Peripheral Nerve Blocks for Cesarean Delivery Analgesia: A Narrative Review
Abstract
:1. Introduction
2. Anatomy and Innervation of the Uterus and Related Structures
2.1. Peripheral Nerve Blocks for Cesarean Section
2.2. Wound Infiltration
2.3. Liposomal Bupivacaine
2.4. Ilioinguinal/Iliohypogastric Blocks
2.5. TAP Block
2.6. Quadratus Lumborum Blocks (QL Block)
2.7. Erector Spinae Plane Blocks
3. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| ESP | Erector spinae plane |
| QL | Quadratus lumborum |
| TAP | Transversus abdominis plane |
| VAS | Visual analog scale |
References
- Gibbons, L.; Belizán, J.; Lauer, J.; Betrán, A.; Merialdi, M.; Althabe, F. The Global Numbers and Costs of Additionally Needed and Unnecessary Caesarean Sections Performed per Year: Overuse as a Barrier to Universal Coverage HEALTH SYSTEMS FINANCING. World Health Rep. 2010, 30. [Google Scholar]
- Gamez, B.H.; Habib, A.S. Predicting Severity of Acute Pain After Cesarean Delivery: A Narrative Review. Anesth. Analg. 2018, 126, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Kainu, J.P.; Sarvela, J.; Tiippana, E.; Halmesmäki, E.; Korttila, K.T. Persistent pain after caesarean section and vaginal birth: A cohort study. Int. J. Obstet. Anesth. 2010, 19, 4–9. [Google Scholar] [CrossRef]
- Bollag, L.; Lim, G.; Sultan, P.; Habib, A.S.; Landau, R.; Zakowski, M.; Tiouririne, M.; Bhambhani, S.; Carvalho, B. Society for Obstetric Anesthesia and Perinatology: Consensus Statement and Recommendations for Enhanced Recovery after Cesarean. Anesth. Analg. 2020, 132, 1362–1377. [Google Scholar] [CrossRef]
- Roofthooft, E.; Joshi, G.P.; Rawal, N.; Van de Velde, M. PROSPECT guideline for elective caesarean section: Updated systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia 2020, 76, 665–680. [Google Scholar] [CrossRef]
- Liu, Q.; Chelly, J.E.; Williams, J.P.; Gold, M.S. Impact of peripheral nerve block with low dose local anesthetics on analgesia and functional outcomes following total knee arthroplasty: A retrospective study. Pain Med. 2015, 16, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.; Cunningham, K.; Fitzgerald, K.; Finnerty, E. Opioid consumption following outpatient upper extremity surgery. J. Hand Surg. 2012, 37, 645–650. [Google Scholar] [CrossRef]
- Williams, B.A.; Kentor, M.L.; Vogt, M.T.; Vogt, W.B.; Coley, K.C.; Williams, J.P.; Roberts, M.S.; Chelly, J.E.; Harner, C.D.; Fu, F.H. Economics of nerve block pain management after anterior cruciate ligament reconstruction: Potential hospital cost savings via associated postanesthesia care unit bypass and same-day discharge. Anesthesiology 2004, 100, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.J.; McDonnell, J.G.; Carvalho, B.; Sharkey, A.; Pawa, A.; Gadsden, J. Essentials of Our Current Understanding: Abdominal Wall Blocks. Reg. Anesth. Pain Med. 2017, 42, 133–183. [Google Scholar] [CrossRef]
