Dual Sensory Impairment: The Correlation between Age Related Macular Degeneration and Sensorineural Hearing Loss
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Quantification of Lesion Morphology
2.3. Audiological Screening
2.3.1. Pure Tone Audiometry
2.3.2. Type of Hearing Impairment
2.4. Statistical Analysis
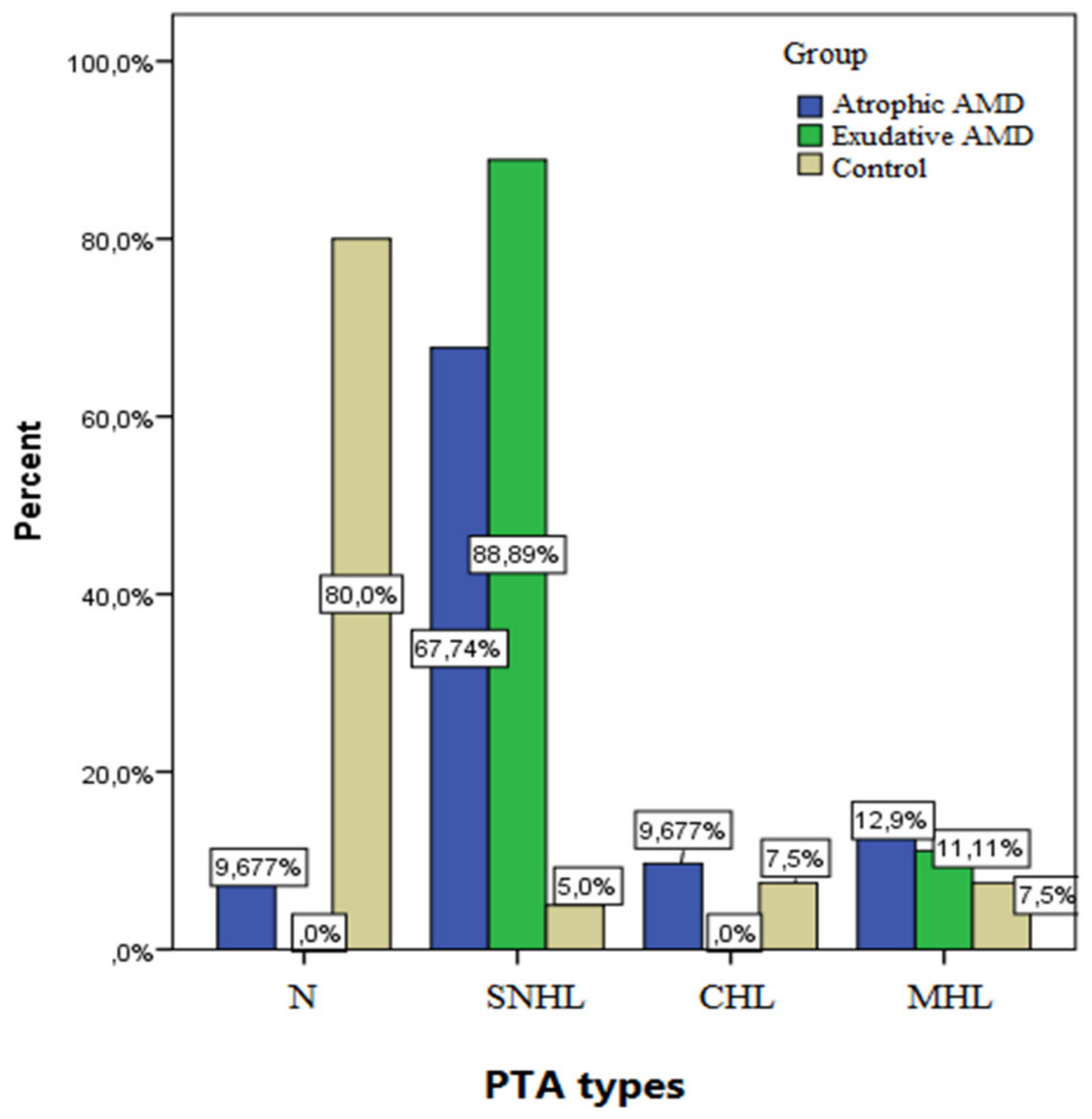
3. Results
4. Discussion
4.1. Synthesis of the Melanic Pigments
4.2. Melanin Functions
4.3. Role of Ocular Melanin
4.4. Distribution and Role of Inner Ear Melanin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chalam, K.V. (Ed.) Fundamentals and Principles of Ophthalmology: Section 2, 2011–2012; American Academy of Ophthalmology: San Francisco, CA, USA, 2011; pp. 303–308. [Google Scholar]
- Kansky, J.J.; Bowling, B. Clinical Ophthalmology—A Systemic Approach, 8th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2011; pp. 598–610. [Google Scholar]
- Maranduca, M.A.; Branisteanu, D.; Serban, D.N.; Branisteanu, D.C.; Stoleriu, G.; Manolache, N.; Serban, I.L. Synthesis and physiological implications of melanic pigments. Oncol. Lett. 2019, 17, 4183–4187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemi, H.; Pourakbari, M.S.; Entezari, M.; Yarmohammadi, M.E. Association of age related macular degeneration and age related hearing impairment. J. Ophthalmic Vis. Res. 2016, 11, 54–60. [Google Scholar]
- Bozkurt, M.K.; Ozturk, B.T.; Kerimoglu, H.; Ersan, I.; Arbag, H.; Bozkurt, B. Association of age-related macular degeneration with age-related hearing loss. J. Laryngol. Otol. 2011, 125, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanins: Skin Pigments and Much More-Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef] [Green Version]
- Rzepka, Z.; Buszman, E.; Beberok, A.; Wrześniok, D. From tyrosine to melanin: Signaling pathways and factors regulating melanogenesis. Postepy Hig. Med. Dosw. 2016, 70, 695–708. [Google Scholar] [CrossRef]
- Hu, D.N.; Simon, J.D.; Sarna, T. Role of ocular melanin in ophtalmic physiology and pathology. Photochem. Photobiol. 2008, 84, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Sarna, T. Properties and function of the ocular melanin: A photobiophysical view. J. Photochem. Photobiol. 1992, 12, 215–258. [Google Scholar] [CrossRef]
- Meyer Zum Gottesberge, A.M. Physiology and pathophysiology of inner ear melanin. Pigment Cell Res. 1988, 1, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Yamazaki, K.; Kanzaki, J.; Hosoda, Y. Ultrastructure of melanocytes in the dark cell area of human vestibular organs: Functional implications of gap junctions, isolated cilia, and annulate lamellae. Anat. Rec. 1994, 240, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M. Cochlear melanocytes and MITF signaling. J. Investig. Dermatol. Symp. Proc. 2001, 6, 95–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Frequency, Hz | Air Conduction, dBHL | Bone Conduction, dBHL |
