Resolution of Papilledema Following Ventriculoperitoneal Shunt or Endoscopic Third Ventriculostomy for Obstructive Hydrocephalus: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hellwig, D.; Grotenhuis, J.A.; Tirakotai, W.; Riegel, T.; Schulte, D.M.; Bauer, B.L.; Bertalanffy, H. Endoscopic third ventriculostomy for obstructive hydrocephalus. Neurosurg. Rev. 2005, 28, 1–34. [Google Scholar] [CrossRef]
- Kulkarni, A.V.; Drake, J.M.; Kestle, J.R.W.; Mallucci, C.L.; Sgouros, S.; Constantini, S. Predicting who will benefit from endoscopic third ventriculostomy compared with shunt insertion in childhood hydrocephalus using the ETV Success Score. J. Neurosurg. Pediatr. 2010, 6, 310–315. [Google Scholar] [CrossRef]
- Ahmed, N.; Alam, S.; Taikul, K.M.; Islam, M.H.; Ahsan, M.A. Subependymal giant cell astrocytoma associated with hyperproteinorrhachia, presented with vp shunt malfunction: A rare clinical entity. J. Neurol. 2020, 10, 97–99. [Google Scholar] [CrossRef]
- Montemurro, N.; Indaimo, A.; Di Carlo, D.T.; Benedetto, N.; Perrini, P. Surgical treatment of long-standing overt ventriculomegaly in adults (LOVA): A comparative case series between ventriculoperitoneal shunt (VPS) and endoscopic third ventriculostomy (ETV). Int. J. Environ. Res. Public Health 2022, 19, 1926. [Google Scholar] [CrossRef]
- Corbett, J.J. Neuro-ophthalmologic complications of hydrocephalus and shunting procedures. Semin. Neurol. 1986, 6, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Lizana, J.; Reinoso, C.M.D.; Aliaga, N.; Marani, W.; Montemurro, N. Bilateral central retinal artery occlusion: An exceptional complication after frontal parasagittal meningioma resection. Surg. Neurol. Int. 2021, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Rigi, M.; Almarzouqi, S.J.; Morgan, M.L.; Lee, A.G. Papilledema: Epidemiology, etiology, and clinical management. Eye Brain 2015, 17, 47–57. [Google Scholar]
- Petrohelos, M.A.; Henderson, J.W. The ocular findings of intracranial tumor. A study of 358 cases. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1950, 55, 89–98. [Google Scholar] [PubMed]
- Sihota, R.; Tandon, R. Diseases of the optic nerve. In Parsons’ Diseases of the Eye, 23rd ed.; Elsevier: New Delhi, India, 2019; pp. 328–352. [Google Scholar]