- Ndiaye, A.; Diop, M.; Ndoye, J.M.; Ndiaye, A.; Mané, L.; Nazarian, S.; Dia, A. Emergence and distribution of the ilioinguinal nerve in the inguinal region: Applications to the ilioinguinal anaesthetic block (about 100 dissections). Surg. Radiol. Anat. SRA 2010, 32, 55–62. [Google Scholar] [CrossRef]
- Klaassen, Z.; Marshall, E.; Tubbs, R.S.; Louis, R.G., Jr.; Wartmann, C.T.; Loukas, M. Anatomy of the ilioinguinal and iliohypogastric nerves with observations of their spinal nerve contributions. Clin. Anat. 2011, 24, 454–461. [Google Scholar] [CrossRef]
- Alkatout, I.; Wedel, T.; Pape, J.; Possover, M.; Dhanawat, J. Review: Pelvic nerves - from anatomy and physiology to clinical applications. Transl. Neurosci. 2021, 12, 362–378. [Google Scholar] [CrossRef] [PubMed]
- Adesope, O.; Ituk, U.; Habib, A.S. Local anaesthetic wound infiltration for postcaesarean section analgesia: A systematic review and meta-analysis. Eur. J. Anaesthesiol. 2016, 33, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Jolly, C.; Jathières, F.; Keïta, H.; Jaouen, E.; Guyot, B.; Torre, A. Cesarean analgesia using levobupivacaine continuous wound infiltration: A randomized trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 194, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Niklasson, B.; Börjesson, A.; Carmnes, U.B.; Segerdahl, M.; Ohman, S.G.; Blanck, A. Intraoperative injection of bupivacaine-adrenaline close to the fascia reduces morphine requirements after cesarean section: A randomized controlled trial. Acta Obstet. Gynecol. Scand. 2012, 91, 1433–1439. [Google Scholar] [CrossRef]
- Tawfik, M.M.; Mohamed, Y.M.; Elbadrawi, R.E.; Abdelkhalek, M.; Mogahed, M.M.; Ezz, H.M. Transversus Abdominis Plane Block Versus Wound Infiltration for Analgesia After Cesarean Delivery: A Randomized Controlled Trial. Anesth. Analg. 2017, 124, 1291–1297. [Google Scholar] [CrossRef]
- Telnes, A.; Skogvoll, E.; Lonnée, H. Transversus abdominis plane block vs. wound infiltration in Caesarean section: A randomised controlled trial. Acta Anaesthesiol. Scand. 2015, 59, 496–504. [Google Scholar] [CrossRef]
- Ranta, P.O.; Ala-Kokko, T.I.; Kukkonen, J.E.; Ohtonen, P.P.; Raudaskoski, T.H.; Reponen, P.K.; Rawal, N. Incisional and epidural analgesia after caesarean delivery: A prospective, placebo-controlled, randomised clinical study. Int. J. Obstet. Anesth. 2006, 15, 189–194. [Google Scholar] [CrossRef]
