Effects of the Antioxidant Quercetin in an Experimental Model of Ulcerative Colitis in Mice
Abstract
1. Introduction
2. Materials and Methods
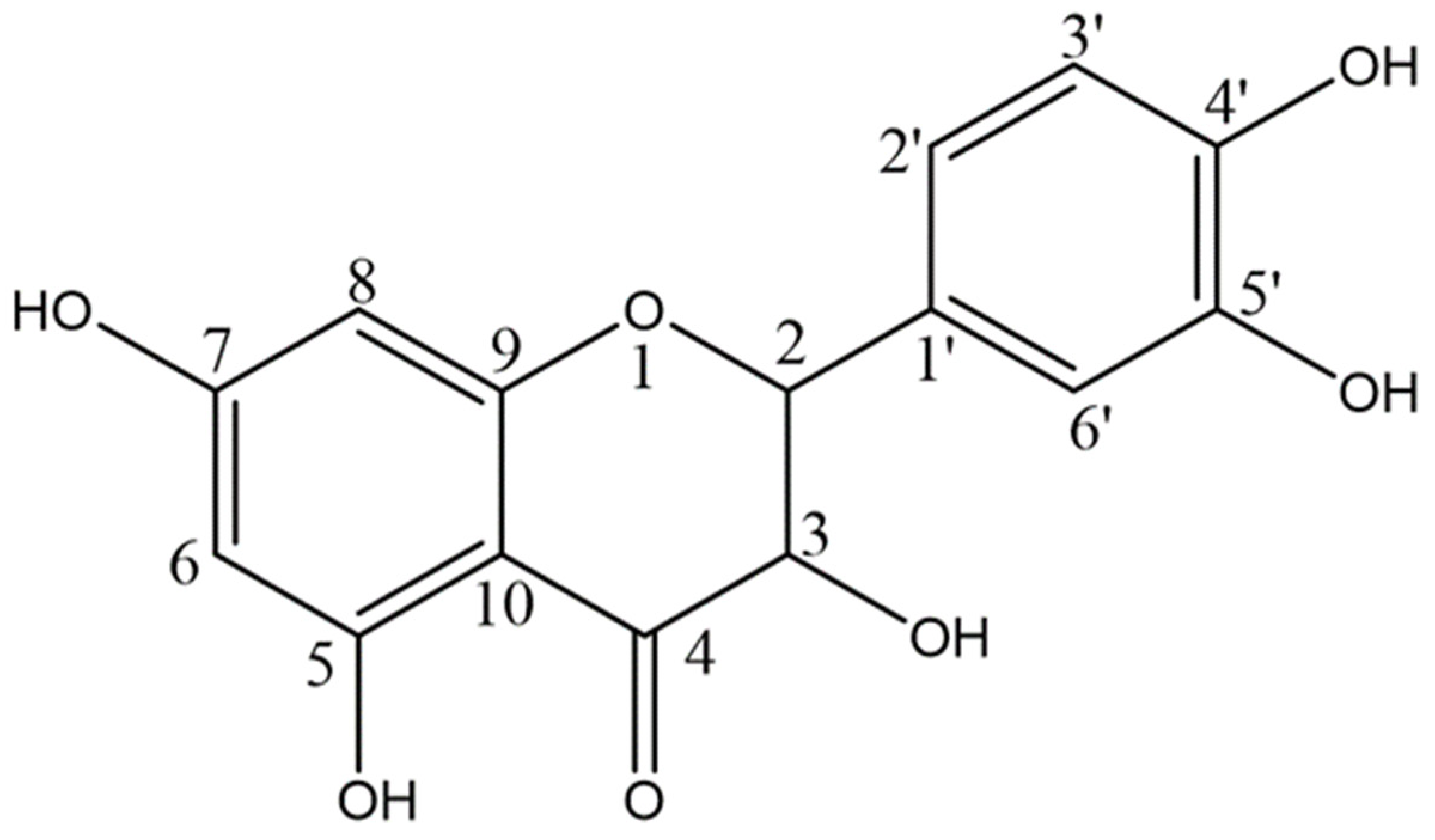
2.1. Quercetin
2.2. Animals
2.3. Experimental Design
2.4. Disease Activity Index
2.5. Tissue Collection and Histology
2.5.1. Immunohistochemistry for Integrin a4β7
2.5.2. Immunohistochemistry for MAdCAM and CD11b
2.6. Statistical Analysis
3. Results
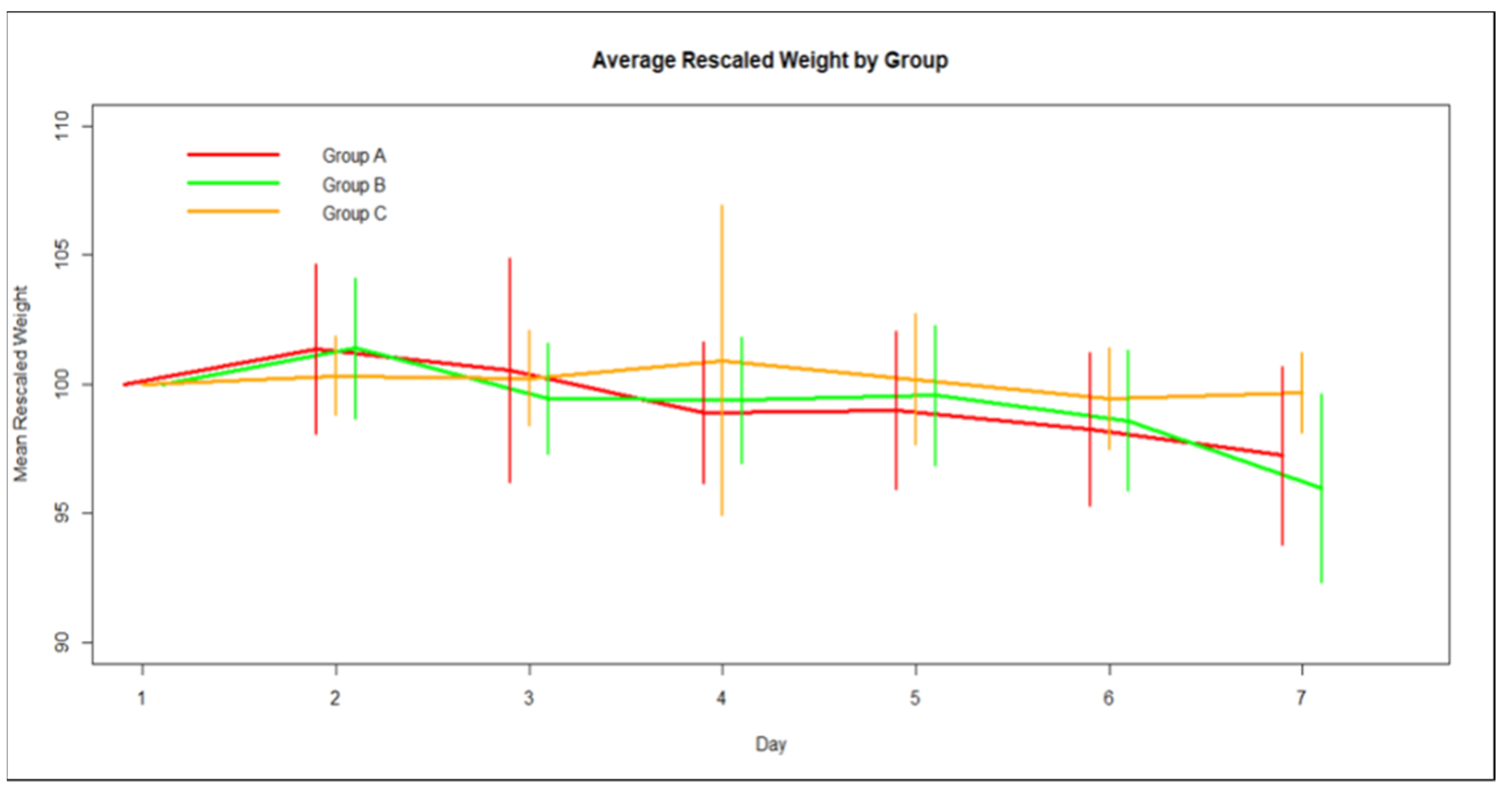
3.1. Weight
3.2. Disease Activity Index
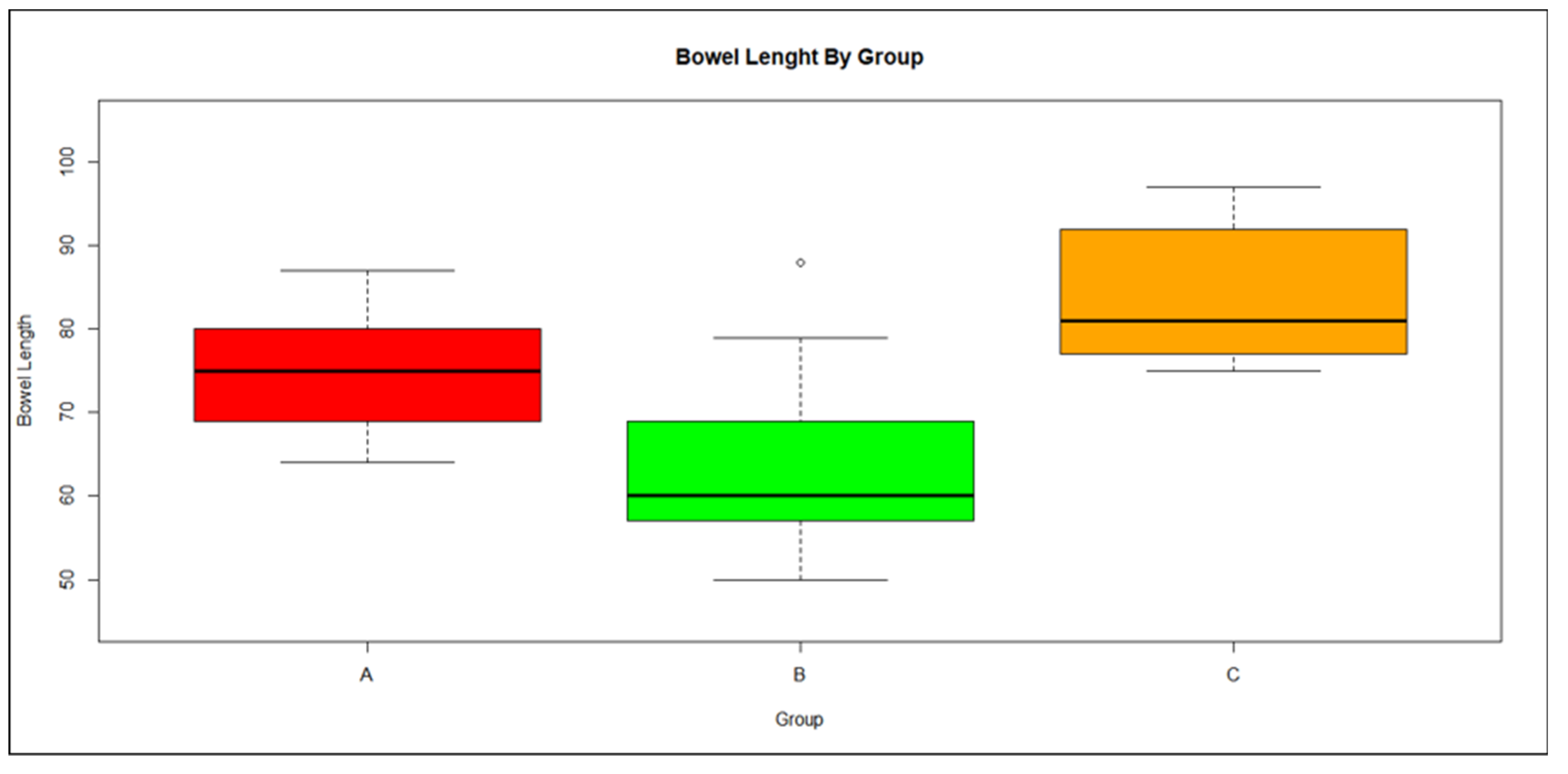
3.3. Bowel Length
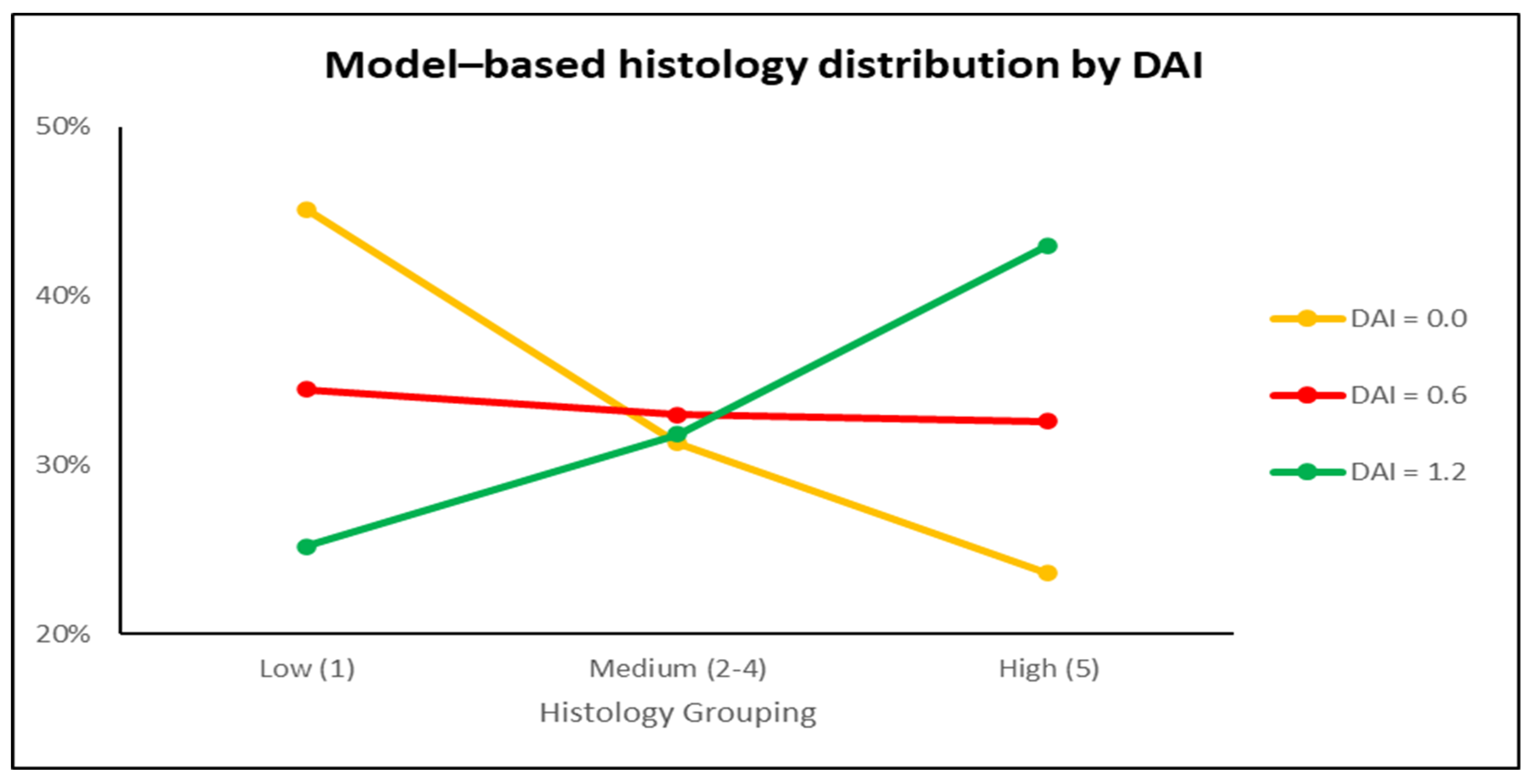
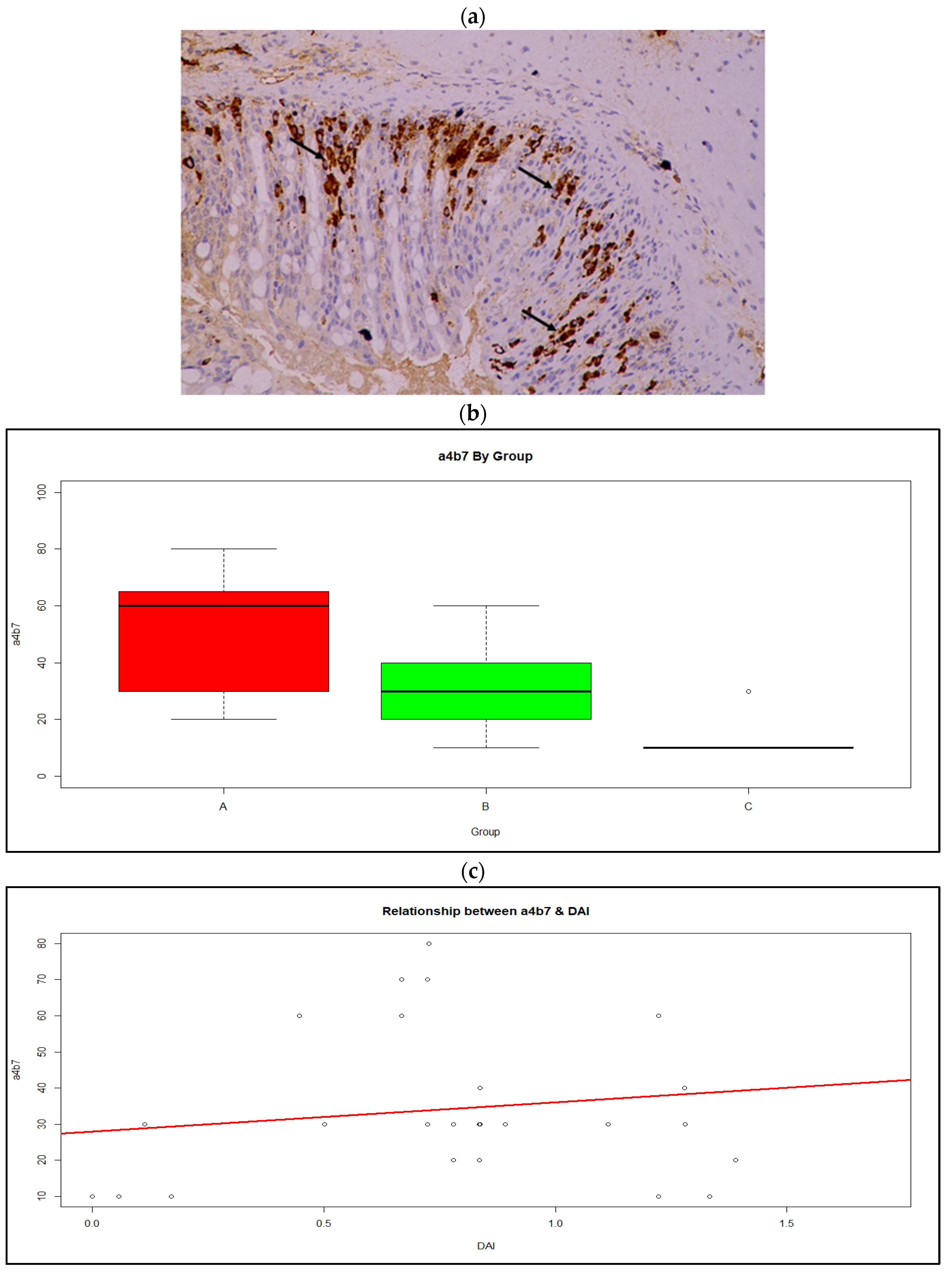
3.4. Histology and Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porter, R.J.; Kalla, R.; Ho, G.-T. Ulcerative colitis: Recent advances in the understanding of disease pathogenesis. F1000Research 2020, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Roessner, A.; Kuester, D.; Malfertheiner, P.; Schneider-Stock, R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol. Res. Pr. 2008, 204, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.; Parker, R.D.; Abdollahi, M. Oxidative Stress and Pathogenesis of Inflammatory Bowel Disease: An Epiphenomenon or the Cause? Dig. Dis. Sci. 2007, 52, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Esworthy, R.S.; Aranda, R.; Martín, M.G.; Doroshow, J.H.; Binder, S.W.; Chu, F.-F. Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am. J. Physiol. Liver Physiol. 2001, 281, G848–G855. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.; Roggenbuck, D.; Laass, M.W. Diagnosis and classification of ulcerative colitis. Autoimmun. Rev. 2014, 13, 463–466. [Google Scholar] [CrossRef]
- Henriksen, M.; Jahnsen, J.; Lygren, I.; Stray, N.; Sauar, J.; Vatn, M.H.; Moum, B.; the IBSEN Study Group C-reactive protein: A predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut 2008, 57, 1518–1523. [Google Scholar] [CrossRef]
