) | 0.06 |
| Na+, mEq/L | 141 (139–142) | 141 (139–142) | 141 (139–142) | 0.87 |
| K+, mEq/L | 4.35 (4.01–4.66) | 4.33 (4.03–4.63) | 4.36 (3.99–4.71) | 0.73 |
| Total cholesterol, mg/dL | 165 ± 41 | 167 ± 41 | 158 ± 40 | 0.11 |
| HbA1c, mmol/mol | 41 ± 11 | 41 ± 12 | 41 ± 8 | 0.99 |
| Uric acid, mg/dL | 5.8 (4.6–6.6) | 5.6 (4.4–6.4) | 6.2 (4.9–7.7) | <0.0001 |
| hs-CRP, mg/dL | 0.16 (0.09–0.40) | 0.15 (0.07–0.36) | 0.24 (0.10–0.70) | 0.045 |
| IL-6, pg/mL | 1.60 (0.01–4.20) | 1.45 (0.10–3.58) | 2.10 (0.50–5.90) | 0.08 |
| Norepinephrine, pg/mL | 230 (155–335) | 223 (160–321) | 240 (117–370) | 0.79 |
| Renin, mIU/L | 14. 8 (7.0–63.4) | 14.6 (7.0–55.4) | 22.0 (6.2–66.6) | 0.61 |
| Aldosterone, ng/dL | 11.0 (8.4–16.4) | 10.4 (7.6–15.4) | 15.2 (9.8–20.8) | 0.010 |
| NT-proBNP, pg/mL | 456 (193–1279) | 332 (160–918) | 1195 (517–2203) | <0.0001 |
| NT-proBNP, pg/mL (sinus rhythm only) | 316 (142–738) | 291 (118–591) | 548 (348–1601) | <0.0001 |
| hs-Troponin T, pg/mL | 17 (9–29) | 15 (9–24) | 27 (14–39) | <0.0001 |
| Urine test (12 missing) | ||||
| Urine osmolality, mOsm/kg | 562 (414–654) | 542 (413–656) | 602 (500–632) | 0.71 |
| uACR, mg/g | 23 (8–66) | 22 (7–63) | 28 (11–98) | 0.23 |
| Albuminuria | 0.50 | |||
| Micro-albuminuria § | 74 (27) | 52 (25) | 22 (34) | |
| Macro-albuminuria § | 25 (9) | 21 (10) | 4 (6) | |
| Spot urinary sodium, mEq/L | 81 (55–115) | 88 (60–126) | 70 (41–88) | <0.0001 |
| FENa, % | 0.55 (0.26–0.88) | 0.56 (0.34–0.92) | 0.44 (0–22–0.77) | 0.001 |
| Therapy (0 missing) | ||||
| Beta-Blocker | 204 (67) | 147 (64) | 57 (77) | 0.027 |
| DHP CCB | 59 (19) | 47 (20) | 12 (16) | 0.44 |
| ACEi or ARB | 167 (55) | 128 (56) | 39 (53) | 0.71 |
