Serum Osteoprotegerin Levels and the Vascular Reactivity Index in Patients with Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Anthropometric Analysis
2.3. Biochemical Investigations
2.4. Endothelial Function Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bundy, J.D.; Li, C.; Stuchlik, P.; Bu, X.; Kelly, T.N.; Mills, K.T.; He, H.; Chen, J.; Whelton, P.K.; He, J. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: A systematic review and network meta-analysis. JAMA Cardiol. 2017, 2, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P. Endothelial dysfunction and hypertension. Hypertension 2014, 64, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial dysfunction in hypertension: Current concepts and clinical implications. Front. Med. 2022, 8, 798958. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Martelli, E.; Enea, I.; Zamboni, M.; Federici, M.; Bracale, U.M.; Sangiorgi, G.; Martelli, A.R.; Messina, T.; Settembrini, A.M. Focus on the most common paucisymptomatic vasculopathic population, from diagnosis to secondary prevention of complications. Diagnostics 2023, 13, 2356. [Google Scholar] [CrossRef] [PubMed]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Lüscher, T.F.; Shechter, M.; Taddei, S.; et al. The assessment of endothelial function: From research into clinical practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Yen, A.A.; Lin, A.W.; Tanaka, H.; Kleis, S. New indices of endothelial function measured by digital thermal monitoring of vascular reactivity: Data from 6084 patients registry. Int. J. Vasc. Med. 2016, 2016, 1348028. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109 (Suppl. S1), III27–III32. [Google Scholar] [CrossRef]
- Ridker, P.M.; Brown, N.J.; Vaughan, D.E.; Harrison, D.G.; Mehta, J.L. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation 2004, 109 (Suppl. S1), IV6–IV19. [Google Scholar] [CrossRef]
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. The role of osteoprotegerin in the crosstalk between vessels and bone: Its potential utility as a marker of cardiometabolic diseases. Pharmacol. Ther. 2018, 182, 115–132. [Google Scholar] [CrossRef]
- Bucay, N.; Sarosi, I.; Dunstan, C.R.; Morony, S.; Tarpley, J.; Capparelli, C.; Scully, S.; Tan, H.L.; Xu, W.; Lacey, D.L.; et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998, 12, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Morony, S.; Tintut, Y.; Zhang, Z.; Cattley, R.C.; Van, G.; Dwyer, D.; Stolina, M.; Kostenuik, P.J.; Demer, L.L. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(−/−) mice. Circulation 2008, 117, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Barbu, C.G.; Arsene, A.L.; Florea, S.; Albu, A.; Sirbu, A.; Martin, S.; Nicolae, A.C.; Burcea-Dragomiroiu, G.T.A.; Popa, D.E.; Velescu, B.S.; et al. Cardiovascular risk assessment in osteoporotic patients using osteoprotegerin as a reliable predictive biochemical marker. Mol. Med. Rep. 2017, 16, 6059–6067. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. The role of osteoprotegerin and its ligands in vascular function. Int. J. Mol. Sci. 2019, 20, 705. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Tsai, J.P.; Lai, Y.H.; Lin, Y.L.; Kuo, C.H.; Wang, C.H.; Hsu, B.G. Serum osteoprotegerin level is positively associated with peripheral artery disease in patients with peritoneal dialysis. Ren. Fail. 2020, 42, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Vita, J.A. Endothelial function. Circulation 2011, 124, e906–e912. [Google Scholar] [CrossRef] [PubMed]
- Little, P.J.; Askew, C.D.; Xu, S.; Kamato, D. Endothelial dysfunction and cardiovascular disease: History and analysis of the clinical utility of the relationship. Biomedicines 2021, 9, 699. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: From mechanism to pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Ahmadi, N.; Hajsadeghi, F.; Gul, K.; Vane, J.; Usman, N.; Flores, F.; Nasir, K.; Hecht, H.; Naghavi, M.; Budoff, M. Relations between digital thermal monitoring of vascular function, the Framingham risk score, and coronary artery calcium score. J. Cardiovasc. Comput. Tomogr. 2008, 2, 382–388. [Google Scholar] [CrossRef]
- Min, H.; Morony, S.; Sarosi, I.; Dunstan, C.R.; Capparelli, C.; Scully, S.; Van, G.; Kaufman, S.; Kostenuik, P.J.; Lacey, D.L.; et al. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J. Exp. Med. 2000, 192, 463–474. [Google Scholar] [CrossRef]
- Dutka, M.; Bobiński, R.; Wojakowski, W.; Francuz, T.; Pająk, C.; Zimmer, K. Osteoprotegerin and RANKL-RANK-OPG-TRAIL signalling axis in heart failure and other cardiovascular diseases. Heart Fail. Rev. 2022, 27, 1395–1411. [Google Scholar] [CrossRef] [PubMed]
- Blázquez-Medela, A.M.; García-Ortiz, L.; Gómez-Marcos, M.A.; Recio-Rodriguez, J.I.; Sánchez-Rodríguez, A.; López-Novoa, J.M.; Martínez-Salgado, C. Osteoprotegerin is associated with cardiovascular risk in hypertension and/or diabetes. Eur. J. Clin. Investig. 2012, 42, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Gunes, M.; Temizkan, S.; Apaydin, T.; Ilgin, C.; Haklar, G.; Gogas Yavuz, D. Serum osteoprotegerin levels, endothelial function and carotid intima-media thickness in type 2 diabetic patients. J. Diabetes Complicat. 2021, 35, 108073. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.H.; Oh, T.R.; Choi, H.S.; Kim, C.S.; Oh, K.H.; Lee, J.; Oh, Y.K.; Jung, J.Y.; Choi, K.H.; Ma, S.K.; et al. Association of circulating osteoprotegerin level with blood pressure variability in patients with chronic kidney disease. J. Clin. Med. 2021, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Xiang, G.D. Changes of plasma concentration of osteoprotegerin and its association with endothelial dysfunction before and after hypouricemic therapy in patients with hyperuricemia. Mod. Rheumatol. 2015, 25, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Shin, Y.G.; Chung, C.H. Elevated serum osteoprotegerin levels are associated with vascular endothelial dysfunction in type 2 diabetes. Diabetes Care 2006, 29, 1664–1666. [Google Scholar] [CrossRef] [PubMed]
- D’Abramo, A.; Zingaropoli, M.A.; Oliva, A.; D’Agostino, C.; Al Moghazi, S.; De Luca, G.; Iannetta, M.; Mastroianni, C.M.; Vullo, V. Immune activation, immunosenescence, and osteoprotegerin as markers of endothelial dysfunction in subclinical HIV-associated atherosclerosis. Mediators Inflamm. 2014, 2014, 192594. [Google Scholar] [CrossRef]
- Abali, R.; Tasdemir, N.; Alpsoy, S.; Tasdemir, U.G.; Guzel, S.; Yuksel, M.A.; Temel Yuksel, I.; Yilmaz, M. No relationship between osteoprotegerin concentrations and endothelial dysfunction in non-obese women with and without polycystic ovary syndrome. Arch. Gynecol. Obstet. 2015, 291, 1075–1080. [Google Scholar] [CrossRef]
- Avbersek-Luznik, I.; Malesic, I.; Rus, I.; Marc, J. Increased levels of osteoprotegerin in hemodialysis patients. Clin. Chem. Lab. Med. 2002, 40, 1019–1023. [Google Scholar] [CrossRef]
- Demir, P.; Erdenen, F.; Aral, H.; Emre, T.; Kose, S.; Altunoglu, E.; Dolgun, A.; Inal, B.B.; Turkmen, A. Serum osteoprotegerin levels related with cardiovascular risk factors in chronic kidney disease. J. Clin. Lab. Anal. 2016, 30, 811–817. [Google Scholar] [CrossRef]
- Morena, M.; Terrier, N.; Jaussent, I.; Leray-Moragues, H.; Chalabi, L.; Rivory, J.P.; Maurice, F.; Delcourt, C.; Cristol, J.P.; Canaud, B.; et al. Plasma osteoprotegerin is associated with mortality in hemodialysis patients. J. Am. Soc. Nephrol. 2006, 17, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Carrero, J.J.; Qureshi, A.R.; Hirai, T.; Takasugi, N.; Ueno, T.; Taniguchi, Y.; Lindholm, B.; Yorioka, N. Plasma osteoprotegerin, arterial stiffness, and mortality in normoalbuminemic Japanese hemodialysis patients. Osteoporos. Int. 2011, 22, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.M.; Hayashi, S.Y.; Riella, M.C.; Lindholm, B. Elevated levels of plasma osteoprotegerin are associated with all-cause mortality risk and atherosclerosis in patients with stages 3 to 5 chronic kidney disease. Braz. J. Med. Biol. Res. 2014, 47, 995–1002. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, Q.X.; Li, J.B.; Huang, N.; Huang, X.W.; Li, Y.L.; Huang, F.X. Elevated osteoprotegerin concentration predicts increased risk of cardiovascular mortality in patients with chronic kidney disease: A systematic review and meta-analysis. Kidney Blood Press. Res. 2020, 45, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial Dysfunction in chronic kidney disease, from biology to clinical outcomes: A 2020 update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef] [PubMed]
- Linden, E.; Cai, W.; He, J.C.; Xue, C.; Li, Z.; Winston, J.; Vlassara, H.; Uribarri, J. Endothelial dysfunction in patients with chronic kidney disease results from advanced glycation end products (AGE)-mediated inhibition of endothelial nitric oxide synthase through RAGE activation. Clin. J. Am. Soc. Nephrol. 2008, 3, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Harlacher, E.; Wollenhaupt, J.; Baaten, C.C.F.M.J.; Noels, H. Impact of uremic toxins on endothelial dysfunction in chronic kidney disease: A systematic review. Int. J. Mol. Sci. 2022, 23, 531. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Lai, Y.H.; Kuo, C.H.; Lin, Y.L.; Tsai, J.P.; Hsu, B.G. Association between serum indoxyl sulfate levels and endothelial function in non-dialysis chronic kidney disease. Toxins 2019, 11, 589. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; He, W.; Liu, W.; Yang, J. Digital microvascular reactivity does not decline with impaired renal function in chronic kidney disease. BMC Nephrol. 2019, 20, 288. [Google Scholar] [CrossRef]
- Herrera, M.D.; Mingorance, C.; Rodríguez-Rodríguez, R.; Alvarez de Sotomayor, M. Endothelial dysfunction and aging: An update. Ageing Res. Rev. 2010, 9, 142–152. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Kiss, T.; Wren, J.D.; Giles, C.B.; Griffin, C.T.; Murfee, W.L.; Pacher, P.; Csiszar, A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 2018, 15, 555–565. [Google Scholar] [CrossRef]
| Characteristics | All Participants (n = 102) | Good Vascular Reactivity (n = 46) | Inermediate Vascular Reactivity (n = 46) | Poor Vascular Reactivity (n = 10) | p Value |
|---|---|---|---|---|---|
| Age (years) | 63.01 ± 8.45 | 62.24 ± 7.55 | 62.27 ± 8.53 | 69.98 ± 9.65 | 0.022 * |
| Height (cm) | 163.84 ± 7.46 | 162.50 ± 7.85 | 165.57 ± 7.38 | 162.00 ± 3.97 | 0.100 |
| Body weight (kg) | 73.31 ± 11.73 | 72.81 ± 10.19 | 75.39 ± 13.30 | 66.07 ± 7.75 | 0.068 |
