High Versus Low Ligation of the Inferior Mesenteric Artery in Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Type of Participants
2.3. Types of Interventions and Comparators
2.4. Type of Outcomes
2.5. Primary Outcomes
2.6. Secondary Outcomes
2.7. Assessment of Risk of Bias in Included Studies
2.8. Data Collection and Analysis
2.9. Summary of Findings Table
3. Results
3.1. Search Results
3.2. Included Studies
3.3. Excluded Studies
3.4. Risk of Bias of Included Studies
3.5. Effects of Interventions
3.5.1. Primary Outcomes
- OM; four RCTs with 649 participants (HL: 327 and LL: 322) were analyzed for OM [14,25,29,31]. We are very uncertain about the effects of HL in reducing OM (HR: 1.24, 95% CI: 0.85–1.83; I2 = 0%; very low CoE) (Table 2). We downgraded the CoE due to serious study limitations and very serious imprecision;
- Postoperative complications; ten RCTs with 1293 participants (HL: 657 and LL: 636) were analyzed for postoperative complications [14,23,24,25,26,27,30,31,32,33]. There may be little to no difference in the postoperative complications between HL and LL (risk ratio (RR): 1.15, 95% CI: 0.87–1.52; I2 = 44%; low CoE) (Table 2). We downgraded the CoE due to serious study limitations and serious inconsistencies.
3.5.2. Secondary Outcomes
- Postoperative mortality; analysis of 1051 (HL: 534 and LL: 517) participants from eight RCTs [14,23,25,26,27,30,31,32] was performed. We are very uncertain about the effect of HL on postoperative mortality (RR: 0.33, 95% CI: 0.03–3.14; I2 = 0%; very low CoE) (Table 2). We downgraded the CoE due to serious study limitations and very serious imprecision;
- Anastomotic leakage; analysis of 1429 (HL: 721 and LL: 708) participants from 12 RCTs [14,22,23,24,25,26,27,28,30,31,32,33] was performed. We are very uncertain about the effects of HL on anastomotic leakage (RR: 1.32, 95% CI: 0.92–1.88; I2 = 0%; very low CoE) (Table 2). We downgraded the CoE due to serious study limitations, serious imprecision, and publication bias.
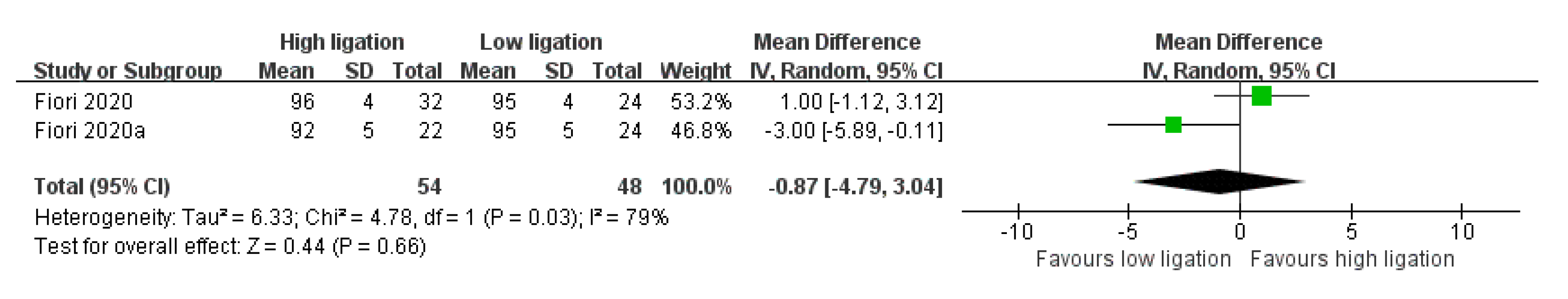
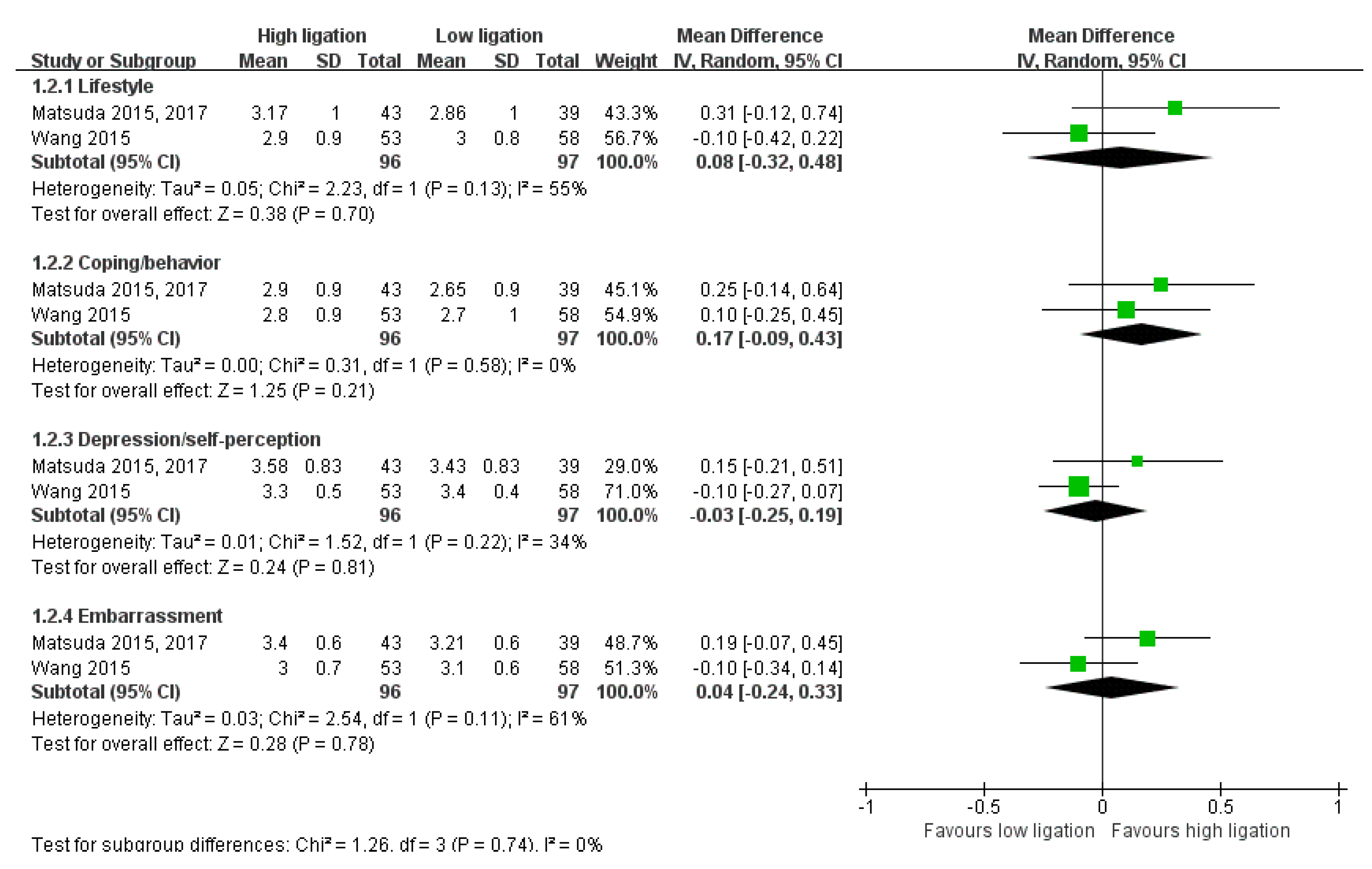
- Functional Outcomes (Short-Term Follow-Up)
- 6.
