Pitavastatin and Lovastatin Exhibit Calcium Channel Blocking Activity Which Potentiate Vasorelaxant Effects of Amlodipine: A New Futuristic Dimension in Statin’s Pleiotropy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Materials
2.3. Animals
2.4. Data Recording
2.5. Solutions
2.6. Pitavastatin, Lovastatin, and Amlodipine Effects on KCL (80 mM) Elicited Contractions
2.7. Pitavastatin, Lovastatin, and Amlodipine Effects on N.E (1 µM) Elicited Contractions
2.8. Combined Effects of Pitavastatin, Lovastatin, and Amlodipine
2.9. Pitavastatin and Lovastatin Effects on Calcium Concentration Response Curves (CCRCs)
2.10. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ach | Acetylcholine |
| CVD | Cardiovascular diseases |
| CCRCs | Calcium Concentration Response Curves |
| EC50 | Median effective concentration |
| FDA | Food and Drug Administration |
| KCL | Potassium chloride |
| KMU | Khyber Medical University |
| mM | Millimole |
| N.E | Norepinephrine |
| NET | Norepinephrine Transporter |
References
- Istvan, E.S.; Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 2001, 292, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Downs, J.R.; Clearfield, M.; Weis, S.; Whitney, E.; Shapiro, D.R.; Beere, P.A.; Langendorferet, A.; Stein, E.A.; Kruyer, W.; Gotto, A.M., Jr.; et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of AFCAPS/TexCAPS. JAMA 1998, 279, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.W.; Chen, J.; Kuron, G.; Hunt, V.; Huff, J.; Hoffman, C.; Rothrock, J.; Lopez, M.; Joshua, H.; Harris, E.; et al. Mevinolin: A highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc. Natl. Acad. Sci. USA 1980, 77, 3957–3961. [Google Scholar] [CrossRef] [PubMed]
- Tobert, J.A. Lovastatin and beyond: The history of the HMG-CoA reductase inhibitors. Nat. Rev. Drug Discov. 2003, 2, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R. The pharmacology of statins. Pharmacol. Res. 2014, 88, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Gotto, A.M., Jr.; Farmer, J.A. Pleiotropic effects of statins: Do they matter? Curr. Opin. Lipidol. 2001, 12, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liao, J.K. Pleiotropic Effects of Statins–Basic Research and Clinical Perspectives. Circ. J. 2010, 74, 818–826. [Google Scholar] [CrossRef]
- Tonelli, M.; Moyé, L.; Sacks, F.M.; Cole, T.; Curhan, G.C. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. J. Am. Soc. Nephrol. 2003, 14, 1605–1613. [Google Scholar] [CrossRef]
- Vidt, D.G.; Cressman, M.D.; Harris, S.; Pears, J.S.; Hutchinson, H.G. Rosuvastatin-induced arrest in progression of renal disease. Cardiology 2004, 102, 52–60. [Google Scholar] [CrossRef]
- Chopra, V.; Rogers, M.A.; Buist, M.; Govindan, S.; Lindenauer, P.K.; Saint, S.; Flanders, S.A. Is statin use associated with reduced mortality after pneumonia? A systematic review and meta-analysis. Am. J. Med. 2012, 125, 1111–1123. [Google Scholar] [CrossRef]
- Laufs, U.; La Fata, V.; Plutzky, J.; Liao, J.K. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 1998, 97, 1129–1135. [Google Scholar] [CrossRef]
- Kinlay, S.; Schwartz, G.G.; Olsson, A.G.; Rifai, N.; Leslie, S.J.; Sasiela, W.J.; Szarek, M.; Libby, P.; Ganz, P. High-dose atorvastatin enhances the decline in inflammatory markers in patients with acute coronary syndromes in the MIRACL study. Circulation 2003, 108, 1560–1566. [Google Scholar] [CrossRef]