- Mizrachi, I.B.B.; Trobe, J.D.; Gebarski, S.S.; Garton, H.J.L. Papilledema in the assessment of ventriculomegaly. J. Neuroophthalmol. 2006, 26, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.; Anand, D.; Dutta, T.K.; Kumar, V.R.R.; Kar, S.S. Retinal nerve fiber layer thickness analysis in cases of papilledema using optical coherence tomography—A case control study. Clin. Neurol. Neurosurg. 2015, 136, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Perrini, P.; Montemurro, N. Congenital absence of a cervical spine pedicle. Neurol India 2016, 64, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.J.; Kardon, R.H.; Lee, A.G.; Frisén, L.; Wall, M. Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography vs. clinical expert assessment using a clinical staging scale. Arch. Ophthalmol. 2010, 12, 705–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funnell, J.P.; Craven, C.L.; D’Antona, L.; Thompson, S.D.; Chari, A.; Thorne, L.; Watkins, L.D.; Toma, A.K. Intracranial pressure in patients with papilloedema. Acta Neurol. Scand. 2018, 138, 137–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebolleda, G.; Munoz-Negrete, F.J. Follow-up of mild papilledema in idiopathic intracranial hypertension with optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5197–5200. [Google Scholar] [CrossRef]
- Bowd, C.; Weinreb, R.N.; Williams, J.M.; Zangwill, L.M. The retinal nerve fiber layer thickness in ocular hypertensive, normal, and glaucomatous eyes with optical coherence tomography. Arch. Ophthalmol. 2000, 118, 22–26. [Google Scholar] [CrossRef]
- Nazir, S.; O’Brien, M.; Qureshi, N.H.; Slape, L.; Green, T.J.; Phillips, P.H. Sensitivity of papilledema as a sign of shunt failure in children. J. AAPOS 2009, 13, 63–66. [Google Scholar] [CrossRef]
- Montemurro, N.; Murrone, D.; Romanelli, B.; Ierardi, A. Postoperative Textiloma Mimicking Intracranial Rebleeding in a Patient with Spontaneous Hemorrhage: Case Report and Review of the Literature. Case Rep. Neurol. 2020, 12, 7–12. [Google Scholar] [CrossRef]
- El-Ghandour, N.M.F. Endoscopic third ventriculostomy versus ventriculoperitoneal shunt in the treatment of obstructive hydrocephalus due to posterior fossa in children. Childs Nerv. Syst. 2011, 27, 117–126. [Google Scholar] [CrossRef]
- Nishiyama, K.; Mori, H.; Tanaka, R. Changes in cerebrospinal fluid hydrodynamics following endoscopic third ventriculostomy for shunt-dependent noncommunicating hydrocephalus. J. Neurosurg. 2003, 98, 1027–1031. [Google Scholar] [CrossRef]
- Schwartz, T.H.; Ho, B.; Prestigiacomo, C.J.; Bruce, J.N.; Feldstein, N.A.; Goodman, R.R. Ventricular volume following third ventriculostomy. J. Neurosurg. 1999, 91, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Podoleanu, A.G. Optical coherence tomography. J. Microsc. 2012, 247, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Chahlavi, A.; El-babaa, S.K.; Luciano, M.G. Adult-onset hydrocephalus. Neurosurg. Clin. N. Am. 2001, 12, 753–760. [Google Scholar] [CrossRef]