| Body mass index (kg/m2) | 27.27 ± 3.70 | 27.61 ± 3.74 | 27.40 ± 3.76 | 25.16 ± 2.70 | 0.158 |
| Vascular reactivity index | 1.93 ± 0.57 | 2.41 ± 0.35 | 1.70 ± 0.18 | 0.82 ± 0.11 | <0.001 * |
| SBP (mmHg) | 135.75 ± 18.67 | 136.52 ± 16.92 | 125.91 ± 20.42 | 131.40 ± 19.29 | 0.735 |
| DBP (mmHg) | 80.23 ± 10.88 | 81.59 ± 9.39 | 79.54 ± 12.05 | 77.10 ± 11.77 | 0.426 |
| Total cholesterol (mg/dL) | 158.00 (139.75–178.00) | 152.50 (139.50–178.00) | 158.00 (138.50–175.75) | 167.50 (142.50–189.50) | 0.657 |
| Triglyceride (mg/dL) | 141.00 (103.50–212.75) | 147.50 (105.50–220.75) | 131.50 (97.25–204.00) | 179.50 (83.75–217.00) | 0.678 |
| HDL-C (mg/dL) | 46.13 ± 10.47 | 46.23 ± 10.39 | 45.61 ± 10.16 | 48.00 ± 12.97 | 0.806 |
| LDL-C (mg/dL) | 89.68 ± 27.99 | 87.98 ± 30.76 | 92.07 ± 26.71 | 86.50 ± 20.91 | 0.732 |
| Fasting glucose (mg/dL) | 113.00 (95.75–154.25) | 115.00 (95.75–155.50) | 111.00 (96.50–165.25) | 107.50 (87.25–144.25) | 0.619 |
| Albumin (mg/dL) | 4.37 ± 0.23 | 4.40 ± 0.25 | 4.35 ± 0.17 | 4.35 ± 0.32 | 0.521 |
| Blood urea nitrogen (mg/dL) | 17.00 (13.75–19.25) | 15.50 (13.00–18.25) | 17.00 (14.00–22.00) | 18.00 (13.25–23.00) | 0.099 |
| Creatinine (mg/dL) | 1.00 (0.80–1.10) | 0.90 (0.78–1.10) | 1.00 (0.90–1.10) | 1.00 (0.96–1.40) | 0.127 |
| eGFR (mL/min) | 81.81 ± 21.91 | 85.77 ± 22.32 | 80.81 ± 20.51 | 68.24 ± 22.43 | 0.065 |
| Osteoprotegerin (pg/mL) | 76.69 (60.48–98.72) | 68.03 (51.78–83.64) | 79.51 (62.92–100.17) | 127.86 (102.63–172.47) | <0.001 * |
| Male, n (%) | 83 (81.4) | 34 (73.9) | 42 (91.3) | 7 (70.0) | 0.063 |
| Diabetes mellitus, n (%) | 52 (51.0) | 26 (56.5) | 21 (45.7) | 5 (50.0) | 0.579 |
| Coronary artery disease, n (%) | 70 (68.6) | 31 (67.4) | 34 (73.9) | 5 (50.0) | 0.326 |
| Smoking, n (%) | 18 (17.6) | 11 (23.9) | 5 (10.9) | 2 (20.0) | 0.255 |
| ACE inhibitor use, n (%) | 20 (19.6) | 8 (17.4) | 11 (23.9) | 1 (10.0) | 0.530 |
| ARB use, n (%) | 49 (48.0) | 25 (54.3) | 18 (39.1) | 6 (60.0) | 0.250 |
| β-blocker use, n (%) | 47 (46.1) | 21 (45.7) | 21 (45.7) | 5 (50.0) | 0.966 |
| CCB use, n (%) | 48 (47.1) | 25 (54.3) | 19 (41.3) | 4 (40.0) | 0.408 |
| Statin use, n (%) | 77 (75.5) | 33 (71.7) | 36 (78.3) | 8 (80.0) | 0.722 |
| Fibrate use, n (%) | 7 (6.9) | 4 (8.7) | 2 (4.3) | 1 (10.0) | 0.653 |
| Variables | Vascular Reactivity Index | ||||
|---|---|---|---|---|---|
| Simple Regression | Multivariable Regression | ||||
| r | p Value | Beta | Adjusted R2 Change | p Value | |
| Male | 0.032 | 0.746 | — | — | — |
| Diabetes mellitus | 0.099 | 0.320 | — | — | — |
| Coronary artery disease | 0.168 | 0.091 | — | — | — |
| Smoking | 0.124 | 0.213 | — | — | — |
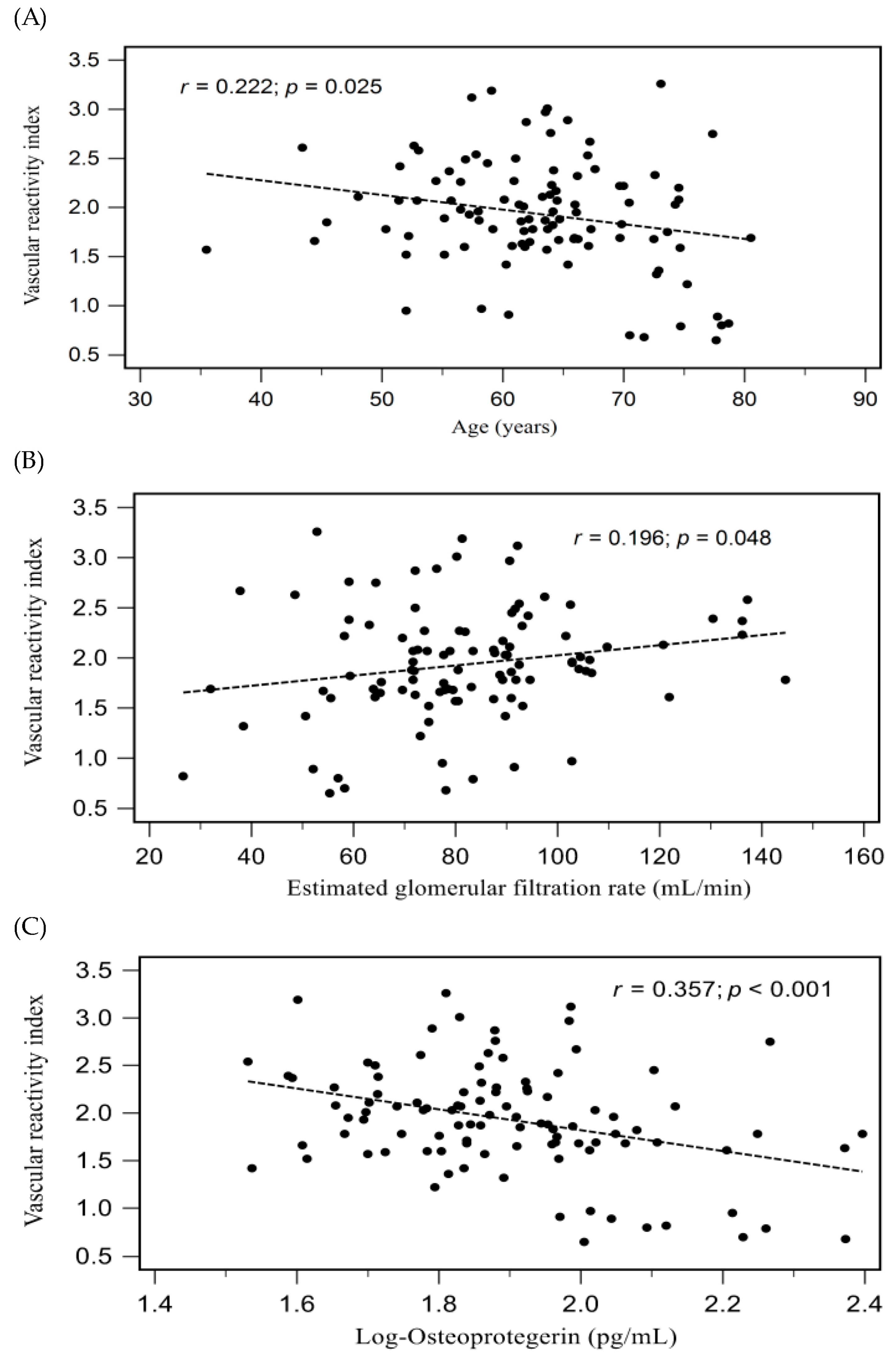