- Kainu, J.P.; Sarvela, J.; Halonen, P.; Puro, H.; Toivonen, H.J.; Halmesmäki, E.; Korttila, K.T. Continuous wound infusion with ropivacaine fails to provide adequate analgesia after caesarean section. Int. J. Obstet. Anesth. 2012, 21, 119–124. [Google Scholar] [CrossRef]
- Carvalho, B.; Lemmens, H.J.; Ting, V.; Angst, M.S. Postoperative subcutaneous instillation of low-dose ketorolac but not hydromorphone reduces wound exudate concentrations of interleukin-6 and interleukin-10 and improves analgesia following cesarean delivery. J. Pain 2013, 14, 48–56. [Google Scholar] [CrossRef]
- Lalmand, M.; Wilwerth, M.; Fils, J.F.; Van der Linden, P. Continuous Ropivacaine Subfascial Wound Infusion Compared With Intrathecal Morphine for Postcesarean Analgesia: A Prospective, Randomized Controlled, Double-Blind Study. Anesth. Analg. 2017, 125, 907–912. [Google Scholar] [CrossRef]
- Beaussier, M.; El’Ayoubi, H.; Schiffer, E.; Rollin, M.; Parc, Y.; Mazoit, J.X.; Azizi, L.; Gervaz, P.; Rohr, S.; Biermann, C.; et al. Continuous preperitoneal infusion of ropivacaine provides effective analgesia and accelerates recovery after colorectal surgery: A randomized, double-blind, placebo-controlled study. Anesthesiology 2007, 107, 461–468. [Google Scholar] [CrossRef]
- Rackelboom, T.; Strat, S.L.; Silvera, S.; Schmitz, T.; Bassot, A.; Goffinet, F.; Ozier, Y.; Beaussier, M.; Mignon, A. Improving continuous wound infusion effectiveness for postoperative analgesia after cesarean delivery: A randomized controlled trial. Obstet. Gynecol. 2010, 116, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Zahn, P.K.; Brennan, T.J. Primary and secondary hyperalgesia in a rat model for human postoperative pain. Anesthesiology 1999, 90, 863–872. [Google Scholar] [CrossRef]
- Dinges, H.C.; Wiesmann, T.; Otremba, B.; Wulf, H.; Eberhart, L.H.; Schubert, A.K. The analgesic efficacy of liposomal bupivacaine compared with bupivacaine hydrochloride for the prevention of postoperative pain: A systematic review and meta-analysis with trial sequential analysis. Reg. Anesth. Pain Med. 2021, 46, 490–498. [Google Scholar] [CrossRef]
- Hussain, N.; Brull, R.; Sheehy, B.; Essandoh, M.K.; Stahl, D.L.; Weaver, T.E.; Abdallah, F.W. Perineural Liposomal Bupivacaine Is Not Superior to Nonliposomal Bupivacaine for Peripheral Nerve Block Analgesia. Anesthesiology 2021, 134, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.W.; Villadiego, L.G.; Lake, Y.N.; Amin, Y.; Timmins, A.E.; Swaim, L.S.; Ashton, D.W. Transversus abdominis plane block with liposomal bupivacaine for pain control after cesarean delivery: A retrospective chart review. J. Pain Res. 2018, 11, 3109–3116. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.S.; Nedeljkovic, S.S.; Horn, J.L.; Smiley, R.M.; Kett, A.G.; Vallejo, M.C.; Song, J.; Scranton, R.; Bao, X. Randomized trial of transversus abdominis plane block with liposomal bupivacaine after cesarean delivery with or without intrathecal morphine. J. Clin. Anesth. 