- Functional Outcomes (Long-Term Follow-Up)
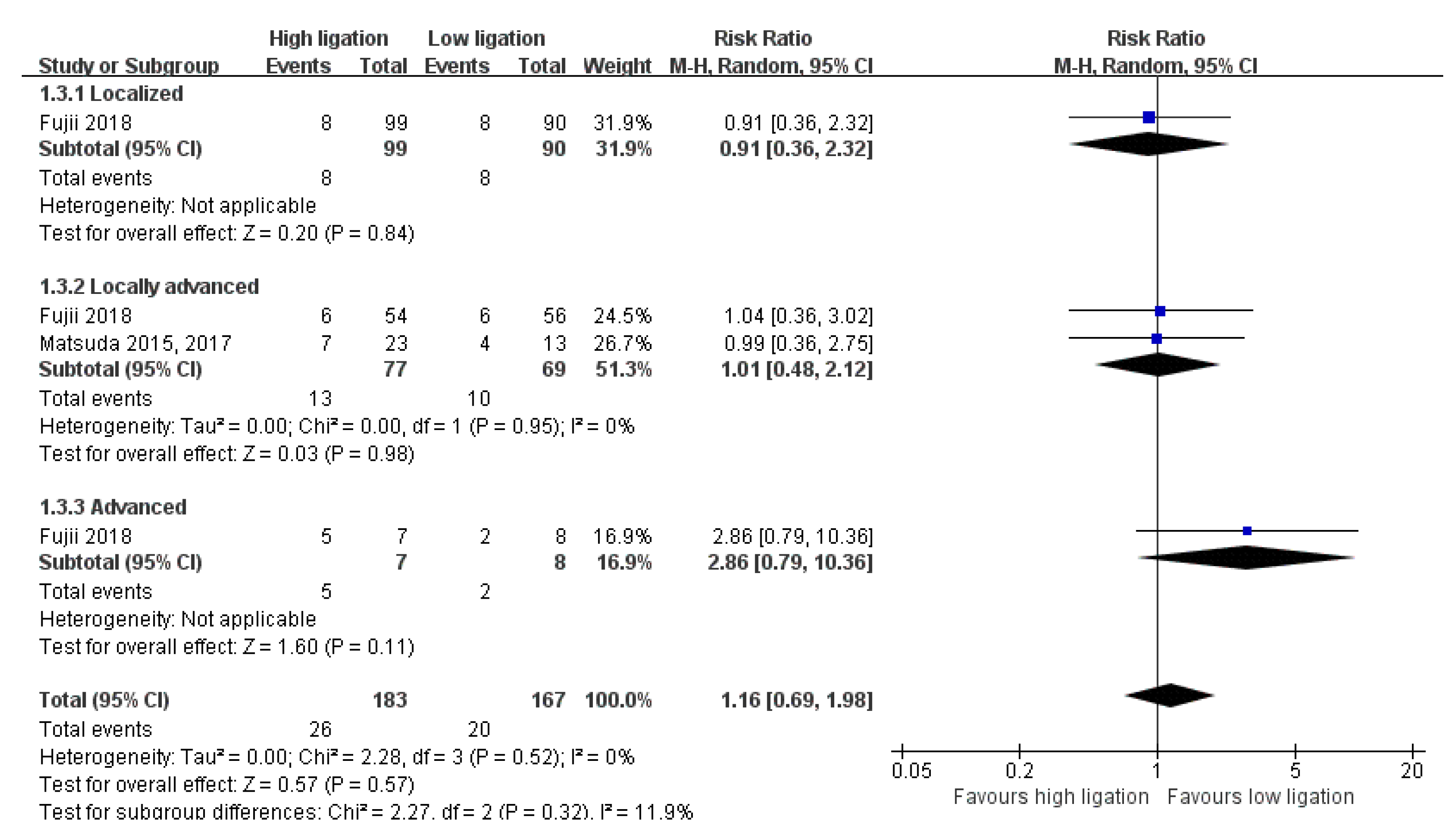
3.6. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Malietzis, G.; Askari, A.; Bernardo, D.; Al-Hassi, H.O.; Clark, S.K. Is right-sided colon cancer different to left-sided colorectal cancer?—A systematic review. Eur. J. Surg. Oncol. 2015, 41, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Su, G. High ligation of the inferior mesenteric artery during sigmoid colon and rectal cancer surgery increases the risk of anastomotic leakage: A meta-analysis. World J. Surg. Oncol. 2018, 16, 157. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, H.; Xin, Y.; Jiang, Y.; Han, Y.; Sheng, H. High ligation of the inferior mesenteric artery and anastomotic leakage in anterior resection for rectal cancer: A systematic review and meta-analysis of randomized controlled trial studies. Colorectal Dis. 2021, 23, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Alici, A.; Kement, M.; Gezen, C.; Akin, T.; Vural, S.; Okkabaz, N.; Basturk, E.; Yegenoglu, A.; Oncel, M. Apical lymph nodes at the root of the inferior mesenteric artery in distal colorectal cancer: An analysis of the risk of tumor involvement and the impact of high ligation on anastomotic integrity. Tech. Coloproctol. 2010, 14, 1–8. [Google Scholar] [CrossRef]
- Kessler, H.; Hohenberger, W. Extended lymphadenectomy in colon cancer is crucial. World J. Surg. 2013, 37, 1789–1798. [Google Scholar] [CrossRef]
- Corder, A.P.; Karanjia, N.D.; Williams, J.D.; Heald, R.J. Flush aortic tie versus selective preservation of the ascending left colic artery in low anterior resection for rectal carcinoma. Br. J. Surg. 1992, 79, 680–682. [Google Scholar] [CrossRef]
- Uehara, K.; Yamamoto, S.; Fujita, S.; Akasu, T.; Moriya, Y. Impact of upward lymph node dissection on survival rates in advanced lower rectal carcinoma. Dig. Surg. 2007, 24, 375–381. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Available online: www.training.cochrane.org/handbook (accessed on 10 March 2021).