- Rizzo, J.L.; Lam, K.V.; Wall, M.; Wilson, M.D. Perimetry, Retinal Nerve Fiber Layer Thickness and Papilledema Grade After Cerebrospinal Fluid Shunting in Patients with Idiopathic Intracranial Hypertension. J. Neuroophthalmol. 2015, 35, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Koktekir, E.; Koktekir, B.; Hakan, K.; Gedik, S.; Akdemir, G. Resolution of papilledema after endoscopic third ventriculostomy versus cerebrospinal fluid shunting in hydrocephalus: A comparative study. J. Neurosurg. 2014, 120, 1465–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montemurro, N.; Santoro, G.; Marani, W.; Petrella, G. Posttraumatic synchronous double acute epidural hematomas: Two craniotomies, single skin incision. Surg. Neurol. Int. 2020, 11, 435. [Google Scholar] [CrossRef]
- Corsalini, M.; Di Venere, D.; Sportelli, P.; Magazzino, D.; Ripa, C.; Cantatore, F.; Cagnetta, G.; De Rinaldis, C.; Montemurro, N.; De Giacomo, A.; et al. Evaluation of prosthetic quality and masticatory efficiency in patients with total removable prosthesis: Study of 12 cases. Oral Implantol. 2018, 11, 230–240. [Google Scholar]
- Carruth, B.P.; Bersani, T.A.; Hurley, P.E.; Ko, M.W. Visual improvement after optic nerve sheath decompression in a case of congenital hydrocephalus and persistent visual loss despite intracranial pressure correction via shunting. Ophthalmic Plast. Reconstr. Surg. 2010, 26, 297–298. [Google Scholar] [CrossRef]
- Chou, S.Y.; Digre, K.B. Neuro-ophthalmic complications of raised intracranial pressure, hydrocephalus, and shunt malfunction. Neurosurg. Clin. N. Am. 1999, 10, 587–608. [Google Scholar] [CrossRef]
- Afonso, J.M.; Falcão, M.; Schlichtenbrede, F.; Falcão-Reis, F.; Silva, S.E.; Schneider, T.M. Spectral Domain-Optical Coherence Tomography as a New Diagnostic Marker for Idiopathic Normal Pressure Hydrocephalus. Front. Neurol. 2017, 1, 172. [Google Scholar] [CrossRef] [Green Version]
- Swanson, J.W.; Aleman, T.S.; Xu, W.; Ying, G.-S.; Pan, W.; Liu, G.T.; Lang, S.-S.; Heuer, G.G.; Storm, P.B.; Bartlett, S.P.; et al. Evaluation of Optical Coherence Tomography to Detect Elevated Intracranial Pressure in Children. JAMA Ophthalmol. 2017, 135, 320–328. [Google Scholar] [CrossRef]
- People, I.K.; Muhlbauer, M.S.; Sanford, R.A.; Kirk, E. Results and complications of intracranial pressure monitoring in 303 children. Pediatr. Neurosurg. 1995, 23, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Langfitt, T.W.; Weinstein, J.D.; Kassell, N.F.; Simeone, F.A. Transmission of Increased Intracranial Pressure. I. Within the Craniospinal Axis. J. Neurosurg. 