2021, 75, 110527. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, M.; Clapp, M.A.; McQuaid-Hanson, E.; Ona, S.; OʼDonnell, T.; James, K.; Bateman, B.T.; Wylie, B.J.; Barth, W.H., Jr. Liposomal Bupivacaine Block at the Time of Cesarean Delivery to Decrease Postoperative Pain: A Randomized Controlled Trial. Obstet. Gynecol. 2018, 132, 70–78. [Google Scholar] [CrossRef]
- Ganta, R.; Samra, S.K.; Maddineni, V.R.; Furness, G. Comparison of the effectiveness of bilateral ilioinguinal nerve block and wound infiltration for postoperative analgesia after caesarean section. Br. J. Anaesth. 1994, 72, 229–230. [Google Scholar] [CrossRef]
- Ahemed, S.A.; Denu, Z.A.; Getinet Kassahun, H.; Yilikal Fentie, D. Efficacy of Bilateral Transversus Abdominis Plane and Ilioinguinal-Iliohypogastric Nerve Blocks for Postcaesarean Delivery Pain Relief under Spinal Anesthesia. Anesthesiol. Res. Pract. 2018, 2018, 1948261. [Google Scholar] [CrossRef] [PubMed]
- Kiran, L.V.; Sivashanmugam, T.; Kumar, V.R.H.; Krishnaveni, N.; Parthasarathy, S. Relative Efficacy of Ultrasound-guided Ilioinguinal-iliohypogastric Nerve Block versus Transverse Abdominis Plane Block for Postoperative Analgesia following Lower Segment Cesarean Section: A Prospective, Randomized Observer-blinded Trial. Anesth. Essays Res. 2017, 11, 713–717. [Google Scholar] [CrossRef]
- Yetneberk, T.; Chekol, B.; Teshome, D. The efficacy of TAP block versus ilioinguinal block for post-cesarean section pain management: A systematic review and meta-analysis. Heliyon 2021, 7, e07774. [Google Scholar] [CrossRef]
- Vallejo, M.C.; Steen, T.L.; Cobb, B.T.; Phelps, A.L.; Pomerantz, J.M.; Orebaugh, S.L.; Chelly, J.E. Efficacy of the bilateral ilioinguinal-iliohypogastric block with intrathecal morphine for postoperative cesarean delivery analgesia. Sci. World J. 2012, 2012, 107316. [Google Scholar] [CrossRef]
- Staker, J.J.; Liu, D.; Church, R.; Carlson, D.J.; Panahkhahi, M.; Lim, A.; LeCong, T. A triple-blind, placebo-controlled randomised trial of the ilioinguinal-transversus abdominis plane (I-TAP) nerve block for elective caesarean section. Anaesthesia 2018, 73, 594–602. [Google Scholar] [CrossRef]
- Tran, D.Q.; Bravo, D.; Leurcharusmee, P.; Neal, J.M. Transversus Abdominis Plane Block: A Narrative Review. Anesthesiology 2019, 131, 1166–1190. [Google Scholar] [CrossRef] [PubMed]
- Hebbard, P. TAP block nomenclature. Anaesthesia 2015, 70, 112–113. [Google Scholar] [CrossRef] [PubMed]
