Management of Complete Heart Block in a Pregnant Woman with Systemic Lupus Erythematosus-Associated Complications: Treatment Considerations and Pitfalls
Abstract
1. Introduction
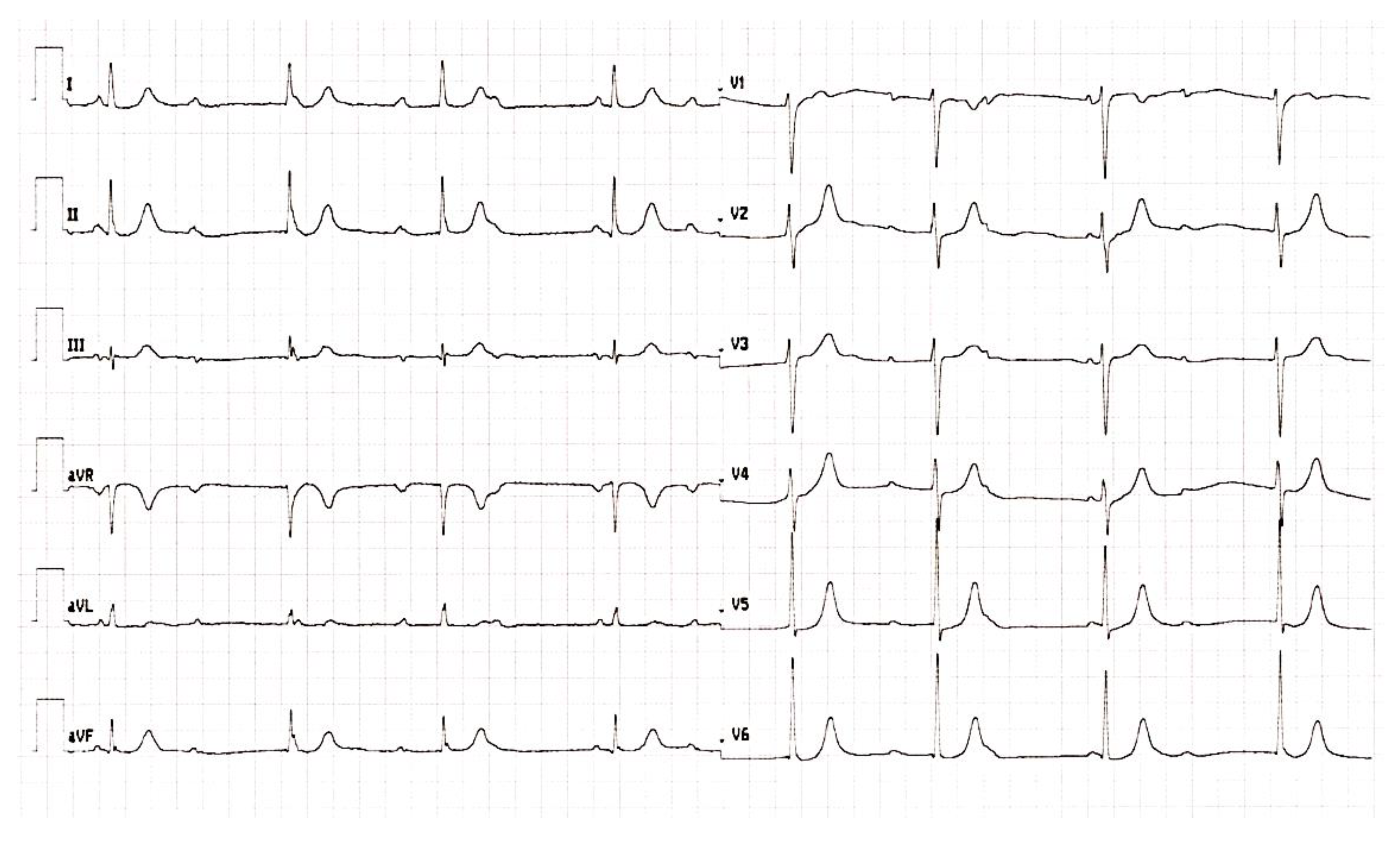
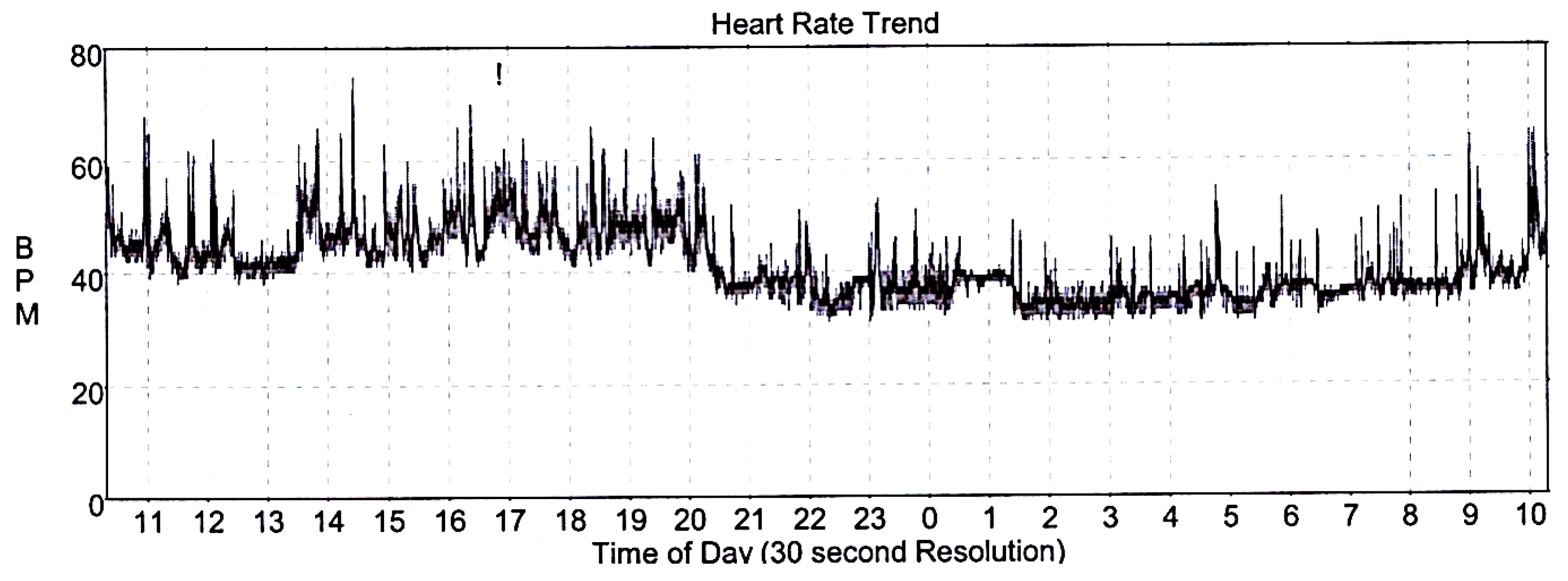
2. Case Report and Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmqvist, F.; Daubert, J.P. First-degree AV block-an entirely benign finding or a potentially curable cause of cardiac disease? Ann. Noninvasive Electrocardiol. 2013, 18, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Wogan, J.M.; Lowenstein, S.R.; Gordon, G.S. Second-degree atrioventricular block: Mobitz type II. J. Emerg. Med. 1993, 11, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Al Taweel, M.; Al Mukhaylid, S.; Alsultan, N. Congenital Complete Heart Block in Adults. J. Adv. Med. Med. Res. 2022, 34, 245–248. [Google Scholar] [CrossRef]
- Fischbach, P.S.; Frias, P.A.; Strieper, M.J.; Campbell, R.M. Natural history and current therapy for complete heart block in children and patients with congenital heart disease. Congenit. Heart Dis. 2007, 2, 224–234. [Google Scholar] [CrossRef]
- Pruetz, J.D.; Miller, J.C.; Loeb, G.E.; Silka, M.J.; Bar-Cohen, Y.; Chmait, R.H. Prenatal diagnosis and management of congenital complete heart block. Birth Defects Res. 2019, 111, 380–388. [Google Scholar] [CrossRef]
- De Carolis, S.; Garufi, C.; Garufi, E.; De Carolis, M.P.; Botta, A.; Tabacco, S.; Salvi, S. Autoimmune Congenital Heart Block: A Review of Biomarkers and Management of Pregnancy. Front. Pediatr. 2020, 8, 607515. [Google Scholar] [CrossRef]
- Brucato, A.; Jonzon, A.; Friedman, D.; Allan, L.D.; Vignati, G.; Gasparini, M.; Stein, J.I.; Montella, S.; Michaelsson, M.; Buyon, J. Proposal for a new definition of congenital complete atrioventricular block. Lupus 2003, 12, 427–435. [Google Scholar] [CrossRef]
- Barra, S.N.C.; Providencia, R.; Paiva, L.; Nascimento, J.; Marques, A.L. A review on advanced atrioventricular block in young or middle-aged adults. Pacing Clin. Electrophysiol. 2012, 35, 1395–1405. [Google Scholar] [CrossRef]
- Bamgboje, A.; Akintan, F.O.; Gupta, N.M.; Kaur, G.; Pekler, G.; Mushiyev, S. Lyme Carditis: A Reversible Cause of Acquired Third-Degree AV Block. Am. J. Case Rep. 2021, 22, e927885. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Kostopoulou, M.; Alunno, A.; Aringer, M.; Bajema, I.; Boletis, J.N.; Cervera, R.; Doria, A.; Gordon, C.; Govoni, M.; et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 2019, 78, 736–745. [Google Scholar] [CrossRef]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019, 71, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Costedoat-Chalumeau, N.; Amoura, Z.; Villain, E.; Cohen, L.; Piette, J.C. Anti-SSA/Ro antibodies and the heart: More than complete congenital heart block? A review of electrocardiographic and myocardial abnormalities and of treatment options. Arthritis Res. Ther. 2005, 7, 69–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Claus, R.; Hickstein, H.; Külz, T.; Lenschow, U.; Meiske, D.; Kotitschke, A.; Thiesen, H.J.; Lorenz, P. Identification and management of fetuses at risk for, or affected by, congenital heart block associated with autoantibodies to SSA (Ro), SSB (La), or an HsEg5-like autoantigen. Rheumatol. Int. 2006, 26, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Villuendas, R.; Martínez-Morillo, M.; Juncà, G.; Teniente-Serra, A.; Diez, C.; Heredia, S.; Riveros-Frutos, A.; Bayés-Genís, A.; Olivé, A. Usefulness of cardiac screening in patients with systemic lupus erythematosus and anti-Ro/SSA antibodies. Lupus 2021, 30, 1596–1602. [Google Scholar] [CrossRef]
- Natsheh, A.; Shimony, D.; Bogot, N.; Nesher, G.; Breuer, G.S. Complete heart block in lupus. Lupus 2019, 28, 1589–1593. [Google Scholar] [CrossRef]
- Petri, M. Pregnancy and Systemic Lupus Erythematosus. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 64, 24–30. [Google Scholar] [CrossRef]
- Munguia-Realpozo, P.; Mendoza-Pinto, C.; Benito, C.S.; Escarcega, R.O.; Garcia-Carrasco, M.; Martinez, S.M.; Morales, I.E.; Romero, J.L.G.; Ruiz-Arguelles, A.; Cervera, R. Systemic lupus erythematosus and hypertension. Autoimmun. Rev. 2019, 18, 102371. [Google Scholar] [CrossRef]
- Rosen, B.; Ovsyshcher, I.A.; Zimlichman, R. Complete atrioventricular block induced by methyldopa. Pacing Clin. Electrophysiol. 1988, 11 Pt 1, 1555–1558. [Google Scholar] [CrossRef]
- Cokkinos, V.; Vorides, M. Impairment of Atrioventricular Conduction by Methyldopa. CHEST J. 1978, 74, 697. [Google Scholar] [CrossRef]
- Hunter, S.; Robson, S.C. Adaptation of the maternal heart in pregnancy. Br. Heart J. 1992, 68, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, B.V.; Chaudhuri, A.; Kulkami, H.L.; Kale, P.A. Therapeutic guidelines for congenital complete heart block presenting in pregnancy. Obstet. Gynecol. 1992, 79 Pt 2, 802–804. [Google Scholar] [PubMed]