1964, 21, 989–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockslager, M.A.; Samuels, B.C.; Allingham, R.R.; Klesmith, Z.A.; Schwaner, S.A.; Forest, C.R.; Ethier, C.R. System for Rapid, Precise Modulation of Intraocular Pressure, toward Minimally-Invasive In Vivo Measurement of Intracranial Pressure. PLoS ONE 2016, 15, e0147020. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, K.; Padungkiatsagul, T.; Moss, H.E. Optical coherence tomography use in idiopathic intracranial hypertension. Ann. Eye Sci. 2020, 5, 7. [Google Scholar] [CrossRef]
- Hoyt, W.F.; Pont, M.E. Pseudopapilledema: Anomalous elevation of optic disk. Pitafalls in diagnosis and management. JAMA 1962, 21, 191–196. [Google Scholar] [CrossRef]
- Montemurro, N.; Condino, S.; Cattari, N.; D’Amato, R.; Ferrari, V.; Cutolo, F. Augmented Reality-Assisted Craniotomy for Parasagittal and Convexity En Plaque Meningiomas and Custom-Made Cranio-Plasty: A Preliminary Laboratory Report. Int. J. Environ. Res. Public Health 2021, 18, 9955. [Google Scholar] [CrossRef]
- Mishra, R.; Narayanan, K.; Umana, G.E.; Montemurro, N.; Chaurasia, B.; Deora, H. Virtual Reality in Neurosurgery: Beyond Neurosurgical Planning. Int. J. Environ. Res. Public Health 2022, 19, 1719. [Google Scholar] [CrossRef]
- Montemurro, N.; Scerrati, A.; Ricciardi, L.; Trevisi, G. The Exoscope in Neurosurgery: An Overview of the Current Literature of Intraoperative Use in Brain and Spine Surgery. J. Clin. Med. 2022, 11, 223. [Google Scholar] [CrossRef]
- Raabe, C.; Fichtner, J.; Beck, J.; Gralla, J.; Raabe, A. Revisiting the rules for freehand ventriculostomy: A virtual reality analysis. J. Neurosurg. 2018, 128, 1250–1257. [Google Scholar] [CrossRef]
- Finger, T.; Schaumann, A.; Schulz, M.; Thomale, U.W. Augmented reality in intraventricular neuroendoscopy. Acta Neurochir. 2017, 159, 1033–1041. [Google Scholar] [CrossRef]
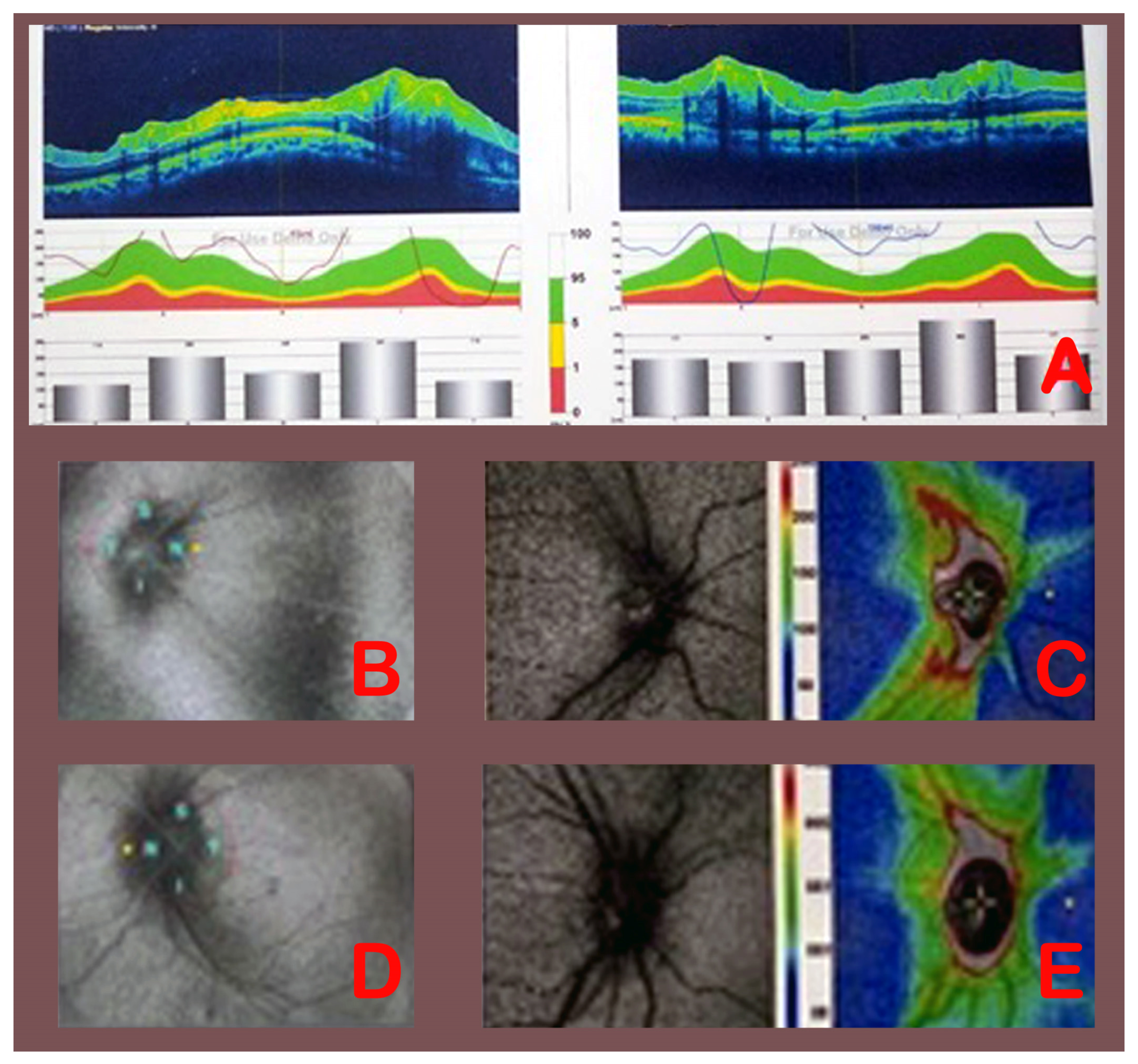

| Grade 1 | Grayish C-shaped halo surrounding the disc; sparing of the temporal disc margin; radial nerve fiber striation disruption. |
| Grade 2 | Halo becomes circumferential; nasal border elevation; no major vessel obscuration. |
| Grade 3 | Obscuration of at least one vessel leaving the disc; elevation of all borders; circumferential halo. |
| Grade 4 | Obscuration of a major vessel on the disc; complete elevation including the cup; circumferential halo. |
| Grade 5 | Obscuration of all vessels on the disc and leaving the disc; all features of Grade 4. |
| Categories | Sub-Categories | Number (%) | |
|---|---|---|---|
| Age | Years | Group A (VPS) | Group B (ETV) |
| 18–24 | 3 (33.3%) | 3 (33.3%) | |
| 25–35 | 3 (33.3%) | 3 (33.3%) | |