- Faiz, S.H.R.; Alebouyeh, M.R.; Derakhshan, P.; Imani, F.; Rahimzadeh, P.; Ghaderi Ashtiani, M. Comparison of ultrasound-guided posterior transversus abdominis plane block and lateral transversus abdominis plane block for postoperative pain management in patients undergoing cesarean section: A randomized double-blind clinical trial study. J. Pain Res. 2018, 11, 5–9. [Google Scholar] [CrossRef]
- Støving, K.; Rothe, C.; Rosenstock, C.V.; Aasvang, E.K.; Lundstrøm, L.H.; Lange, K.H. Cutaneous Sensory Block Area, Muscle-Relaxing Effect, and Block Duration of the Transversus Abdominis Plane Block: A Randomized, Blinded, and Placebo-Controlled Study in Healthy Volunteers. Reg. Anesth. Pain Med. 2015, 40, 355–362. [Google Scholar] [CrossRef]
- Belavy, D.; Cowlishaw, P.J.; Howes, M.; Phillips, F. Ultrasound-guided transversus abdominis plane block for analgesia after Caesarean delivery. Br. J. Anaesth. 2009, 103, 726–730. [Google Scholar] [CrossRef]
- Baaj, J.M.; Alsatli, R.A.; Majaj, H.A.; Babay, Z.A.; Thallaj, A.K. Efficacy of ultrasound-guided transversus abdominis plane (TAP) block for postcesarean section delivery analgesia--a double-blind, placebo-controlled, randomized study. Middle East J. Anaesthesiol. 2010, 20, 821–826. [Google Scholar]
- Tan, T.T.; Teoh, W.H.; Woo, D.C.; Ocampo, C.E.; Shah, M.K.; Sia, A.T. A randomised trial of the analgesic efficacy of ultrasound-guided transversus abdominis plane block after caesarean delivery under general anaesthesia. Eur. J. Anaesthesiol. 2012, 29, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Eslamian, L.; Jalili, Z.; Jamal, A.; Marsoosi, V.; Movafegh, A. Transversus abdominis plane block reduces postoperative pain intensity and analgesic consumption in elective cesarean delivery under general anesthesia. J. Anesth. 2012, 26, 334–338. [Google Scholar] [CrossRef]
- El-Boghdadly, K.; Desai, N.; Halpern, S.; Blake, L.; Odor, P.M.; Bampoe, S.; Carvalho, B.; Sultan, P. Quadratus lumborum block vs. transversus abdominis plane block for caesarean delivery: A systematic review and network meta-analysis. Anaesthesia 2021, 76, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Chandon, M.; Bonnet, A.; Burg, Y.; Barnichon, C.; DesMesnards-Smaja, V.; Sitbon, B.; Foiret, C.; Dreyfus, J.F.; Rahmani, J.; Laloë, P.A.; et al. Ultrasound-guided Transversus Abdominis plane block versus continuous wound infusion for post-caesarean analgesia: A randomized trial. PLoS ONE 2014, 9, e103971. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.; Jolly, C.; Dumoulin, J.L.; Meftah, R.B.; Blanié, P.; Laloë, P.A.; Tabary, N.; Fischler, M.; Le Guen, M. Convulsions in 2 patients after bilateral ultrasound-guided transversus abdominis plane blocks for cesarean analgesia. Reg. Anesth. Pain Med. 2014, 39, 248–251. [Google Scholar] [CrossRef]