|---|---|---|
| 125 | 80 | - |
| 250 | 100 | 45 |
| 500 | 115 | 60 |
| 1000 | 120 | 70 |
| 2000 | 120 | 70 |
| 4000 | 115 | 70 |
| 8000 | 100 | 40 |
| Frequency (Hz) | Mean (dB) | SD | p |
|---|---|---|---|
| 125 Case | 19.48 | 15.64 | <0.001 |
| Control | 8.12 | 6.12 | |
| 250 Case | 20.2 | 15.3 | 0.001 |
| Control | 7.40 | 5.9 | |
| 1000 Case | 19.5 | 18.2 | 0.004 |
| Control | 14.8 | 14.1 | |
| 2000 Case | 24.2 | 22.2 | 0.017 |
| Control | 18.7 | 7.5 | |
| 4000 Case | 36.4 | 24.6 | <0.001 |
| Control | 26.8 | 20.3 | |
| 8000 Case | 48.2 | 26.2 | <0.001 |
| Control | 30.8 | 25.9 |
| Types of AMD | Frequency (Hz) | Mean | SD | p |
|---|---|---|---|---|
| Nonexudative | 125 | 17.37 | 12.9 | <0.001 |
| Exudative | 31.14 | 21.1 | ||
| Nonexudative | 250 | 12.94 | 11.8 | 0.004 |
| Exudative | 23.26 | 20.3 | ||
| Nonexudative | 1000 | 16.03 | 15.23 | <0.001 |
| Exudative | 29.84 | 19.4 | ||
| Nonexudative | 2000 | 20.31 | 19.89 | 0.013 |
| Exudative | 35.21 | 25.16 | ||
| Nonexudative | 4000 | 38.55 | 25.32 | 0.006 |
| Exudative | 49.23 | 27.44 | ||
| Nonexudative | 8000 | 21.45 | 14.92 | <0.001 |
| Exudative | 6.48 | 4.23 |
| Agiographic Pattern (n) | Frequency 125 | Frequency 250 | Frequency 1000 | Frequency 2000 | Frequency 4000 | Frequency 8000 |
|---|---|---|---|---|---|---|
| Drusen (2) | ||||||
| Mean | 17.45 | 12.23 | 18.13 | 24.13 | 39.16 | 48.72 |
| SD | 2.36 | 1.45 | 2.01 | 2.12 | 2.41 | 1.98 |
| Control (40) | ||||||
| Mean | 8.12 | 7.40 | 14.8 | 18.7 | 26.8 | 30.8 |
| SD | 6.12 | 5.9 | 14.1 | 17.5 | 20.3 | 25.9 |
| p | <0.001 | |||||
| GA (7) | ||||||
| Mean | 14.85 | 10.02 | 13.66 | 13.35 | 28.19 | 35.42 |
| SD | 1.89 | 7.55 | 7.82 | 1.14 | 2.13 | 1.15 |
| Control (40) | ||||||
| Mean | 8.12 | 7.40 | 14.8 | 18.7 | 26.8 | 30.8 |
| SD | 6.12 | 5.9 | 14.1 | 17.5 | 20.3 | 25.9 |
| p | <0.001 | |||||
| Occult CNV (9) | ||||||
| Mean | 15.68 | 15.78 | 23.16 | 14.65 | 29.31 | 36.43 |
| SD | 2.13 | 1.23 | 2.29 | 2.14 | 1.88 | 2.24 |
| Control (40) | ||||||
| Mean | 8.12 | 7.40 | 14.8 | 18.7 | 26.8 | 30.8 |
| SD | 6.12 | 5.9 | 14.1 | 17.5 | 20.3 | 25.9 |
| p | 0.02 | |||||
| Classic CNV (5) | ||||||
| Mean | 36.12 | 26.14 | 32.24 | 41.71 | 55.13 | 58.63 |
| SD | 2.16 | 2.12 | 1.86 | 2.01 | 3.12 | 2.19 |
| Control (40) | ||||||
| Mean | 8.12 | 7.40 | 14.8 | 18.7 | 26.8 | 30.8 |