| MRAs | 91 (30) | 55 (24) | 36 (49) | <0.0001 |
| ARNI | 32 (11) | 24 (10) | 8 (11) | 0.91 |
| Statins | 159 (52) | 124 (54) | 35 (47) | 0.36 |
| Thiazides/thiazide-like diuretics | 41 (13) | 36 (16) | 5 (7) | 0.054 |
| Loop diuretics | 154 (51) | 98 (43) | 56 (76) | <0.0001 |
| Furosemide equivalent dose | 0.4 | |||
| 1–50 mg | 109 (36) | 66 (29) | 43 (58) | |
| 51–100 mg | 29 (9) | 20 (9) | 9 (13) | |
| >100 mg | 16 (6) | 12 (5) | 4 (5) | |
| SGLT2i | 19 (6) | 13 (6) | 6 (8) | 0.44 |
| Insulin | 17 (6) | 15 (7) | 2 (3) | 0.23 |
| Variable | Missing | Whole Population (n = 304) | Continuous RVF (n = 230) | Discontinuous RVF (n = 74) | p-Value |
|---|---|---|---|---|---|
| Left ventricle size and function | |||||
| LVMi, g/m2 | 0 | 121 ± 33 | 120 ± 30 | 124 ± 40 | 0.43 |
| RWT | 0 | 0.39 ± 0.09 | 0.38 ± 0.09 | 0.35 ± 0.09 | 0.017 |
| LV EDVi, mL | 0 | 78 ± 28 | 76 ± 25 | 83 ± 35 | 0.15 |
| LV EF, % | 0 | 61 ± 12 | 62 ± 12 | 55 ± 13 | <0.0001 |
| LV GLS, % | 0 | 14.1 ± 4.4 | 14.6 ± 4.2 | 12.1 ± 4.8 | 0.005 |
| Stroke volume, mL/beat | 0 | 54 ± 30 | 54 ± 30 | 53 ± 29 | 0.88 |
| Cardiac output, L/min | 0 | 3.8 ± 2.6 | 3.7 ± 2.6 | 3.9 ± 2.3 | 0.60 |
| Mitral E wave, cm/s | 0 | 105 ± 75 | 96 ± 77 | 131 ± 61 | <0.0001 |
| Average e’, cm/s | 0 | 8.7 ± 2.6 | 9.1 ± 2.8 | 8.4 ± 2.6 | 0.08 |
| Average E/e’ | 0 | 10.8 (8.3–15.5) | 10.5 (8.1–14.1) | 13.5 (9.0–20.0) | <0.0001 |
| Left atrium size and function | |||||
| LAVi, mL/m2 | 0 | 43 ± 16 | 40 ± 14 | 51 ± 18 | <0.0001 |
| LA reservoir strain, % | 0 | 24 ± 11 | 26 ± 11 | 17 ± 8 | <0.0001 |
| Right ventricle and pulmonary circulation | |||||
| TAPSE, mm | 0 | 20 ± 4 | 20 ± 4 | 19 ± 4 | 0.26 |
| FAC, % | 0 | 50 ± 9 | 51 ± 8 | 45 ± 10 | <0.0001 |