- Mack, D.R.; Saul, B.; Boyle, B.; Griffiths, A.; Sauer, C.; Markowitz, J.; LeLeiko, N.; Keljo, D.; Rosh, J.R.; Baker, S.S.; et al. Analysis of Using the Total White Blood Cell Count to Define Severe New-onset Ulcerative Colitis in Children. J. Craniofacial Surg. 2020, 71, 354–360. [Google Scholar] [CrossRef]
- Cohen, R.D.; Yu, A.P.; Wu, E.Q.; Xie, J.; Mulani, P.M.; Chao, J. Systematic review: The costs of ulcerative colitis in Western countries. Aliment. Pharmacol. Ther. 2010, 31, 693–707. [Google Scholar] [CrossRef]
- Ungaro, R.; Colombel, J.-F.; Lissoos, T.; Peyrin-Biroulet, L. A Treat-to-Target Update in Ulcerative Colitis: A Systematic Review. Am. J. Gastroenterol. 2019, 114, 874–883. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B.; et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar] [CrossRef]
- Ham, M.; Moss, A.C. Mesalamine in the treatment and maintenance of remission of ulcerative colitis. Expert Rev. Clin. Pharmacol. 2012, 5, 113–123. [Google Scholar] [CrossRef]
- Ho, G.T.; Chiam, P.; Drummond, H.; Loane, J.; Arnott, I.D.; Satsangi, J. The efficacy of corticosteroid therapy in inflammatory bowel disease: Analysis of a 5-year UK inception cohort. Aliment. Pharmacol. Ther. 2006, 24, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Zenlea, T.; Peppercorn, M.A. Immunosuppressive therapies for inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 3146–3152. [Google Scholar] [CrossRef] [PubMed]
- Money, S. The Risks of Chronic Corticosteroid Exposure. J. Pain Palliat. Care Pharmacother. 2017, 31, 160–161. [Google Scholar] [CrossRef] [PubMed]