| SD | 6.12 | 5.9 | 14.1 | 17.5 | 20.3 | 25.9 |
| p | 0.012 | |||||
| Serous PED (2) | ||||||
| Mean | 17.31 | 16.23 | 15.96 | 24.09 | 41.12 | 45.61 |
| SD | 1.23 | 1.34 | 1.89 | 2.21 | 2.36 | 2.15 |
| Control (40) | ||||||
| Mean | 8.12 | 7.40 | 14.8 | 18.7 | 26.8 | 30.8 |
| SD | 6.12 | 5.9 | 14.1 | 17.5 | 20.3 | 25.9 |
| p | 0.06 | |||||
| Fibrovascular PED (7) | ||||||
| Mean | 36.24 | 27.81 | 36.12 | 41.86 | 54.23 | 38.23 |
| SD | 2.46 | 1.87 | 2.13 | 2.11 | 2.53 | 3.12 |
| Control (40) | ||||||
| Mean | 8.12 | 7.40 | 14.8 | 18.7 | 26.8 | 30.8 |
| SD | 6.12 | 5.9 | 14.1 | 17.5 | 20.3 | 25.9 |
| p | <0.001 | |||||
| Drusenoid PED (1) | ||||||
| Mean | 16.23 | 14.16 | 25.66 | 34.12 | 29.98 | 32.66 |
| SD | 2.16 | 3.56 | 6.45 | 6.89 | 5.14 | 7.69 |
| Control (40) | ||||||
| Mean | 8.12 | 7.40 | 14.8 | 18.7 | 26.8 | 30.8 |
| SD | 6.12 | 5.9 | 14.1 | 17.5 | 20.3 | 25.9 |
| p | 0.035 | |||||
| Haemorrhagic PED (5) | ||||||
| Mean | 29.45 | 26.55 | 27.00 | 35.23 | 41.44 | 36.78 |
| SD | 1.22 | 1.14 | 2.16 | 1.17 | 1.11 | 2.19 |
| Control (40) | ||||||
| Mean | 8.12 | 7.40 | 14.8 | 18.7 | 26.8 | 30.8 |
| SD | 6.12 | 5.9 | 14.1 | 17.5 | 20.3 | 25.9 |
| p | <0.001 | |||||
| Scar (2) | ||||||
| Mean | 2.47 | 27.54 | 28.19 | 36.25 | 39.82 | 41.22 |
| SD | 1.36 | 2.14 | 1.16 | 2.18 | 2.13 | 2.27 |
| Control (40) | ||||||
| Mean | 8.12 | 7.40 | 14.8 | 18.7 | 26.8 | 30.8 |
| SD | 6.12 | 5.9 | 14.1 | 17.5 | 20.3 | 25.9 |
| p | <0.001 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Istrate, M.; Hasbei-Popa, M.; Iliescu, D.A.; Ghiță, A.C.; Vlaicu, B.; Ghiță, M.A. Dual Sensory Impairment: The Correlation between Age Related Macular Degeneration and Sensorineural Hearing Loss. Medicina 2022, 58, 291. https://doi.org/10.3390/medicina58020291
Istrate M, Hasbei-Popa M, Iliescu DA, Ghiță AC, Vlaicu B, Ghiță MA. Dual Sensory Impairment: The Correlation between Age Related Macular Degeneration and Sensorineural Hearing Loss. Medicina. 2022; 58(2):291. https://doi.org/10.3390/medicina58020291
Chicago/Turabian StyleIstrate, Marina, Mihai Hasbei-Popa, Daniela Adriana Iliescu, Ana Cristina Ghiță, Brigitha Vlaicu, and Mihai Aurelian Ghiță. 2022. "Dual Sensory Impairment: The Correlation between Age Related Macular Degeneration and Sensorineural Hearing Loss" Medicina 58, no. 2: 291. https://doi.org/10.3390/medicina58020291
APA StyleIstrate, M., Hasbei-Popa, M., Iliescu, D. A., Ghiță, A. C., Vlaicu, B., & Ghiță, M. A. (2022). Dual Sensory Impairment: The Correlation between Age Related Macular Degeneration and Sensorineural Hearing Loss. Medicina, 58(2), 291. https://doi.org/10.3390/medicina58020291