- Griffiths, J.D.; Barron, F.A.; Grant, S.; Bjorksten, A.R.; Hebbard, P.; Royse, C.F. Plasma ropivacaine concentrations after ultrasound-guided transversus abdominis plane block. Br. J. Anaesth. 2010, 105, 853–856. [Google Scholar] [CrossRef]
- Rosenberg, P.H.; Veering, B.T.; Urmey, W.F. Maximum recommended doses of local anesthetics: A multifactorial concept. Reg. Anesth. Pain Med. 2004, 29, 564–575, discussion 524. [Google Scholar] [CrossRef]
- Ng, S.C.; Habib, A.S.; Sodha, S.; Carvalho, B.; Sultan, P. High-dose versus low-dose local anaesthetic for transversus abdominis plane block post-Caesarean delivery analgesia: A meta-analysis. Br. J. Anaesth. 2018, 120, 252–263. [Google Scholar] [CrossRef]
- Abdallah, F.W.; Chan, V.W.; Brull, R. Transversus abdominis plane block: A systematic review. Reg. Anesth. Pain Med. 2012, 37, 193–209. [Google Scholar] [CrossRef]
- Elsharkawy, H.; El-Boghdadly, K.; Barrington, M. Quadratus Lumborum Block: Anatomical Concepts, Mechanisms, and Techniques. Anesthesiology 2019, 130, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Blanco, R.; Ansari, T.; Riad, W.; Shetty, N. Quadratus Lumborum Block Versus Transversus Abdominis Plane Block for Postoperative Pain After Cesarean Delivery: A Randomized Controlled Trial. Reg. Anesth. Pain Med. 2016, 41, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, H.; El-Boghdadly, K.; Kolli, S.; Esa, W.A.S.; DeGrande, S.; Soliman, L.M.; Drake, R.L. Injectate spread following anterior sub-costal and posterior approaches to the quadratus lumborum block: A comparative cadaveric study. Eur. J. Anaesthesiol. 2017, 34, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Balocco, A.L.; López, A.M.; Kesteloot, C.; Horn, J.L.; Brichant, J.F.; Vandepitte, C.; Hadzic, A.; Gautier, P. Quadratus lumborum block: An imaging study of three approaches. Reg. Anesth. Pain Med. 2021, 46, 35–40. [Google Scholar] [CrossRef]
- Elsharkawy, H.; Ahuja, S.; DeGrande, S.; Maheshwari, K.; Chan, V. Subcostal approach to anterior quadratus lumborum block for pain control following open urological procedures. J. Anesth. 2019, 33, 148–154. [Google Scholar] [CrossRef]
- Blanco, R.; Ansari, T.; Girgis, E. Quadratus lumborum block for postoperative pain after caesarean section: A randomised controlled trial. Eur. J. Anaesthesiol. 2015, 32, 812–818. [Google Scholar] [CrossRef]
- Krohg, A.; Ullensvang, K.; Rosseland, L.A.; Langesæter, E.; Sauter, A.R. The Analgesic Effect of Ultrasound-Guided Quadratus Lumborum Block After Cesarean Delivery: A Randomized Clinical Trial. Obstet. Anesth. Dig. 2018, 126, 559–565. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, K.; Zhang, Y.; Chen, G.; Zhou, Y. Quadratus lumborum block for postoperative analgesia after cesarean section: A meta-analysis of randomized controlled trials with trial sequential analysis. Sci. Rep. 2021, 11, 18104. [Google Scholar] [CrossRef]