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. Grade Handbook. Available online: https://gdt.gradepro.org/app/handbook/handbook.html#h.m9385o5z3li7 (accessed on 10 March 2022).
- Hajibandeh, S.; Hajibandeh, S.; Maw, A. Meta-analysis and trial sequential analysis of randomized controlled trials comparing high and low ligation of the inferior mesenteric artery in rectal cancer surgery. Dis. Colon Rectum 2020, 63, 988–999. [Google Scholar] [CrossRef] [PubMed]
- Kruszewski, W.J.; Szajewski, M.; Ciesielski, M.; Buczek, T.; Kawecki, K.; Walczak, J. Level of inferior mesenteric artery ligation does not affect rectal cancer treatment outcomes despite better cancer-specific survival after low ligation-randomized trial results. Colorectal Dis. 2021, 23, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Inomata, M.; Hara, T.; Mizusawa, J.; Katayama, H.; Shida, D.; Ohue, M.; Ito, M.; Kinugasa, Y.; Saida, Y.; et al. Clinical impact of d3 lymph node dissection with left colic artery (lca) preservation compared to d3 without lca preservation: Exploratory subgroup analysis of data from jcog0404. Ann. Gastroenterol. Surg. 2020, 4, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Komen, N.; Slieker, J.; de Kort, P.; de Wilt, J.H.; van der Harst, E.; Coene, P.P.; Gosselink, M.P.; Tetteroo, G.; de Graaf, E.; van Beek, T.; et al. High tie versus low tie in rectal surgery: Comparison of anastomotic perfusion. Int. J. Colorectal Dis. 2011, 26, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Samalavicius, N.E.; Dulskas, A.; Uselis, S.; Smolskas, E.; Smailyte, G.; Lunevicius, R. High vascular ligation in left-sided colon cancer surgery issafe and adequate. Eur. Surg. 2018, 50, 221–227. [Google Scholar] [CrossRef]
- Akagi, T.; Hara, T.; Inomata, M.; Mizusawa, J.; Katayama, H.; Shida, D.; Ohue, M.; Hamaguchi, T.; Ito, M.; Kinugasa, Y.; et al. Clinical impact of d3 lymph node dissection preserving left colic artery (lca) compared to d3 without preserving lca: Exploratory subgroup analysis of data from randomized controlled trial of laparoscopic versus open surgery for colon cancer from japan clinical oncology group study jcog0404. J. Clin. Oncol. 2019, 37, 653. [Google Scholar] [CrossRef]
- Planelles-Soler, P.; Mora-Lopez, L.; Hannaoui, N.; Serra-Pla, S.; Dominguez-Garcia, A.; Muñoz-Rodriguez, J.; Prats-Lopez, J.; Navarro-Soto, S.; Serra-Aracil, X. Prospecitve Controlled and Randomized Study of the Genitourinary Function after Rectal Cancer Surgery in Relation to the Dissection of the Inferior Mesenteric Vessels. Available online: https://clinicaltrials.gov/ct2/show/NCT03520088 (accessed on 1 October 2021).
- A Prospective Clinical Study for Laparoscopic d3 Dissection with Preservation of Left Colic Artery in Rectal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02753465 (accessed on 1 October 2021).
- Park, J.S. Anastomotic Leakage after Laparoscopic Anterior Resection for Rectal Cancer with High Versus Low Ligation of Inferior Mesenteric Artery: A Randomized Multicenter Trial. Available online: https://trialsearch.who.int/?TrialID=KCT0003523 (accessed on 1 October 2021).