| RV free wall longitudinal strain, % | 9 | 27 ± 8 | 28 ± 6 | 25 ± 6 | <0.0001 |
| 3D-RV EF, % | 9 | 57 ± 9 | 59 ± 8 | 51 ± 9 | <0.0001 |
| Systolic PAP, mmHg | 4 | 38 ± 14 | 34 ± 11 | 47 ± 16 | <0.0001 |
| Diastolic PAP, mmHg | 6 | 11 ± 5 | 9 ± 4 | 15 ± 5 | <0.0001 |
| Mean PAP, mmHg | 6 | 20 ± 7 | 18 ± 5 | 26 ± 9 | <0.0001 |
| TAPSE/sPAP, mm/mmHg | 0 | 0.60 ± 0.23 | 0.64 ± 0.23 | 0.47 ± 0.20 | <0.0001 |
| ePVR, WU | 6 | 1.6 ± 0.9 | 1.5 ± 0.8 | 2.0 ± 1.0 | 0.002 |
| ePAWP, mmHg | 6 | 11.5 ± 5.1 | 10.0 ± 3.5 | 16.3 ± 6.3 | <0.0001 |
| Congestion assessment | 0 | ||||
| IVC, mm | 15 (15–18) | 15 (15–18) | 20 (17–23) | <0.0001 | |
| IVC ≥21 mm | 40 (13) | 12 (4) | 28 (38) | <0.0001 | |
| IVC collapse <50% | 35 (10) | 10 (4) | 25 (34) | <0.0001 | |
| B-lines | 1 (0–5) | 1 (0–4) | 6 (1–15) | <0.0001 |
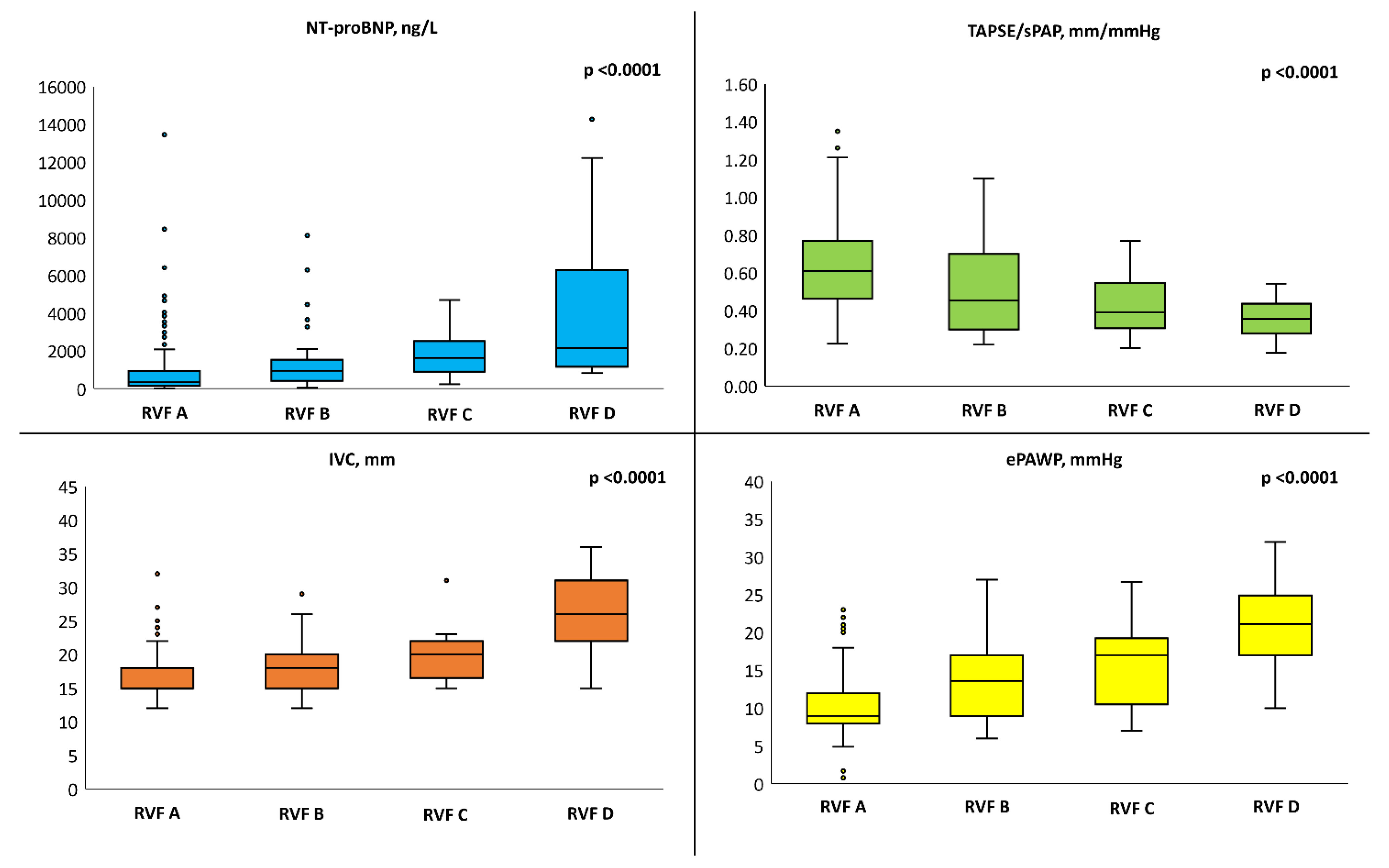
| Variable | RVF A (n = 230) | RVF B (n = 39) | RVF C (n = 18) | RVF D (n = 17) | p-Value |
|---|---|---|---|---|---|
| NT-proBNP §, ng/L | 332 (160–918) | 768 (379–1522) * | 1520 (783–2550) * | 2147 (1427–4172) *^° | <0.0001 |
| uACR, mg/g | 15 (8–53) | 22 (8–63) | 26 (13–65) | 38 (27–121) * | 0.03 |
| Serum creatinine, mg/dL | 0.93 (0.79–1.18) | 0.96 (0.92–1.25) | 1.08 (0.93–1.25) | 1.14 (0.96–1.39) * | 0.03 |
| Average E/e’ | 10.5 (8.1–14.1) | 12.7 (10.1–16.7) | 13.1 (8.8–18.6) | 15.8 (10.2–21.3) * | 0.003 |
| LAVi, mL/m2 | 40 ± 14 | 49 ± 16 * | 50 ± 13 * | 60 ± 18 *^ | <0.0001 |
| Systolic PAP, mmHg | 34 ± 11 | 44 ± 16 * | 49 ± 17 * | 54 ± 13 *^ | <0.0001 |
| ePVR, WU | 1.5 ± 0.8 | 1.8 ± 0.9 | 2.1 ± 1.0 | 2.4 ± 1.1 * | 0.016 |
| ePAWP, mmHg | 10.0 ± 3.5 | 14.5 ± 5.9 * | 15.7 ± 5.8 * | 21.2 ± 5.8 *^° | <0.0001 |