- Hidaka, N.; Chiba, Y.; Kurita, T.; Satoh, S.; Nakano, H. Is intrapartum temporary pacing required for women with complete atrioventricular block? An analysis of seven cases. BJOG 2006, 113, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Adekanye, O.; Srinivas, K.; Collis, R.E. Bradyarrhythmias in pregnancy: A case report and review of management. Int. J. Obstet. Anesth. 2007, 16, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Thaman, R.; Curtis, S.; Faganello, G.; Szantho, G.V.; Turner, M.S.; Trinder, J.; Sellers, S.; Stuart, G.A. Cardiac outcome of pregnancy in women with a pacemaker and women with untreated atrioventricular conduction block. Europace 2011, 13, 859–863. [Google Scholar] [CrossRef]
- Suri, V.; Keepanasseril, A.; Aggarwal, N.; Vijayvergiya, R.; Chopra, S.; Rohilla, M. Maternal complete heart block in pregnancy: Analysis of four cases and review of management. J. Obstet. Gynaecol. Res. 2009, 35, 434–437. [Google Scholar] [CrossRef]
- Carrión-Camacho, M.R.; Marín-León, I.; Molina-Doñoro, J.M.; González-López, J.R. Safety of Permanent Pacemaker Implantation: A Prospective Study. J. Clin. Med. 2019, 8, 35. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, D.; Sarkar, A. Complete Heart Block and Pregnancy Outcome: An Analysis from Eastern India. SOJ Gynaecol. Obstet. Womens Health 2015, 1, 5. [Google Scholar] [CrossRef]
- Pedrinazzi, C.; Gazzaniga, P.; Durin, O.; Tovena, D.; Inama, G. Implantation of a permanent pacemaker in a pregnant woman under the guidance of electrophysiologic signals and transthoracic echocardiography. J. Cardiovasc. Med. 2008, 9, 1169–1172. [Google Scholar] [CrossRef]
- Jordaens, L.J.; Vandenbogaerde, J.F.; Van De Bruaene, P.; De Buyzere, M. Transesophageal echocardiography for insertion of a physiological pacemaker in early pregnancy. Pacing Clin. Electrophysiol. 1990, 13, 955–957. [Google Scholar] [CrossRef]
- Guo, P.; Qiu, J.; Wang, Y.; Chen, G.; Proietti, R.; Fadhle, A.S.; Zhao, C.; Wen Wang, D. Zero-fluoroscopy permanent pacemaker implantation using Ensite NavX system: Clinical viability or fanciful technique? Pacing Clin. Electrophysiol. 