| Age (years) | −0.222 | 0.025 * | — | — | — |
| Height (cm) | −0.061 | 0.545 | — | — | — |
| Body weight (kg) | 0.004 | 0.971 | — | — | — |
| Body mass index (kg/m2) | 0.057 | 0.570 | — | — | — |
| Systolic blood pressure (mmHg) | 0.075 | 0.454 | — | — | — |
| Diastolic blood pressure (mmHg) | 0.141 | 0.158 | — | — | — |
| Log-TCH (mg/dL) | −0.096 | 0.335 | — | — | — |
| Log-Triglyceride (mg/dL) | 0.041 | 0.680 | — | — | — |
| HDL-C (mg/dL) | −0.006 | 0.955 | — | — | — |
| LDL-C (mg/dL) | −0.027 | 0.790 | — | — | — |
| Log-Glucose (mg/dL) | 0.093 | 0.352 | — | — | — |
| Albumin (mg/dL) | 0.138 | 0.168 | — | — | — |
| Log-BUN (mg/dL) | −0.189 | 0.057 | — | — | — |
| Log-Creatinine (mg/dL) | −0.190 | 0.056 | — | — | — |
| eGFR (mL/min) | 0.196 | 0.048 * | — | — | — |
| Log-Osteoprotegerin (pg/mL) | −0.357 | <0.001 * | −0.357 | 0.119 | <0.001 * |
| Model | Osteoprotegerin (per 1 pg/mL of Increase) for Vascular Reactivity Dysfunction | Osteoprotegerin (per 1 pg/mL of Increase) for Poor Vascular Reactivity | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Crude model | 1.020 (1.006–1.034) | 0.005 * | 1.024 (1.010–1.038) | 0.001 * |
| Adjusted model | 1.025 (1.005–1.041) | 0.003 * | 1.028 (1.011–1.045) | 0.001 * |
| Vascular ReactivityDysfunction | ||||||
| AUC (95% CI) | Cut-off | Sen (%) | Spe (%) | PPV (%) | NPV (%) | |
| Osteoprotegerin (pg/mL) | 0.679 (0.575–0.783) | 89.72 | 51.8 | 82.6 | 78.4 | 58.5 |
| Poor Vascular Reactivity | ||||||
| AUC (95% CI) | Cut-off | Sen (%) | Spe (%) | PPV (%) | NPV (%) | |
| Osteoprotegerin (pg/mL) | 0.905 (0.843–0.968) | 93.11 | 100.0 | 78.3 | 33.4 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-L.; Huang, P.-Y.; Tsai, J.-P.; Wang, J.-H.; Hsu, B.-G. Serum Osteoprotegerin Levels and the Vascular Reactivity Index in Patients with Hypertension. Medicina 2023, 59, 1794. https://doi.org/10.3390/medicina59101794
Chen Y-L, Huang P-Y, Tsai J-P, Wang J-H, Hsu B-G. Serum Osteoprotegerin Levels and the Vascular Reactivity Index in Patients with Hypertension. Medicina. 2023; 59(10):1794. https://doi.org/10.3390/medicina59101794
Chicago/Turabian StyleChen, Yen-Liang, Po-Yu Huang, Jen-Pi Tsai, Ji-Hung Wang, and Bang-Gee Hsu. 2023. "Serum Osteoprotegerin Levels and the Vascular Reactivity Index in Patients with Hypertension" Medicina 59, no. 10: 1794. https://doi.org/10.3390/medicina59101794
APA StyleChen, Y.-L., Huang, P.-Y., Tsai, J.-P., Wang, J.-H., & Hsu, B.-G. (2023). Serum Osteoprotegerin Levels and the Vascular Reactivity Index in Patients with Hypertension. Medicina, 59(10), 1794. https://doi.org/10.3390/medicina59101794