- Clunn, G.F.; Sever, P.S.; Hughes, A.D. Calcium channel regulation in vascular smooth muscle cells: Synergistic effects of statins and calcium channel blockers. Int. J. Cardiol. 2010, 139, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Begum, R.; Faisal, M.S.; Khan, A.; Nabi, M.; Shehzadi, G.; Ullah, S.; Ali, W. Current statins show calcium channel blocking activity through voltage gated channels. BMC Pharmacol. Toxicol. 2016, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Ali, W.; Ullah, A.; Ahmad, S.; Alsaiari, A.A.; Almehmadi, M.; Abdulaziz, O.; Allahyani, M.; Aljuaid, A. Atorvastatin and Fluvastatin Potentiate Blood Pressure Lowering Effect of Amlodipine through Vasorelaxant Phenomenon. Medicina 2023, 59, 1023. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Ali, W.; Ullah, A.; Ahmad, S.; Hussain Haya Ali, W. Rosuvastatin and Simvastatin Potentiate Antihypertensive Effect of Amlodipine Through Vasorelaxation Phenomenon. Pak. J. Pharm. Sci. 2023, 36, 953–961. [Google Scholar] [PubMed]
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA 2002, 288, 2998–3007. [Google Scholar]
- Musha, S.; Watanabe, M.; Ishida, Y.; Kato, S.; Konishi, M.; Tomoda, A. A phenoxazine compound, 2-amino-4, 4α-dihydro-4α-7-dimethyl-3H-phenoxazine-3-one reverses the phenylephrine or high-K+ induced contraction of smooth muscles in rat aorta and guinea pig tenia cecum. Biol. Pharm. Bull. 2005, 28, 1521–1523. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gilani, A.H.; Khan A ullah Jabeen, Q.; Subhan, F.; Ghafar, R. Antispasmodic and blood pressure lowering effects of Valeriana wallichii are mediated through K+ channel activation. J. Ethnopharmacol. 2005, 100, 347–352. [Google Scholar] [CrossRef]
- Kousar, M.; Salma, U.; Khan, T.; Shah, A.J. Antihypertensive Potential of Tartaric Acid and Exploration of Underlying Mechanistic Pathways. Dose-Response 2022, 20, 15593258221135728. [Google Scholar] [CrossRef]
- Malik, A.; Mehmood, M.H.; Channa, H.; Akhtar, M.S.; Gilani, A.H. Pharmacological basis for the medicinal use of polyherbal formulation and its ingredients in cardiovascular disorders using rodents. BMC Complement Altern. Med. 2017, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Khan A ullah Gilani, A.H. Antispasmodic and bronchodilator activities of Artemisia vulgaris are mediated through dual blockade of muscarinic receptors and calcium influx. J. Ethnopharmacol. 2009, 126, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Fuster, V. The global burden of cardiovascular diseases and risks: A compass for global action. J. Am. Coll. Cardiology 2020, 76, 2980–2981. [Google Scholar] [CrossRef] [PubMed]
- Margolis, K.L.; Davis, B.R.; Baimbridge, C.; Ciocon, J.O.; Cuyjet, A.B.; Dart, R.A.; Einhorn, P.T.; Ford, C.E.; Gordon, D.; Hartney, T.J.; et al. Long-Term Follow-Up of Moderately Hypercholesterolemic Hypertensive Patients Following Randomization to Pravastatin vs Usual Care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). J. Clin. Hypertens. 2013, 15, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Pietz, K.; Battleman, D.S.; Beyth, R.J. Prevalence of comorbid hypertension and dyslipidemia and associated cardiovascular disease. Heart Dis. 2004, 2, 3. [Google Scholar]
- Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: Results of prospectively designed overviews of randomised trials. Lancet 2000, 356, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.E.; Ashiya, M. Antihypertensive therapies. Nat. Rev. Drug Discov. 2007, 6, 597–598. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Wang, H.; Yang, H.; Yin, W.; Xu, S.; Jiang, T.; Wang, M.; Wu, F.; Yu, W. Inhibition of the norepinephrine transporter rescues vascular hyporeactivity to catecholamine in obstructive jaundice. Eur. J. Pharmacol. 2021, 900, 174055. [Google Scholar] [CrossRef]
- (PDF) Realism about PDB [Internet]. Available online: https://www.researchgate.net/publication/6152919_Realism_about_PDB (accessed on 14 August 2023).
- ChemIDplus and the Drug Information Portal Content Available from PubChem Only Starting December 2022 [Internet]. U.S. National Library of Medicine. Available online: https://www.nlm.nih.gov/pubs/techbull/tb.html (accessed on 14 August 2023).