- Falasco, G.; Zinicola, R.; Forbes, A. Immunosuppressants in distal ulcerative colitis. Aliment. Pharmacol. Ther. 2002, 16, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wu, K.; Liang, X.; Liang, Y.; Li, R. Infliximab clinically treating ulcerative colitis: A systematic review and meta-analysis. Pharmacol. Res. 2019, 148, 104455. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Baert, F.; Danese, S.; Krznarić, Ž.; Kobayashi, T.; Yao, X.; Chen, J.; Rosario, M.; Bhatia, S.; Kisfalvi, K.; et al. Efficacy and Safety of Vedolizumab Subcutaneous Formulation in a Randomized Trial of Patients with Ulcerative Colitis. Gastroenterology 2020, 158, 562–572.e12. [Google Scholar] [CrossRef]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.-F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.-J.; Danese, S.; et al. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef]
- Singh, S.; George, J.; Boland, B.S.; Casteele, N.V.; Sandborn, W.J. Primary Non-Response to Tumor Necrosis Factor Antagonists is Associated with Inferior Response to Second-line Biologics in Patients with Inflammatory Bowel Diseases: A Systematic Review and Meta-analysis. J. Crohn’s Colitis 2018, 12, 635–643. [Google Scholar] [CrossRef]
- Anwar, S.; Almatroudi, A.; Alsahli, M.A.; Khan, M.A.; Khan, A.A.; Rahmani, A.H. Natural Products: Implication in Cancer Prevention and Treatment through Modulating Various Biological Activities. Anti-Cancer Agents Med. Chem. 2020, 20, 2025–2040. [Google Scholar] [CrossRef]
- Anwar, S.; Almatroudi, A.; Allemailem, K.S.; Joseph, R.J.; Khan, A.A.; Rahmani, A.H. Protective Effects of Ginger Extract against Glycation and Oxidative Stress-Induced Health Complications: An In Vitro Study. Processes 2020, 8, 468. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Khosravan, V. Anticonvulsant effect of Crocus sativus L. stigmas aqueous and ethanolic extracts in mice. Arch. Iranian Med. Arch Iran Med. 2001, 5, 44–47. [Google Scholar]