- Niu, J.W.; Ning, W.; Wang, W.Y.; Pei, D.P.; Meng, F.Q.; Liu, Z.Z.; Cai, D.G. Clinical effect of preservation of the left colonic artery in laparoscopic anterior resection for rectal cancer. Zhonghua Yi Xue Za Zhi 2016, 96, 3582–3585. [Google Scholar] [CrossRef]
- Fiori, E.; Crocetti, D.; Lamazza, A.; De Felice, F.; Scotti, G.B.; Sterpetti, A.V.; Mingoli, A.; Sapienza, P.; De Toma, G. Defecatory dysfunction after colon cancer resection: The role of inferior mesenteric artery tie. Anticancer Res. 2020, 40, 2969–2974. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.; Zhang, H.; Wang, Y.; Yuan, Z.; Di, C. Effect of ligation level of inferior mesenteric artery on postoperative defecation function in patients with rectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2015, 18, 1132–1135. [Google Scholar]
- Feng, W.; Zong, Y.; Zhao, J.; Li, W.; Miao, Y.; Xu, Z.; Xu, Z.; Sun, J.; Zheng, M.; Lu, A. High versus low ligation of the inferior mesenteric artery during laparoscopic rectal cancer surgery: A prospective study of surgical and oncological outcomes. J. Surg. Oncol. 2021, 123 (Suppl. S1), S76–S80. [Google Scholar] [CrossRef]
- Fiori, E.; Crocetti, D.; Lamazza, A.; De Felice, F.; Sterpetti, A.V.; Irace, L.; Mingoli, A.; Sapienza, P.; De Toma, G. Is low inferior mesenteric artery ligation worthwhile to prevent urinary and sexual dysfunction after total mesorectal excision for rectal cancer? Anticancer Res. 2020, 40, 4223–4228. [Google Scholar] [CrossRef] [PubMed]
- Mari, G.M.; Crippa, J.; Cocozza, E.; Berselli, M.; Livraghi, L.; Carzaniga, P.; Valenti, F.; Roscio, F.; Ferrari, G.; Mazzola, M.; et al. Low ligation of inferior mesenteric artery in laparoscopic anterior resection for rectal cancer reduces genitourinary dysfunction: Results from a randomized controlled trial (highlow trial). Ann. Surg. 2019, 269, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, D.; He, L.; Zhang, Y.; Zhao, S.; Zhang, L.; Sun, X.; Suo, J. Marginal artery stump pressure in left colic artery-preserving rectal cancer surgery: A clinical trial. ANZ J. Surg. 2017, 87, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Yokoyama, S.; Hotta, T.; Takifuji, K.; Watanabe, T.; Tamura, K.; Mitani, Y.; Iwamoto, H.; Mizumoto, Y.; Yamaue, H. Oncological outcomes following rectal cancer surgery with high or low ligation of the inferior mesenteric artery. Gastrointest. Tumors 2017, 4, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Hotta, T.; Takifuji, K.; Yokoyama, S.; Oku, Y.; Watanabe, T.; Mitani, Y.; Ieda, J.; Mizumoto, Y.; Yamaue, H. Randomized clinical trial of defaecatory function after anterior resection for rectal cancer with high versus low ligation of the inferior mesenteric artery. Br. J. Surg. 2015, 102, 501–508. [Google Scholar] [CrossRef]
- Fujii, S.; Ishibe, A.; Ota, M.; Watanabe, K.; Watanabe, J.; Kunisaki, C.; Endo, I. Randomized clinical trial of high versus low inferior mesenteric artery ligation during anterior resection for rectal cancer. BJS Open 2018, 2, 195–202. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, S.; Huang, J.; Huang, P.; Peng, S.; Lin, J.; Li, T.; Wang, J.; Huang, M. Accurate low ligation of inferior mesenteric artery and root lymph node dissection according to different vascular typing in laparoscopic radical resection of rectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2018, 21, 46–52. [Google Scholar]
- Wu, Y.-J.; Li, M. Clinical research of laparoscopic low anterior resection with preservation of the left colonic artery. Zhonghua Wei Chang Wai Ke Za Zhi 2017, 20, 1313–1315. [Google Scholar]
- Rockwood, T.H.; Church, J.M.; Fleshman, J.W.; Kane, R.L.; Mavrantonis, C.; Thorson, A.G.; Wexner, S.D.; Bliss, D.; Lowry, A.C. Fecal incontinence quality of life scale: Quality of life instrument for patients with fecal incontinence. Dis. Colon Rectum 2000, 43, 9–16. [Google Scholar] [CrossRef]
- Rullier, E.; Zerbib, F.; Marrel, A.; Amouretti, M.; Lehur, P.A. Validation of the french version of the fecal incontinence quality-of-life (fiql) scale. Gastroenterol. Clin. Biol. 2004, 28, 562–568. [Google Scholar] [CrossRef]
- Altomare, D.F.; Rinaldi, M.; Giardiello, G.G.; Donelli, A.; Petrolino, M.; Villani, R.D.; Masin, A.; Melega, E.; Ratto, C.; Memeo, V. Italian translation and prospective validation of fecal incontinence quality of life (fiql) index. Chir. Ital. 2005, 57, 153–158. [Google Scholar] [PubMed]
- Shi, H.Y.; Lee, K.T.; Lee, H.H.; Uen, Y.H.; Na, H.L.; Chao, F.T.; Chiu, C.C. The minimal clinically important difference in the gastrointestinal quality-of-life index after cholecystectomy. Surg. Endosc. 2009, 23, 2708–2712. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Liong, M.L.; Lim, K.K.; Leong, W.S.; Yuen, K.H. The minimum clinically important difference of the international consultation on incontinence questionnaires (iciq-ui sf and iciq-lutsqol). Urology 2019, 133, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.J.; Williford, W.O.; Chang, Y.; Machi, M.; Jones, K.M.; Walker-Corkery, E.; Lepor, H. Benign prostatic hyperplasia specific health status measures in clinical research: How much change in the american urological association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J. Urol. 1995, 154, 1770–1774. [Google Scholar] [CrossRef]