- Briasoulis, A.; Agarwal, V.; Valachis, A.; Messerli, F.H. Antihypertensive effects of statins: A meta-analysis of prospective controlled studies. J. Clin. Hypertens. 2013, 15, 310–320. [Google Scholar] [CrossRef]
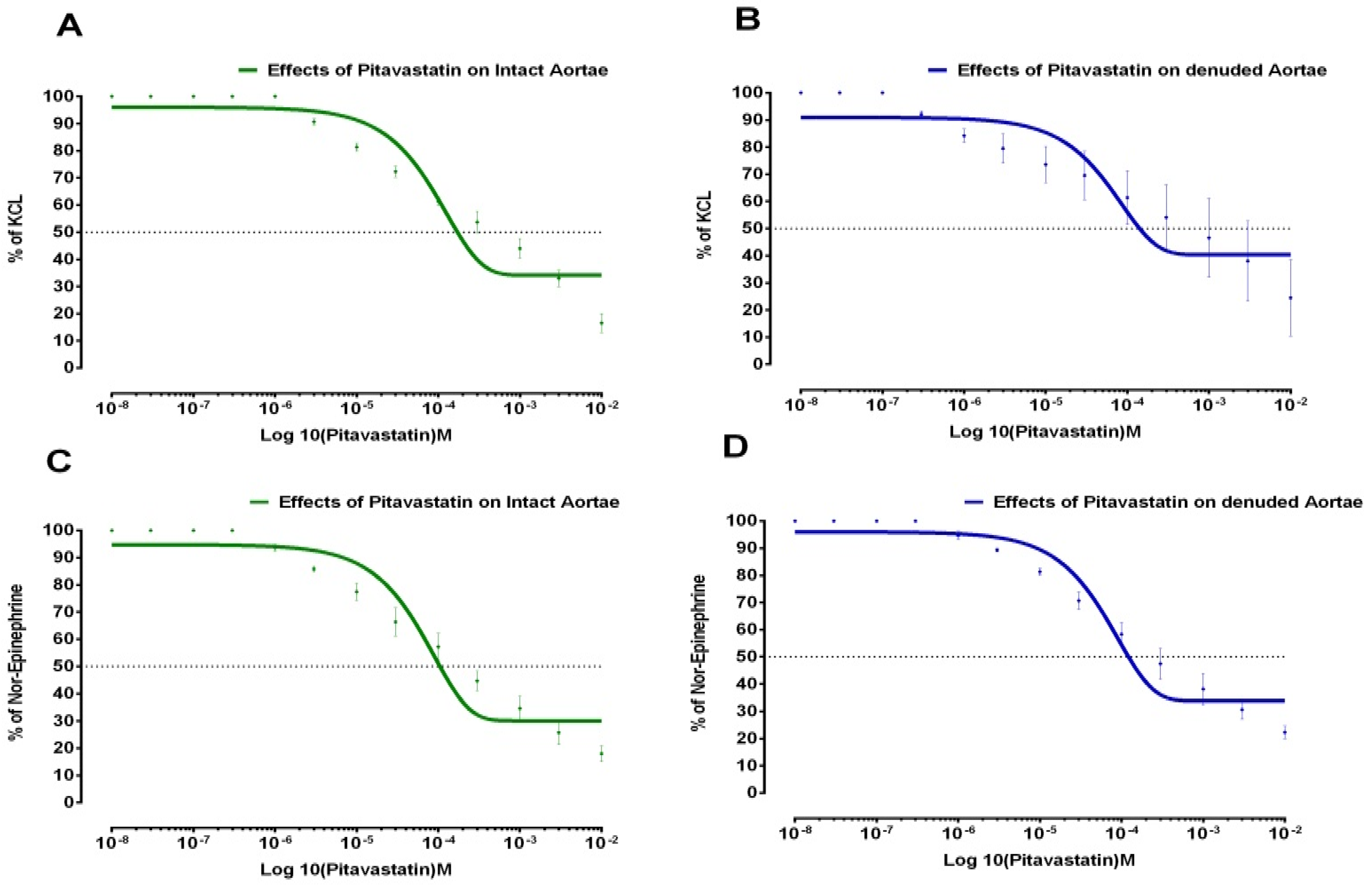



| Drugs | Aortae Status | % of KCL (Control Max) | % of NE (Control Max) | EC50 ± SD KCL-Induced (Molar) | EC50 ± SD NE-Induced (Molar) |
|---|---|---|---|---|---|
| Pitavastatin | Intact | 34 | 30 | 1.76 × 10−4 ± 0.28 | 1.05 × 10−4 ± 0.20 |
| Denuded | 40 | 33 | 1.41 × 10−4 ± 0.07 | 1.16 × 10−4 ± 0.17 | |
| Lovastatin | Intact | 24 | 34.7 | 7.44 × 10−5 ± 0.16 | 5.68 × 10−5 ± 0.10 |
| Denuded | 35.5 | 34 | 4.55 × 10−5 ± 0.10 | 4.90 × 10−5 ± 0.08 | |
| Amlodipine | Intact | 40 | 40.5 | 2.3 × 10−5 ± 1.11 | 2.52 × 10−5 ± 1.81 |
| Denuded | 39 | 22.9 | 3.29 × 10−5 ± 1.69 | 3.83 × 10−5 ± 1.52 |
| Drugs | Aortae Status | % of KCL (Control Max) | % of NE (Control Max) | EC50 KCL-Induced (Molar) | EC50 NE-Induced (Molar) |
|---|---|---|---|---|---|
| Amlodipine + Pitavastatin | Intact | 14.6 | 4.73 | 6.11 × 10−5 ± 0.19 | 3.10 × 10−6 ± 1.99 |
| Denuded | 14.9 | 11.2 | 1.36 × 10−5 ± 0.04 | 1.52 × 10−6 ± 0.99 | |
| Amlodipine + Lovastatin | Intact | 5.26 | 6.60 | 8.11 × 10−6 ± 0.02 | 2.58 × 10−5 ± 0.08 |
| Denuded | 9.01 | 10.3 | 5.21 × 10−6 ± 0.01 | 1.15 × 10−5 ± 0.04 | |
| Pitavastatin + Amlodipine | Intact | 6.07 | 14.9 | 4.84 × 10−6 ± 0.01 | 1.97 × 10−5 ± 0.01 |
| Denuded | 6.33 | 9.28 | 2.17 × 10−5 ± 0.08 | 3.02 × 10−5 ± 0.70 | |
| Lovastatin + Amlodipine | Intact | 9.28 | 11.4 | 1.54 × 10−5 ± 0.06 | 1.06 × 10−5 ± 1.15 |
| Denuded | 9.81 | 12.2 | 8.95 × 10−6 ± 0.03 | 2.28 × 10−5 ± 0.70 |
| Statins | CCRCs Specifications | EC50 Log [Ca++] M |
|---|---|---|