- Hosseinzadeh, H.; Karimi, G.; Niapoor, M. Antidepressant effects of Crocus sativus stigma extracts and its constituents, crocin and safranal, in mice. J. Med. Plants 2004, 3, 48–58. [Google Scholar]
- Tayman, C.; Tonbul, A.; Kosus, A.; Hirfanoglu, I.M.; Uysal, S.; Haltas, H.; Tatli, M.M.; Andiran, F. N-acetylcysteine may prevent severe intestinal damage in necrotizing enterocolitis. J. Pediatr. Surg. 2012, 47, 540–550. [Google Scholar] [CrossRef]
- Ozdemir, R.; Yurttutan, S.; Sarı, F.N.; Uysal, B.; Unverdi, H.G.; Canpolat, F.E.; Erdeve, O.; Dilmen, U. Antioxidant effects of N-acetylcysteine in a neonatal rat model of necrotizing enterocolitis. J. Pediatr. Surg. 2012, 47, 1652–1657. [Google Scholar] [CrossRef]
- Xu, M.; Duan, X.-Y.; Chen, Q.-Y.; Fan, H.; Hong, Z.-C.; Deng, S.-J.; Nan, Z.; Wu, H.; Dong, Y.-L.; Liu, Y.-J.; et al. Effect of compound sophorae decoction on dextran sodium sulfate (DSS)-induced colitis in mice by regulating Th17/Treg cell balance. Biomed. Pharmacother. 2018, 109, 2396–2408. [Google Scholar] [CrossRef]
- Daskalaki, M.G.; Vyrla, D.; Harizani, M.; Doxaki, C.; Eliopoulos, A.G.; Roussis, V.; Ioannou, E.; Tsatsanis, C.; Kampranis, S.C. Neorogioltriol and Related Diterpenes from the Red Alga Laurencia Inhibit Inflammatory Bowel Disease in Mice by Suppressing M1 and Promoting M2-Like Macrophage Responses. Mar. Drugs 2019, 17, 97. [Google Scholar] [CrossRef]
- Bai, X.; Gou, X.; Cai, P.; Xu, C.; Cao, L.; Zhao, Z.; Huang, M.; Jin, J. Sesamin Enhances Nrf2-Mediated Protective Defense against Oxidative Stress and Inflammation in Colitis via AKT and ERK Activation. Oxidative Med. Cell. Longev. 2019, 2019, 2432416. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Shaik, Y.B.; Castellani, M.L.; Perrella, A.; Conti, F.; Salini, V.; Tete, S.; Madhappan, B.; Vecchiet, J.; A De Lutiis, M.; Caraffa, A.; et al. Role of quercetin (a natural herbal compound) in allergy and inflammation. J. Boil. Regul. Homeost. Agents 2006, 20, 47–52. [Google Scholar]