2018, 41, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Kojic, E.M.; Hardarson, T.; Sigfusson, N.; Sigvaldason, H. The prevalence and prognosis of third-degree atrioventricular conduction block: The Reykjavik study. J. Intern. Med. 1999, 246, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Tselios, K.; Gladman, D.D.; Harvey, P.; Su, J.; Urowitz, M.B. Severe brady-arrhythmias in systemic lupus erythematosus: Prevalence, etiology and associated factors. Lupus 2018, 27, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Ploux, S.; Strik, M.; Caillol, T.; Ramirez, F.D.; Abu-Alrub, S.; Marchand, H.; Buliard, S.; Haïssaguerre, M.; Bordachar, P. Beyond the wrist: Using a smartwatch electrocardiogram to detect electrocardiographic abnormalities. Arch. Cardiovasc. Dis. 2022, 115, 29–36. [Google Scholar] [CrossRef]
- Yerasi, C.; O’Donoghue, S.; Satler, L.F.; Waksman, R. Apple Watch detecting high-grade block after transcatheter aortic valve implantation. Eur. Heart J. 2020, 41, 1096. [Google Scholar] [CrossRef]

| Autoantibodies | Physical Parameters | |||||
|---|---|---|---|---|---|---|
| ANA | Anti-Ds DNA | Anti-Ss DNA | Anti-ENA | BP (mmHg) | HR (bpm) | |
| At the time of the diagnosis | 1:1600 | + | + | +/- | 180/100 | 65 |
| Clinically stable disease (3 years on maintenance treatment) | 1:100 | + | + | - | 130/75 | 70 |
| 1 month prior to the PM implantation | 1:1600 | + | + | - | 140/75 | 40 |
| 5 months after the PM implantation | 1:3200 | + | n/a | - | 135/70 | 85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rihackova, E.; Vysocanova, P.; Rihacek, M.; Kucerova, D.; Blahovcova, T.; Kala, P. Management of Complete Heart Block in a Pregnant Woman with Systemic Lupus Erythematosus-Associated Complications: Treatment Considerations and Pitfalls. Medicina 2023, 59, 88. https://doi.org/10.3390/medicina59010088
Rihackova E, Vysocanova P, Rihacek M, Kucerova D, Blahovcova T, Kala P. Management of Complete Heart Block in a Pregnant Woman with Systemic Lupus Erythematosus-Associated Complications: Treatment Considerations and Pitfalls. Medicina. 2023; 59(1):88. https://doi.org/10.3390/medicina59010088
Chicago/Turabian StyleRihackova, Eva, Petra Vysocanova, Michal Rihacek, Dominika Kucerova, Tereza Blahovcova, and Petr Kala. 2023. "Management of Complete Heart Block in a Pregnant Woman with Systemic Lupus Erythematosus-Associated Complications: Treatment Considerations and Pitfalls" Medicina 59, no. 1: 88. https://doi.org/10.3390/medicina59010088
APA StyleRihackova, E., Vysocanova, P., Rihacek, M., Kucerova, D., Blahovcova, T., & Kala, P. (2023). Management of Complete Heart Block in a Pregnant Woman with Systemic Lupus Erythematosus-Associated Complications: Treatment Considerations and Pitfalls. Medicina, 59(1), 88. https://doi.org/10.3390/medicina59010088