| Pitavastatin | Control | −2.7 ± 0.1 |
| Test Concentration (7.42 × 10−7 M) | −2.6 ± 0.14 ** | |
| Test concentration (1.21 × 10−6 M) | −2.1 ± 0.15 ** | |
| Lovastatin | Control | −2.7 ± 0.08 |
| Test Concentration (6.74 × 10−7 M) | −2.5 ± 0.10 ** | |
| Test concentration (2.74 × 10−6 M) | −2.3 ± 0.12 ** | |
| Verapamil | Control | −2.4 ± 0.11 |
| Test Concentration (0.03 µM) | −1.41 ± 0.22 ** | |
| Test concentration (0.1 µM) | −0.70 ± 0.2 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, W.; Ali, N.; Ullah, A.; Rahman, S.U.; Ahmad, S. Pitavastatin and Lovastatin Exhibit Calcium Channel Blocking Activity Which Potentiate Vasorelaxant Effects of Amlodipine: A New Futuristic Dimension in Statin’s Pleiotropy. Medicina 2023, 59, 1805. https://doi.org/10.3390/medicina59101805
Ali W, Ali N, Ullah A, Rahman SU, Ahmad S. Pitavastatin and Lovastatin Exhibit Calcium Channel Blocking Activity Which Potentiate Vasorelaxant Effects of Amlodipine: A New Futuristic Dimension in Statin’s Pleiotropy. Medicina. 2023; 59(10):1805. https://doi.org/10.3390/medicina59101805
Chicago/Turabian StyleAli, Wajid, Niaz Ali, Abid Ullah, Shafiq Ur Rahman, and Shujaat Ahmad. 2023. "Pitavastatin and Lovastatin Exhibit Calcium Channel Blocking Activity Which Potentiate Vasorelaxant Effects of Amlodipine: A New Futuristic Dimension in Statin’s Pleiotropy" Medicina 59, no. 10: 1805. https://doi.org/10.3390/medicina59101805
APA StyleAli, W., Ali, N., Ullah, A., Rahman, S. U., & Ahmad, S. (2023). Pitavastatin and Lovastatin Exhibit Calcium Channel Blocking Activity Which Potentiate Vasorelaxant Effects of Amlodipine: A New Futuristic Dimension in Statin’s Pleiotropy. Medicina, 59(10), 1805. https://doi.org/10.3390/medicina59101805