- Kinker, A.T.C.A.U.S.S.B. Quercetin: A Promising Treatment for the Common Cold. J. Anc. Dis. Prev. Remedies 2014, 2, 111. [Google Scholar] [CrossRef]
- Del Follo-Martinez, A.; Banerjee, N.; Li, X.; Safe, S.; Mertens-Talcott, S. Resveratrol and Quercetin in Combination Have Anticancer Activity in Colon Cancer Cells and Repress Oncogenic microRNA-27a. Nutr. Cancer 2013, 65, 494–504. [Google Scholar] [CrossRef] [PubMed]
- K, R.M.; Ghosh, B. Quercetin inhibits LPS-induced nitric oxide and tumor necrosis factor-α production in murine macrophages. Int. J. Immunopharmacol. 1999, 21, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Mani, I.; Iversen, L.; Ziboh, V.A. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostaglandins Leukot. Essent. Fat. Acids 1998, 58, 17–24. [Google Scholar] [CrossRef]
- Lee, K.M.; Hwang, M.K.; Lee, D.E.; Lee, K.W.; Lee, H.J. Protective Effect of Quercetin against Arsenite-Induced COX-2 Expression by Targeting PI3K in Rat Liver Epithelial Cells. J. Agric. Food Chem. 2010, 58, 5815–5820. [Google Scholar] [CrossRef]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of Quercetin: Problems and Promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef]
- Pralhad, T.; Rajendrakumar, K. Study of freeze-dried quercetin–cyclodextrin binary systems by DSC, FT-IR, X-ray diffraction and SEM analysis. J. Pharm. Biomed. Anal. 2004, 34, 333–339. [Google Scholar] [CrossRef]
- Zheng, Y.; Haworth, I.S.; Zuo, Z.; Chow, M.S.; Chow, A.H. Physicochemical and Structural Characterization of Quercetin-β-Cyclodextrin Complexes. J. Pharm. Sci. 2005, 94, 1079–1089. [Google Scholar] [CrossRef]