- Pangthipampai, P.; Dejarkom, S.; Poolsuppasit, S.; Luansritisakul, C.; Tangchittam, S. Bilateral posterior Quadratus Lumborum block for pain relief after cesarean delivery: A randomized controlled trial. BMC Anesthesiol. 2021, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Lu, D.; Yang, X.; Zhou, Z.; Chen, X.; Chen, K.; Zhou, X.; Feng, X. Postoperative analgesic effects of various quadratus lumborum block approaches following cesarean section: A randomized controlled trial. J. Pain Res. 2019, 12, 2305–2312. [Google Scholar] [CrossRef]
- Hussain, N.; Brull, R.; Weaver, T.; Zhou, M.; Essandoh, M.; Abdallah, F.W. Postoperative Analgesic Effectiveness of Quadratus Lumborum Block for Cesarean Delivery under Spinal Anesthesia. Anesthesiology 2021, 134, 72–87. [Google Scholar] [CrossRef]
- Murouchi, T.; Iwasaki, S.; Yamakage, M. Quadratus Lumborum Block: Analgesic Effects and Chronological Ropivacaine Concentrations After Laparoscopic Surgery. Reg. Anesth. Pain Med. 2016, 41, 146–150. [Google Scholar] [CrossRef]
- Ueshima, H.; Hiroshi, O. Incidence of lower-extremity muscle weakness after quadratus lumborum block. J. Clin. Anesth. 2018, 44, 104. [Google Scholar] [CrossRef]
- Sá, M.; Cardoso, J.M.; Reis, H.; Esteves, M.; Sampaio, J.; Gouveia, I.; Carballada, P.; Pinheiro, C.; Machado, D. Quadratus lumborum block: Are we aware of its side effects? A report of 2 cases. Rev. Bras. Anestesiol. 2018, 68, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, Y.; Tanaka, N.; Suzuka, T.; Yamanaka, T.; Iwata, M.; Ozu, N.; Kawaguchi, M. Anterior Quadratus Lumborum Block and Quadriceps Strength: A Prospective Cohort Study. J. Clin. Med. 2023, 12, 3837. [Google Scholar] [CrossRef] [PubMed]
- De Cassai, A.; Tonetti, T. Local anesthetic spread during erector spinae plane block. J. Clin. Anesth. 2018, 48, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Dostbil, A.; Ince, I.; Altinpulluk, E.Y.; Perez, M.F.; Peksoz, U.; Cimilli, G.; Kasali, K.; Atalay, C.; Ozmen, O.; Sahin, T.; et al. Analgesic effect of erector spinae plane block after cesarean section: A randomized controlled trial. Niger. J. Clin. Pract. 2023, 26, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Malawat, A.; Verma, K.; Jethava, D.; Jethava, D.D. Erector spinae plane block and transversus abdominis plane block for postoperative analgesia in cesarean section: A prospective randomized comparative study. J. Anaesthesiol. Clin. Pharmacol. 2020, 36, 201–206. [Google Scholar] [CrossRef]