- Spaliviero, M.; Strom, K.H.; Gu, X.; Araki, M.; Culkin, D.J.; Wong, C. Does greenlight hps() laser photoselective vaporization prostatectomy affect sexual function? J. Endourol. 2010, 24, 2051–2057. [Google Scholar] [CrossRef]
- Krychman, M.; Rowan, C.G.; Allan, B.B.; Durbin, S.; Yacoubian, A.; Wilkerson, D. Effect of single-session, cryogen-cooled monopolar radiofrequency therapy on sexual function in women with vaginal laxity: The viveve i trial. J. Womens Health 2018, 27, 297–304. [Google Scholar] [CrossRef]
- Peng, J.; Wu, H.; Li, X.; Sheng, W.; Huang, D.; Guan, Z.; Wang, M.; Cai, S. Prognostic significance of apical lymph node metastasis in patients with node-positive rectal cancer. Colorectal Dis. 2013, 15, e13–e20. [Google Scholar] [CrossRef]
- Newland, R.C.; Dent, O.F.; Lyttle, M.N.; Chapuis, P.H.; Bokey, E.L. Pathologic determinants of survival associated with colorectal cancer with lymph node metastases: A multivariate analysis of 579 patients. Cancer 1994, 73, 2076–2082. [Google Scholar] [CrossRef]
- Huh, J.W.; Kim, Y.J.; Kim, H.R. Distribution of lymph node metastases is an independent predictor of survival for sigmoid colon and rectal cancer. Ann. Surg. 2012, 255, 70–78. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese society for cancer of the colon and rectum (jsccr) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef]
- Jonnada, P.K.; Karunakaran, M.; Rao, D. Outcomes of level of ligation of inferior mesenteric artery in colorectal cancer: A systematic review and meta-analysis. Future Oncol. 2021, 17, 3645–3661. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, M.K.; Gamboa, A.C.; Regenbogen, S.E.; Holder-Murray, J.; Abdel-Misih, S.R.Z.; Hawkins, A.T.; Silviera, M.L.; Maithel, S.K.; Balch, G.C. A US Rectal Cancer Consortium Study of Inferior Mesenteric Artery Versus Superior Rectal Artery Ligation: How High Do We Need to Go? Dis. Colon Rectum. 2021, 64, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Han, S.R.; Lee, C.S.; Bae, J.H.; Lee, H.J.; Yoon, M.R.; Al-Sawat, A.; Lee, D.S.; Lee, I.K.; Lee, Y.S. Quantitative evaluation of colon perfusion after high versus low ligation in rectal surgery by indocyanine green: A pilot study. Surg. Endosc. 2022, 36, 3511–3519. [Google Scholar] [CrossRef]
- Reddy, S.H.; Gupta, V.; Yadav, T.D.; Singh, G.; Sahni, D. Lengthening of left colon after rectal resection: What all is adequate? A prospective cohort study. Int. J. Surg. 2016, 31, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Thum-umnuaysuk, S.; Boonyapibal, A.; Geng, Y.Y.; Pattana-Arun, J. Lengthening of the colon for low rectal anastomosis in a cadaveric study: How much can we gain? Tech. Coloproctol. 2013, 17, 377–381. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Hirai, T.; Komori, K.; Kato, T. Survival benefit of high ligation of the inferior mesenteric artery in sigmoid colon or rectal cancer surgery. Br. J. Surg. 2006, 93, 609–615. [Google Scholar] [CrossRef]
- Chew, M.H.; Yeh, Y.T.; Lim, E.; Seow-Choen, F. Pelvic autonomic nerve preservation in radical rectal cancer surgery: Changes in the past 3 decades. Gastroenterol. Rep. 2016, 4, 173–185. [Google Scholar] [CrossRef]
- Abdelli, A.; Tillou, X.; Alves, A.; Menahem, B. Genito-urinary sequelae after carcinological rectal resection: What to tell patients in 2017. J. Visc. Surg. 2017, 154, 93–104. [Google Scholar] [CrossRef]
- Yin, T.C.; Chen, Y.C.; Su, W.C.; Chen, P.J.; Chang, T.K.; Huang, C.W.; Tsai, H.L.; Wang, J.Y. Low Ligation Plus High Dissection Versus High Ligation of the Inferior Mesenteric Artery in Sigmoid Colon and Rectal Cancer Surgery: A Meta-Analysis. Front. Oncol. 2021, 11, 774–782. [Google Scholar] [CrossRef]
- Tryliskyy, Y.; Wong, C.S.; Demykhova, I.; Tyselskyi, V.; Kebkalo, A.; Poylin, V. Systematic review and meta-analysis of randomized controlled trials evaluating the effect of the level of ligation of inferior mesenteric artery on functional outcomes in rectal cancer surgery. Int. J. Colorectal Dis. 2022, 37, 709–718. [Google Scholar] [CrossRef]
- Thorlund, K.; Walter, S.D.; Johnston, B.C.; Furukawa, T.A.; Guyatt, G.H. Pooling health-related quality of life outcomes in meta-analysis-a tutorial and review of methods for enhancing interpretability. Res. Synth. Methods 2011, 2, 188–203. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, T.C.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2011, 372, n71. [Google Scholar] [CrossRef]





| (a) | |||||||||||||||||||||
| Study Name | Trial Period (Year to Year) | Study Design/Setting/Country | Description of Participants | Intervention and Comparator | Duration of Follow-Up (Months) | Total Number Analyzed | Age (Mean ± Standard Deviation) | ||||||||||||||
| Feng 2021 [25] | 2016 to 2018 | RCT/likely single-center/China | Patients 18–75 years old with histologically proven adenocarcinoma, a rectal lesion (distal margin 5–15 cm from the anus), clinical stage I–III disease (based on CT or MRI), and a Karnofsky score of ≥80 (unable to perform strenuous physical activity but ambulatory and able to perform light or sedentary work) | HL | 24 | 47 | 60.5 ± 10.2 | ||||||||||||||
| LL | 48 | 59.8 ± 8.9 | |||||||||||||||||||
| Fiori 2020 [23] | 2013 to 2018 | RCT/single-center/Italy | Patients with stage II, stage III, M0, and sigmoidal cancer treated by laparoscopic surgery | HL | 60 | 32 | 67.0 ± 9.0 | ||||||||||||||