- Liu, M.; Dong, L.; Chen, A.; Zheng, Y.; Sun, D.; Wang, X.; Wang, B. Inclusion complexes of quercetin with three β-cyclodextrins derivatives at physiological pH: Spectroscopic study and antioxidant activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 854–860. [Google Scholar] [CrossRef]
- Kellici, T.F.; Chatziathanasiadou, M.V.; Diamantis, D.; Chatzikonstantinou, A.V.; Andreadelis, I.; Christodoulou, E.; Valsami, G.; Mavromoustakos, T.; Tzakos, A.G. Mapping the interactions and bioactivity of quercetin⿿(2-hydroxypropyl)-β-cyclodextrin complex. Int. J. Pharm. 2016, 511, 303–311. [Google Scholar] [CrossRef]
- Diamantis, D.A.; Ramesova, S.; Chatzigiannis, C.M.; Degano, I.; Gerogianni, P.S.; Karadima, K.E.; Perikleous, S.; Rekkas, D.; Gerothanassis, I.P.; Galaris, D.; et al. Exploring the oxidation and iron binding profile of a cyclodextrin encapsulated quercetin complex unveiled a controlled complex dissociation through a chemical stimulus. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2018, 1862, 1913–1924. [Google Scholar] [CrossRef]
- Christodoulou, E.; Ntountaniotis, D.; Leonis, G.; Mavromoustakos, T.; Valsami, G. Application of Neutralization and Freeze-Drying Technique for the Preparation of the Beneficial in Drug Delivery 2-Hydroxypropyl-β-Cyclodextrin Complexes with Bioactive Molecules. In Supramolecules in Drug Discovery and Drug Delivery; Mavromoustakos, T., Tzakos, A.G., Durdagi, S., Eds.; Humana: New York, NY, USA, 2020; Volume 2207. [Google Scholar] [CrossRef]
- Ohkusa, T. Production of experimental ulcerative colitis in hamsters by dextran sulfate sodium and changes in intestinal mi-croflora. Nihon Shokakibyo Gakkai Zasshi 1985, 82, 1327–1336. (in Japanese). [Google Scholar] [PubMed]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef] [PubMed]