| 36–50 | 3 (33.3%) | 3 (33.3%) | |
| Sex | Male | 6 (66.7%) | 7 (77.8%) |
| Female | 3 (33.3%) | 2 (22.2%) | |
| Etiology of obstructive hydrocephalus | CPA tumor | 2 (22.2%) | 2 (22.2%) |
| Posterior fossa tumor | 3 (33.3%) | 1 (11.1%) | |
| Aqueductal stenosis | 2 (22.2%) | 4 (44.4%) | |
| Pineal tumor | 2 (22.2%) | 2 (22.2%) | |
| Clinical presentation | Papilledema | 9 (100%) | 9 (100%) |
| Headache | 9 (100%) | 9 (100%) | |
| Vomiting | 6 (66.7%) | 5 (27.7%) | |
| Gait disturbance | 4 (44.4%) | 2 (22.2%) | |
| Hearing loss | 4 (44.4%) | 2 (22.2%) | |
| Case No. | Surgical Treatment | Preop Frisen Rt | Preop RNFL Rt (µm) | Postop Frisen Rt | Postop RNFL Rt (µm) | Preop Frisen Lt | Preop RNFL Lt (µm) | Postop Frisen Lt | Postop RNFL Lt (µm) | RNFL Change Rt (µm) | RNFL Change Lt (µm) | Frisen Change Rt | Frisen Change Lt |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | VPS | Grade 5 | 300 | Grade 3 | 197 | Grade 5 | 300 | Grade 2 | 176 | 103 | 124 | 2 | 3 |
| 2. | VPS | Grade 3 | 151 | Grade 1 | 105 | Grade 1 | 124 | Grade 1 | 107 | 46 | 17 | 2 | 0 |
| 3. | VPS | Grade 1 | 121 | Grade 1 | 109 | Grade 4 | 239 | Grade 1 | 97 | 12 | 142 | 0 | 3 |
| 4. | VPS | Grade 3 | 180 | Grade 2 | 171 | Grade 3 | 199 | Grade 3 | 165 | 9 | 34 | 1 | 0 |
| 5. | VPS | Grade 1 | 99 | Grade 1 | 96 | Grade 3 | 166 | Grade 1 | 116 | 3 | 50 | 0 | 2 |
| 6. | VPS | Grade 1 | 116 | Grade 1 | 102 | Grade 3 | 176 | Grade 1 | 106 | 14 | 70 | 0 | 2 |
| 7. | VPS | Grade 3 | 175 | Grade 2 | 154 | Grade 4 | 212 | Grade 1 | 125 | 21 | 87 | 1 | 3 |
| 8. | VPS | Grade 3 | 165 | Grade 1 | 119 | Grade 2 | 165 | Grade 1 | 119 | 46 | 46 | 2 | 1 |
| 9. | VPS | Grade 2 | 132 | Grade 1 | 103 | Grade 1 | 120 | Grade 1 | 107 | 29 | 13 | 1 | 0 |
| 10. | ETV | Grade 2 | 107 | Grade 1 | 90 | Grade 2 | 103 | Grade 1 | 64 | 17 | 39 | 1 | 1 |
| 11. | ETV | Grade 4 | 184 | Grade 2 | 136 | Grade 3 | 165 | Grade 2 | 130 | 48 | 35 | 2 | 1 |
| 12. | ETV | Grade 3 | 175 | Grade 2 | 144 | Grade 4 | 212 | Grade 1 | 115 | 31 | 97 | 1 | 3 |
| 13. | ETV | Grade 3 | 176 | Grade 1 | 102 | Grade 2 | 116 | Grade 1 | 92 | 74 | 24 | 2 | 1 |
| 14. | ETV | Grade 3 | 175 | Grade 1 | 104 | Grade 4 | 215 | Grade 1 | 102 | 71 | 113 | 1 | 3 |
| 15. | ETV | Grade 3 | 175 | Grade 1 | 105 | Grade 4 | 212 | Grade 1 | 107 | 70 | 105 | 2 | 3 |
| 16. | ETV | Grade 2 | 149 | Grade 1 | 103 | Grade 2 | 143 | Grade 1 | 107 | 46 | 36 | 1 | 1 |
| 17. | ETV | Grade 3 | 178 | Grade 2 | 148 | Grade 3 | 172 | Grade 1 | 112 | 30 | 60 | 1 | 2 |
| 18. | ETV | Grade 1 | 107 | Grade 1 | 63 | Grade 1 | 112 | Grade 1 | 68 | 44 | 44 | 0 | 0 |