| LL | 24 | 68.0 ± 10.0 | |||||||||||||||||||
| Fiori 2020a [26] | 2013 to 2019 | RCT/single-center/Italy | Patients treated with curative laparoscopic resection for pT2N0M0, rectal adenocarcinoma, and laparoscopic TME | HL | 60 | 22 | 68.0 ± 9.0 | ||||||||||||||
| LL | 24 | 68.0 ± 11.0 | |||||||||||||||||||
| Fujii 2018 [31] | 2006 to 2012 | RCT/single-center/Japan | Patients aged 20 years or above, with histologically proven adenocarcinoma of the rectum. | HL | 60 | 164 | 65.9 ± 10.4 | ||||||||||||||
| LL | 160 | 65.6 ± 11.5 | |||||||||||||||||||
| Guo 2017 [28] | 2013 to 2013 | RCT/single-center/China | Patients with only solitary radical resectable rectal cancers 3–20 cm from the anus as their first malignant neoplasm | HL | NR | 29 | NR | ||||||||||||||
| LL | 28 | NR | |||||||||||||||||||
| Kruszewski 2021 [14] | 2010 to 2016 | RCT/single-center/Poland | Patients who underwent radical surgery due to rectal or rectosigmoid adenocarcinoma | HL | More than 60 months | 65 | 64.0 ± 9.0 | ||||||||||||||
| LL | 65 | 65.0 ± 8.5 | |||||||||||||||||||
| Mari 2019 [27] | 2014 to 2016 | RCT/multi-center/Italy | Patient 18 years of age or older, BMI < 30, ASA I, II, III, Elective laparoscopic LAR + TME, and no evidence of metastatic disease | HL | 12 | 101 | 67.0 (34.0–87.0) a,b | ||||||||||||||
| LL | 95 | 68.0 (35.0–86.0) a,b | |||||||||||||||||||
| Matsuda 2015 [30] c | 2008 to 2011 | RCT/single-center/Japan | Patients scheduled for anterior resection with reconstruction using the double-stapling technique for rectal cancer | HL | 12 | 51 | 69.0 (45.0–85.0) d | ||||||||||||||
| LL | 49 | 67.0 (45.0–89.0) d | |||||||||||||||||||
| Matsuda 2017 [29] c | 2008 to 2011 | RCT/single-center/Japan | Patients with curable rectal cancer located <15 cm from the anus and patients with end-to-end anastomosis reconstructed by the double-stapling technique | HL | 36 | 51 | 69.0 d | ||||||||||||||
| LL | 49 | 67.0 d | |||||||||||||||||||
| Niu 2016 [22] | 2009 to 2015 | RCT/single-center/China | All patients with rectal cancer confirmed by preoperative colonoscopic pathology | HL | NR | 45 | 49.9 ± 8.2 | ||||||||||||||
| LL | 54 | 51.3 ± 6.3 | |||||||||||||||||||
| Wang 2015 [24] | 2013 to 2013 | RCT/single-center/China | Patients with rectal cancer undergoing low anterior resection, R0 resection, and end-to-end double anastomosis | HL | 12 | 63 | 56.8 ± 14.2 | ||||||||||||||
| LL | 65 | 58.6 ± 13.7 | |||||||||||||||||||
| Wu 2017 [33] | 2014 to 2016 | RCT/single-center/China | Patients with low rectal cancer without invasion or adhesion to other organs or structures; patients under the age of 70 years and able to tolerate laparoscopic surgery; patients without severe cardiopulmonary disease, renal dysfunction, dyshepatia, or metabolic disorders; without metastasis; without intestinal obstruction, perforation, or gastroenteritis; and with no history of radiotherapy or chemotherapy | HL | NR | 50 | 58.4 ± 9.3 | ||||||||||||||
| LL | 46 | 59.1 ± 9.1 | |||||||||||||||||||
| Zhou 2018 [32] | 2015 to 2016 | RCT/single-center/China | Patients with rectal cancer who were confirmed to have complete resection of the primary tumor and no distant metastasis, 2 to 15 cm from the anus, after preoperative examination; patients aged 18 to 75 years old who could undergo laparoscopic surgery and who had no obvious contraindications to surgery | HL | 1 | 52 | 52.7 ± 12.9 | ||||||||||||||
| LL | 52 | 53.9 ± 13.5 | |||||||||||||||||||
| (b) | |||||||||||||||||||||
| Study | Procedure | Tumor Location | Stage | Neoadjuvant CRT | Adjuvant CTx | Protective Stoma | ALND for LL | ||||||||||||||
| 0/I | II | III | IV | HL | LL | HL | LL | HL | LL | ||||||||||||
| HL | LL | HL | LL | HL | LL | HL | LL | ||||||||||||||
| Feng 2021 [25] | Laparoscopic LAR | Rectum | 21 a | 25 a | 12 a | 13 a | 14 a | 10 a | Excluded | Excluded | NR | NR | Yes | ||||||||
| Fiori 2020 [23] | Laparoscopic anterior rectosigmoid resection | Sigmoid | Excluded | 10 a | 8 a | 22 a | 16 a | Excluded | NR | NR | NR | NR | |||||||||
| Fiori 2020a [26] | Laparoscopic AR | Rectum | 22 b | 24 b | Excluded | Excluded | Excluded | Excluded | NR | NR | NR | ||||||||||
| Fujii 2018 [31] | Laparoscopic or open AR | Rectum | 60 b | 60 b | 43 b | 36 b | 54 b | 56 b | 7 b | 8 b | Excluded | 39 b | 46 b | 36 b | 47 b | Yes | |||||
| Guo 2017 [28] | Laparoscopic resection | Rectum | NR | Excluded c | NR | 10 b | 10 b | Yes | |||||||||||||
| Kruszewski 2021 [14] | Laparoscopic or open, AR or HP or APR | Rectum or rectosigmoid | 32 a | 23 a | 14 a | 18 a | 19 a | 24 a | Excluded | 42 a | 43 a | 25 b | 27 b | 3 b | 2 b | No | |||||
| Mari 2019 [27] d | Laparoscopic LAR | Rectum | 44 e | 60 e | 25 a | 21 a | 39 e | 19 e | 3 a | 3 a | 30 a | 25 a | 56 a | 42 a | NR | Yes | |||||
| Matsuda 2015 [30] f | Laparoscopic or open AR | Rectum | 9 e | 17 e | 15 e | 17 e | 23 e | 13 e | 4 e | 2 e | 2 b | 5 b | NR | 20 b | 19 b | NR | |||||
| Matsuda 2017 [29] f | Laparoscopic or open AR | Rectum | 9 e | 17 e | 15 e | 17 e | 23 e | 13 e | 4 e | 2 e | 2 a | 5 a | 29 a | 20 a | NR | NR | |||||
| Niu 2016 [22] | Laparoscopic AR | Rectum | 14 a | 19 a | 22 a | 25 a | 9 a | 8 a | NR | Excluded | NR | 4 e | 0 e | Yes | |||||||