- Shrum, B.; Anantha, R.V.; Xu, S.X.; Donnelly, M.; Haeryfar, S.M.; McCormick, J.K.; Mele, T. A robust scoring system to evaluate sepsis severity in an animal model. BMC Res. Notes 2014, 7, 233. [Google Scholar] [CrossRef]
- Murthy, S.N.S.; Cooper, H.S.; Shim, H.; Shah, R.S.; Ibrahim, S.A.; Sedergran, D.J. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig. Dis. Sci. 1993, 38, 1722–1734. [Google Scholar] [CrossRef]
- Rogerio, A.D.P.; Kanashiro, A.; Fontanari, C.; Da Silva, E.V.G.; Lucisano-Valim, Y.M.; Soares, E.G.; Faccioli, L.H. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm. Res. 2007, 56, 402–408. [Google Scholar] [CrossRef]
- Kleemann, R.; Verschuren, L.; Morrison, M.; Zadelaar, S.; van Erk, M.J.; Wielinga, P.Y.; Kooistra, T. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis 2011, 218, 44–52. [Google Scholar] [CrossRef]
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Il Farm. 2001, 56, 683–687. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Sreedhar, R.; Giridharan, V.V.; Watanabe, K. Molecular targets of quercetin with anti-inflammatory properties in atopic dermatitis. Drug Discov. Today 2016, 21, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Shiratora, Y.; Aoki, S.; Takada, H.; Kiriyama, H.; Ohto, K.; Hai, K.; Teraoka, H.; Matano, S.; Matsumoto, K.; Kamii, K. Oxygen-Derived Free Radical Generating Capacity of Polymorphonuclear Cells in Patients with Ulcerative Colitis. Digestion 1989, 44, 163–171. [Google Scholar] [CrossRef]
- Comalada, M.; Camuesco, D.; Sierra, S.; Ballester, I.; Xaus, J.; Gálvez, J.; Zarzuelo, A. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-κB pathway. Eur. J. Immunol. 2005, 35, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Han, C.; Jin, J.; Qin, K.; Zhang, H.; Li, T.; Li, N.; Cao, X. Integrin CD11b attenuates colitis by strengthening Src-Akt pathway to polarize anti-inflammatory IL-10 expression. Sci. Rep. 2016, 6, 26252. [Google Scholar] [CrossRef] [PubMed]