| Surgical Treatment | Eye | RNFL Thickness Mean ± SD (Micrometers) | p Value | |
|---|---|---|---|---|
| Pre-Operative | Post-Operative | |||
| VPS | Right | 159.8 ± 59.4 | 128.4 ± 36.3 | 0.016 |
| VPS | Left | 189.0 ± 56.6 | 124.2 ± 27.6 | 0.003 |
| ETV | Right | 158.4 ± 30.7 | 110.5 ± 27.5 | <0.001 |
| ETV | Left | 161.1 ± 45.2 | 99.6 ± 21.6 | 0.001 |
| Eye | Pre-Operative Frisen Grading | Post-Operative Frisen Grading | Total | Spearman Rho | p Value | ||
|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | |||||
| Right | Grade 1 | 3 | 0 | 0 | 3 | 0.721 | 0.028 |
| Grade 2 | 1 | 0 | 0 | 1 | |||
| Grade 3 | 2 | 2 | 0 | 4 | |||
| Grade 5 | 0 | 0 | 1 | 1 | |||
| Left | Grade 1 | 2 | 0 | 0 | 2 | ||
| Grade 2 | 1 | 0 | 0 | 1 | |||
| Grade 3 | 2 | 0 | 1 | 3 | 0.375 | 0.333 | |
| Grade 4 | 1 | 1 | 0 | 2 | |||
| Grade 5 | 0 | 0 | 1 | 1 | |||
| Eye | Pre-Operative Frisen Grading | Post-Operative Frisen Grading | Total | Spearman Rho | p Value | ||
|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | |||||
| Right | Grade 1 | 1 | 0 | 0 | 1 | 0.6 | 0.13 |
| Grade 2 | 2 | 0 | 0 | 2 | |||
| Grade 3 | 2 | 2 | 1 | 5 | |||
| Grade 4 | 0 | 0 | 1 | 1 | |||
| Left | Grade 1 | 1 | 0 | 0 | 1 | ||
| Grade 2 | 2 | 1 | 0 | 3 | 0.7 | 1.0 | |
| Grade 3 | 1 | 1 | 0 | 2 | |||
| Grade 4 | 1 | 1 | 1 | 3 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, S.; Montemurro, N.; Ashfaq, M.; Ghosh, D.; Sarker, A.C.; Khan, A.H.; Dey, S.; Chaurasia, B. Resolution of Papilledema Following Ventriculoperitoneal Shunt or Endoscopic Third Ventriculostomy for Obstructive Hydrocephalus: A Pilot Study. Medicina 2022, 58, 281. https://doi.org/10.3390/medicina58020281
Das S, Montemurro N, Ashfaq M, Ghosh D, Sarker AC, Khan AH, Dey S, Chaurasia B. Resolution of Papilledema Following Ventriculoperitoneal Shunt or Endoscopic Third Ventriculostomy for Obstructive Hydrocephalus: A Pilot Study. Medicina. 2022; 58(2):281. https://doi.org/10.3390/medicina58020281
Chicago/Turabian StyleDas, Sukriti, Nicola Montemurro, Musannah Ashfaq, Dipankar Ghosh, Asit Chandra Sarker, Akhlaque Hossain Khan, Sharbari Dey, and Bipin Chaurasia. 2022. "Resolution of Papilledema Following Ventriculoperitoneal Shunt or Endoscopic Third Ventriculostomy for Obstructive Hydrocephalus: A Pilot Study" Medicina 58, no. 2: 281. https://doi.org/10.3390/medicina58020281
APA StyleDas, S., Montemurro, N., Ashfaq, M., Ghosh, D., Sarker, A. C., Khan, A. H., Dey, S., & Chaurasia, B. (2022). Resolution of Papilledema Following Ventriculoperitoneal Shunt or Endoscopic Third Ventriculostomy for Obstructive Hydrocephalus: A Pilot Study. Medicina, 58(2), 281. https://doi.org/10.3390/medicina58020281