| Wang 2015 [24] | Laparoscopic or open LAR | Rectum | NR | Excluded c | Excluded | Excluded | Yes | ||||||||||||||
| Wu 2017 [33] | Laparoscopic resection | Rectum | 5 a | 4 a | 32 a | 29 a | 13 a | 13 a | Excluded | Excluded | NR | NR | NR | ||||||||
| Zhou 2018 [32] | Laparoscopic resection | Rectum | 2 a | 4 a | 27 a | 23 a | 23 a | 25 a | NR | Likely excluded c | NR | 13 a | 17 a | NR | |||||||
| Patient or population: Colorectal cancer surgery Setting: Randomized controlled trials Intervention: High ligation Comparison: Low ligation | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Number of Participants (Studies) | Certainty of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects | What Happened? | |
| Risk with Low Ligation | Risk Difference with High Ligation | |||||
| Overall mortality Follow-up: range 2 years to 5 years MCID: 2% absolute difference | 649 (4 RCTs) | ⨁◯◯◯ VERY LOW a,b | HR: 1.24 (0.85 to 1.83) | 146 per 1000 | 32 more per 1000 (20 fewer to 105 more) | We are very uncertain about the effects of HL on improving overall mortality |
| Postoperative complications Follow-up: 30 days MCID: 5% absolute difference | 1293 (10 RCTs) | ⨁⨁◯◯ LOW a,c,d | RR: 1.15 (0.87 to 1.52) | 280 per 1000 | 42 more per 1000 (36 fewer to 146 more) | There may be little to no difference in postoperative complications between HL and LL |
| Disease recurrence Follow-up: range 1 year to 5 years MCID: 2% absolute difference | 862 (6 RCTs) | ⨁◯◯◯ VERY LOW a,b | HR: 1.17 (0.83 to 1.63) | 146 per 1000 | 23 more per 1000 (23 fewer to 81 more) | We are very uncertain about the effects of HL on improving disease recurrence |
| Cancer-specific mortality Follow-up: 5 years MCID: 2% absolute difference | 118 (1 RCT) | ⨁◯◯◯ VERY LOW a,f | HR: 3.03 (1.18 to 7.77) | 102 per 1000 | 176 more per 1000 (17 more to 464 more) | We are very uncertain about the effects of HL on improving cancer-specific mortality |
| Postoperative mortality Follow-up: 30 days MCID: 2% absolute difference | 1051 (8 RCTs) | ⨁◯◯◯ VERY LOW a,f | RR: 0.33 (0.03 to 3.14) | 4 per 1000 | 3 fewer per 1000 (4 fewer to 8 more) | We are very uncertain about the effects of HL on improving postoperative mortality |
| Anastomotic leakage Follow-up: 30 days MCID: 5% absolute difference | 1429 (12 RCTs) | ⨁◯◯◯ VERY LOW a,e,g | RR: 1.32 (0.92 to 1.88) | 65 per 1000 | 21 more per 1000 (5 fewer to 57 more) | We are very uncertain about the effects of HL on improving anastomotic leakage |
| The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MCID: minimal clinically important difference; RCT: randomized controlled trial; HR: hazard ratio; HL: high ligation; LL: low ligation; RR: risk ratio | ||||||
| GRADE working group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of the effect | ||||||
| Patient or population: Patients who underwent colorectal cancer surgery Setting: Randomized controlled trials Intervention: High ligation Comparison: Low ligation | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Number of Participants (Studies) | Certainty of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects | What Happened? | |
| Risk with Low Ligation | Risk Difference with High Ligation | |||||
| Defecatory dysfunction (incontinence) assessed with the JWIS Scale from 0 (best) to 20 (worst) Follow-up: range of 3 to 6 months MCID: 1 points a | 307 (4 RCTs) | ⨁⨁◯◯ LOW b,c | - | JWIS ranged from 0.17 to 4.3 | MD: 0.42 higher (0.2 higher to 0.63 higher) | There may be little to no difference in defecatory dysfunction (incontinence) between HL and LL |
| Defecatory dysfunction (constipation) assessed with the AWCS Scale from 0 (best) to 30 (worst) Follow-up: 6 months MCID: 1.5 points a | 102 (2 RCTs) | ⨁⨁◯◯ LOW b,d | - | AWCS ranged from 6.0 to 6.2 | MD: 1.63 higher (0.85 higher to 2.42 higher) | HL may reduce defecatory function (constipation) |
| Defecatory dysfunction (overall quality of life) assessed with the GIQLI Scale from 0 (worst) to 144 (best) Follow-up: 1 month MCID: 6.5 points e | 196 (1 RCT) | ⨁⨁◯◯ LOW b,c | - | Mean GIQLI was 133.15 | MD: 1.13 lower (3.32 lower to 1.06 higher) | There may be little to no difference in defecatory dysfunction (overall quality of life) between HL and LL |
| Urinary dysfunction (incontinence) assessed with the ICIQ-UI Scale from 0 (best) to 21 (worst) Follow-up: range of 1 to 6 months MCID: 4 points f | 242 (2 RCTs) | ⨁⨁◯◯ LOW b,c | - | ICIQ ranged from 0.5 to 4.76 | MD: 1.44 higher (0.7 higher to 2.17 higher) | There may be little to no difference in urinary dysfunction (incontinence) between HL and LL |
| Urinary dysfunction (urinary symptom) assessed with the IPSS Scale from 0 (best) to 35 (worst) Follow-up: 1 month MCID: 3 points g | 196 (1 RCT) | ⨁⨁◯◯ LOW b,d | - | Mean IPSS was 20.12 | MD: 1.69 higher (0.27 lower to 3.65 higher) | There may be little to no difference in urinary dysfunction (urinary symptom) between HL and LL |
| Sexual dysfunction (male) assessed with the IIEF-5 Scale from 1 (worst) to 25 (best) Follow-up: range of 1 to 6 months MCID: 5 points h | 158 (2 RCTs) | ⨁⨁◯◯ LOW b,d | - | IIEF ranged from 13 to 16.41 | MD: 3.73 lower (5.46 lower to 2.01 lower) | There may be little to no difference in male sexual dysfunction between HL and LL |