- Roosenboom, B.; van Lochem, E.G.; Meijer, J.; Smids, C.; Nierkens, S.; Brand, E.C.; van Erp, L.W.; Kemperman, L.G.; Groenen, M.J.; Horje, C.S.H.T.; et al. Development of Mucosal PNAd+ and MAdCAM-1+ Venules during Disease Course in Ulcerative Colitis. Cells 2020, 9, 891. [Google Scholar] [CrossRef] [PubMed]



| Score | Body Weight Decrease (%) | Stool Consistency | Presence of Blood |
|---|---|---|---|
| 0 | <1 | Normal | Normal |
| 1 | 1–5 | ||
| 2 | 5–10 | Loose stools | |
| 3 | 10–20 | ||
| 4 | >20 | Diarrhoea | Gross bleeding |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kottakis, G.; Kambouri, K.; Giatromanolaki, A.; Valsami, G.; Kostomitsopoulos, N.; Tsaroucha, A.; Pitiakoudis, M. Effects of the Antioxidant Quercetin in an Experimental Model of Ulcerative Colitis in Mice. Medicina 2023, 59, 87. https://doi.org/10.3390/medicina59010087
Kottakis G, Kambouri K, Giatromanolaki A, Valsami G, Kostomitsopoulos N, Tsaroucha A, Pitiakoudis M. Effects of the Antioxidant Quercetin in an Experimental Model of Ulcerative Colitis in Mice. Medicina. 2023; 59(1):87. https://doi.org/10.3390/medicina59010087
Chicago/Turabian StyleKottakis, George, Katerina Kambouri, Alexandra Giatromanolaki, Georgia Valsami, Nikolaos Kostomitsopoulos, Alexandra Tsaroucha, and Michael Pitiakoudis. 2023. "Effects of the Antioxidant Quercetin in an Experimental Model of Ulcerative Colitis in Mice" Medicina 59, no. 1: 87. https://doi.org/10.3390/medicina59010087
APA StyleKottakis, G., Kambouri, K., Giatromanolaki, A., Valsami, G., Kostomitsopoulos, N., Tsaroucha, A., & Pitiakoudis, M. (2023). Effects of the Antioxidant Quercetin in an Experimental Model of Ulcerative Colitis in Mice. Medicina, 59(1), 87. https://doi.org/10.3390/medicina59010087