- Boules, M.L.; Goda, A.S.; Abdelhady, M.A.; Abu El-Nour Abd El-Azeem, S.A.; Hamed, M.A. Comparison of Analgesic Effect Between Erector Spinae Plane Block and Transversus Abdominis Plane Block After Elective Cesarean Section: A Prospective Randomized Single-Blind Controlled Study. J. Pain Res. 2020, 13, 1073–1080. [Google Scholar] [CrossRef]
- Hamed, M.A.; Yassin, H.M.; Botros, J.M.; Abdelhady, M.A. Analgesic Efficacy of Erector Spinae Plane Block Compared with Intrathecal Morphine After Elective Cesarean Section: A Prospective Randomized Controlled Study. J. Pain Res. 2020, 13, 597–604. [Google Scholar] [CrossRef]
- Ribeiro Junior, I.D.V.; Carvalho, V.H.; Brito, L.G.O. Erector spinae plane block for analgesia after cesarean delivery: A systematic review with meta-analysis. Braz. J. Anesthesiol. 2022, 72, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Selvi, O.; Tulgar, S. Ultrasound guided erector spinae plane block as a cause of unintended motor block. Rev. Esp. De Anestesiol. Y Reanim. 2018, 65, 589–592. [Google Scholar] [CrossRef] [PubMed]

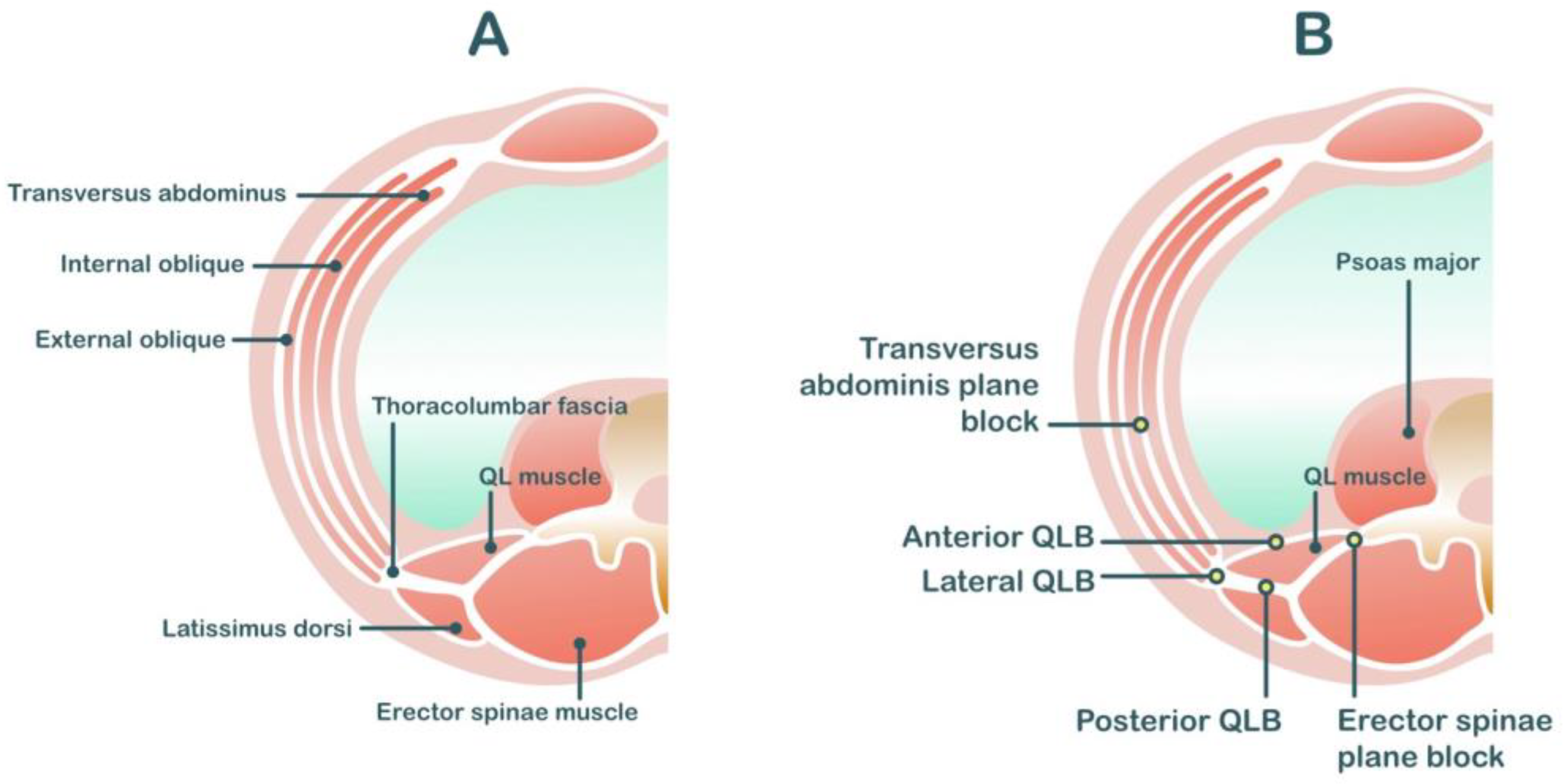
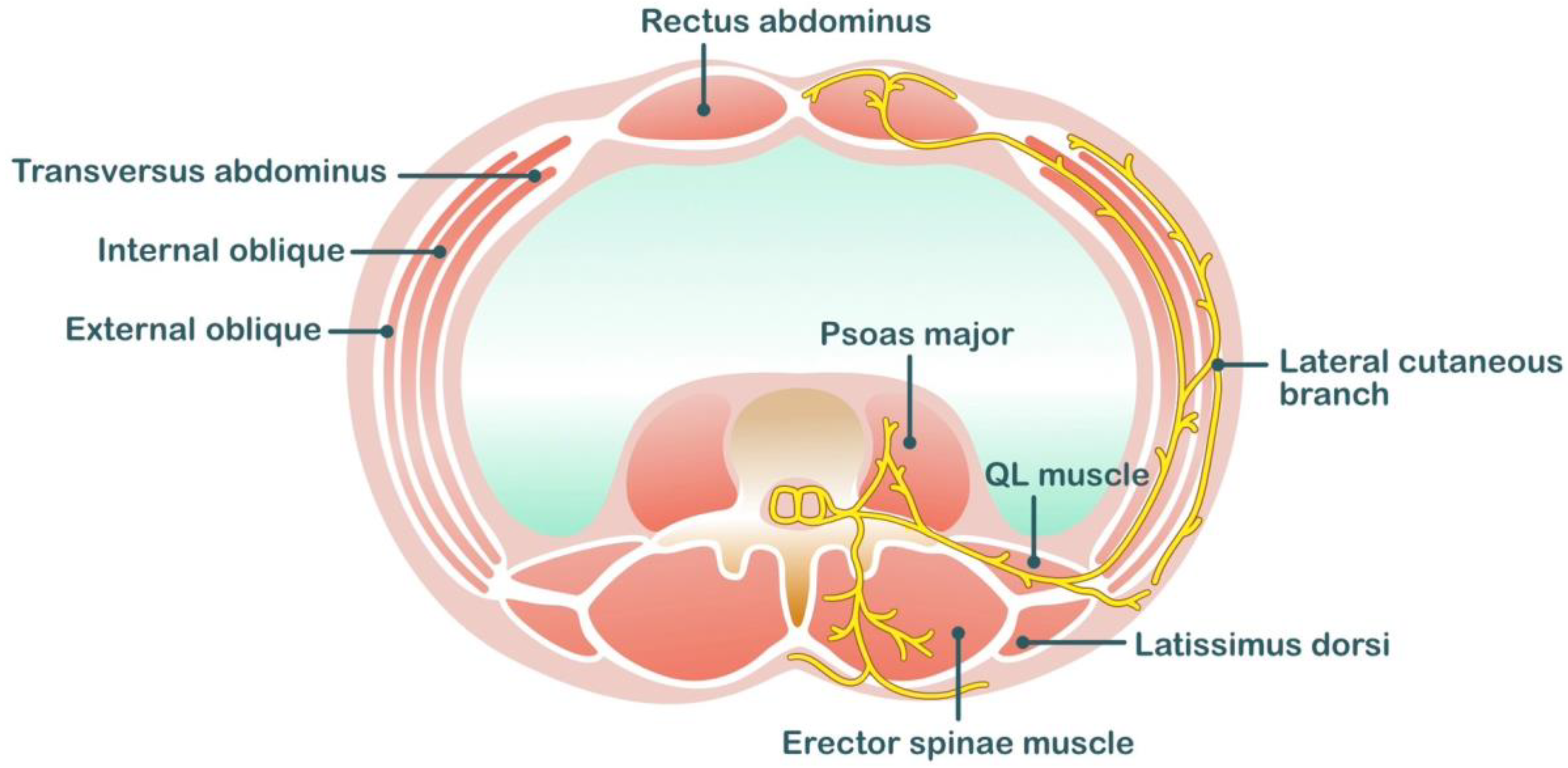
| Peripheral Nerve Block Techniques | Dermatomal Coverage | Somatic Analgesia | Visceral Analgesia | Potential Risks | Analgesic Efficacy |
|---|---|---|---|---|---|
Wound infiltration technique
| Variable | + | - |
|
|
| Liposomal bupivacaine | Variable | + | - |
|
|
| Ilioinguinal/iliohypogastric blocks (II-IH blocks) | T12-L1 | + | - |
|
|
| Transversus abdominis plane block (TAP block) | T10-L1 | + | - |
| |
| Quadratus lumborum block (QL block)
| Depends on the approach, varying from T6 to L4 | + | Possibly provides visceral analgesia |
| |
| Erector spinae plane block | Variable | + | Possibly provides visceral analgesia |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangkum, L.; Tangjitbampenbun, A.; Chalacheewa, T.; Brennan, K.; Liu, H. Peripheral Nerve Blocks for Cesarean Delivery Analgesia: A Narrative Review. Medicina 2023, 59, 1951. https://doi.org/10.3390/medicina59111951
Sangkum L, Tangjitbampenbun A, Chalacheewa T, Brennan K, Liu H. Peripheral Nerve Blocks for Cesarean Delivery Analgesia: A Narrative Review. Medicina. 2023; 59(11):1951. https://doi.org/10.3390/medicina59111951
Chicago/Turabian StyleSangkum, Lisa, Amornrat Tangjitbampenbun, Theerawat Chalacheewa, Kristin Brennan, and Henry Liu. 2023. "Peripheral Nerve Blocks for Cesarean Delivery Analgesia: A Narrative Review" Medicina 59, no. 11: 1951. https://doi.org/10.3390/medicina59111951
APA StyleSangkum, L., Tangjitbampenbun, A., Chalacheewa, T., Brennan, K., & Liu, H. (2023). Peripheral Nerve Blocks for Cesarean Delivery Analgesia: A Narrative Review. Medicina, 59(11), 1951. https://doi.org/10.3390/medicina59111951