| Sexual dysfunction (female) assessed with the FSFI Scale from 2 (worst) to 36 (best) Follow-up: 6 months MCID: 4.6 points i | 46 (1 RCT) | ⨁⨁◯◯ LOW b,d | Mean FSFI was 17 | MD: 5 lower (7.03 lower to 2.97 lower) | HL may reduce female sexual function compared with LL | |
| The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FIQL: Fecal Incontinence Quality of Life Scale; MCID: minimal clinically important difference; RCT: randomized controlled trial; MD: mean difference; HL: high ligation; LL: low ligation; JWIS: Jorge-Wexner Incontinence Score; AWCS: Agachan-Wexner Constipation Score; GIQLI: Gastrointestinal Quality of Life Index; ICIQ-UI: International Consultation on Incontinence Questionnaire—Urinary Incontinence; IPSS: International Prostate Symptom Score; IIEF-5: International Index of Erectile Function-5; FSFI: Female Sexual Function Index | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of the effect | ||||||
| Patient or population: Patients who underwent colorectal cancer surgery Setting: Randomized controlled trials Intervention: High ligation Comparison: Low ligation | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Number of Participants (Studies) | Certainty of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects | What Happened? | |
| Risk with Low Ligation | Risk Difference with High Ligation | |||||
| Defecatory dysfunction (incontinence) assessed with the JWIS Scale from 0 (best) to 20 (worst) Follow-up: 12 months MCID: 1 points a | 295 (4 RCTs) | ⨁⨁◯◯ LOW b,c | - | JWIS ranged from 0.10 to 3.8 | MD: 0.11 higher (0.25 lower to 0.47 higher) | There may be little to no difference in defecatory dysfunction (incontinence) between HL and LL |
| Defecatory dysfunction (constipation) assessed with the AWCS Scale from 0 (best) to 30 (worst) Follow-up: 12 months MCID: 1.5 points a | 102 (2 RCTs) | ⨁⨁◯◯ LOW b,d | - | Mean AWCS was 6 | MD: 1.61 higher (0.83 higher to 2.39 higher) | HL may reduce defecatory function (constipation) compared with LL |
| Defecatory dysfunction (overall quality of life) assessed with the GIQLI Scale from 0 (worst) to 144 (best) Follow-up: 9 months MCID: 6.5 points e | 196 (1 RCT) | ⨁⨁◯◯ LOW b,c | - | Mean GIQLI was 137.15 | MD: 4.3 lower (6.34 lower to 2.26 lower) | There may be little to no difference in defecatory dysfunction (overall quality of life) between HL and LL |
| Urinary dysfunction (incontinence) assessed with the ICIQ-UI Scale from 0 (best) to 21 (worst) Follow-up: range of 9 to 12 months MCID: 4 points f | 242 (2 RCTs) | ⨁⨁◯◯ LOW b,c | - | ICIQ-UI ranged from 0.6 to 4.34 | MD: 1.90 higher (0.82 higher to 2.99 higher) | There may be little to no difference in urinary dysfunction (incontinence) between HL and LL |
| Urinary dysfunction (urinary symptoms) assessed with the IPSS Scale from 0 (best) to 35 (worst) Follow-up: 9 months MCID: 3 points g | 196 (1 RCT) | ⨁⨁◯◯ LOW b,d | - | Mean IPSS was 18.82 | MD: 4.72 higher (2.43 higher to 7.01 higher) | HL may aggravate urinary symptoms compared with LL |
| Sexual dysfunction (male) assessed with the IIEF-5 Scale from 1 (worst) to 25 (best) Follow-up: 9 to 12 months MCID: 5 points h | 158 (2 RCTs) | ⨁⨁◯◯ LOW b,d | - | IIEF ranged from 13 to 17.76 | MD: 5.11 lower (6.85 lower to 3.37 lower) | HL may reduce male erectile function compared with LL |
| Sexual dysfunction (female) assessed with the FSFI Scale from 2 (worst) to 36 (best) Follow-up: 12 months MCID: 4.6 points i | 46 (1 RCT) | ⨁⨁◯◯ LOW b,d | - | Mean FSFI was 18 | MD: 5 lower (6.74 lower to 3.26 lower) | HL may reduce female sexual function compared with LL |
| The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FIQL: Fecal Incontinence Quality of Life Scale; MCID: minimal clinically important difference; RCT: randomized controlled trial; MD: mean difference; HL: high ligation; LL: low ligation; JWIS: Jorge-Wexner Incontinence Score; AWCS: Agachan-Wexner Constipation Score; GIQLI: Gastrointestinal Quality of Life Index; ICIQ-UI: International Consultation on Incontinence Questionnaire—Urinary Incontinence; IPSS: International Prostate Symptom Score; IIEF-5: International Index of Erectile Function-5; FSFI: Female Sexual Function Index | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of the effect | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; An, S.; Kim, M.H.; Jung, J.H.; Kim, Y. High Versus Low Ligation of the Inferior Mesenteric Artery in Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis. Medicina 2022, 58, 1143. https://doi.org/10.3390/medicina58091143
Kim K, An S, Kim MH, Jung JH, Kim Y. High Versus Low Ligation of the Inferior Mesenteric Artery in Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis. Medicina. 2022; 58(9):1143. https://doi.org/10.3390/medicina58091143
Chicago/Turabian StyleKim, Kwangmin, Sanghyun An, Myung Ha Kim, Jae Hung Jung, and Youngwan Kim. 2022. "High Versus Low Ligation of the Inferior Mesenteric Artery in Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis" Medicina 58, no. 9: 1143. https://doi.org/10.3390/medicina58091143
APA StyleKim, K., An, S., Kim, M. H., Jung, J. H., & Kim, Y. (2022). High Versus Low Ligation of the Inferior Mesenteric Artery in Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis. Medicina, 58(9), 1143. https://doi.org/10.3390/medicina58091143