| IVC, mm | 15 (15–18) | 18 (15–20) * | 20 (18–22) * | 27 (25–31) *^° | <0.0001 |
| B-lines | 1 (0–4) | 3 (0–10) | 6 (1–15) * | 10 (1–18) * | <0.0001 |
| TAPSE/sPAP | 0.64 ± 0.23 | 0.52 ± 0.24 * | 0.43 ± 0.17 * | 0.36 ± 0.10 *^° | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Punta, L.; De Biase, N.; Mazzola, M.; Filidei, F.; Balletti, A.; Armenia, S.; Di Fiore, V.; Buralli, S.; Galeotti, G.G.; De Carlo, M.; et al. Bio-Humoral and Non-Invasive Haemodynamic Correlates of Renal Venous Flow Patterns across the Heart Failure Spectrum. Medicina 2023, 59, 1704. https://doi.org/10.3390/medicina59101704
Del Punta L, De Biase N, Mazzola M, Filidei F, Balletti A, Armenia S, Di Fiore V, Buralli S, Galeotti GG, De Carlo M, et al. Bio-Humoral and Non-Invasive Haemodynamic Correlates of Renal Venous Flow Patterns across the Heart Failure Spectrum. Medicina. 2023; 59(10):1704. https://doi.org/10.3390/medicina59101704
Chicago/Turabian StyleDel Punta, Lavinia, Nicolò De Biase, Matteo Mazzola, Francesco Filidei, Alessio Balletti, Silvia Armenia, Valerio Di Fiore, Simona Buralli, Gian Giacomo Galeotti, Marco De Carlo, and et al. 2023. "Bio-Humoral and Non-Invasive Haemodynamic Correlates of Renal Venous Flow Patterns across the Heart Failure Spectrum" Medicina 59, no. 10: 1704. https://doi.org/10.3390/medicina59101704
APA StyleDel Punta, L., De Biase, N., Mazzola, M., Filidei, F., Balletti, A., Armenia, S., Di Fiore, V., Buralli, S., Galeotti, G. G., De Carlo, M., Giannini, C., Masi, S., & Pugliese, N. R. (2023). Bio-Humoral and Non-Invasive Haemodynamic Correlates of Renal Venous Flow Patterns across the Heart Failure Spectrum. Medicina, 59(10), 1704. https://doi.org/10.3390/medicina59101704







