Corticosteroid-Induced Liver Injury in Adult-Onset Still’s Disease
Abstract
1. Introduction
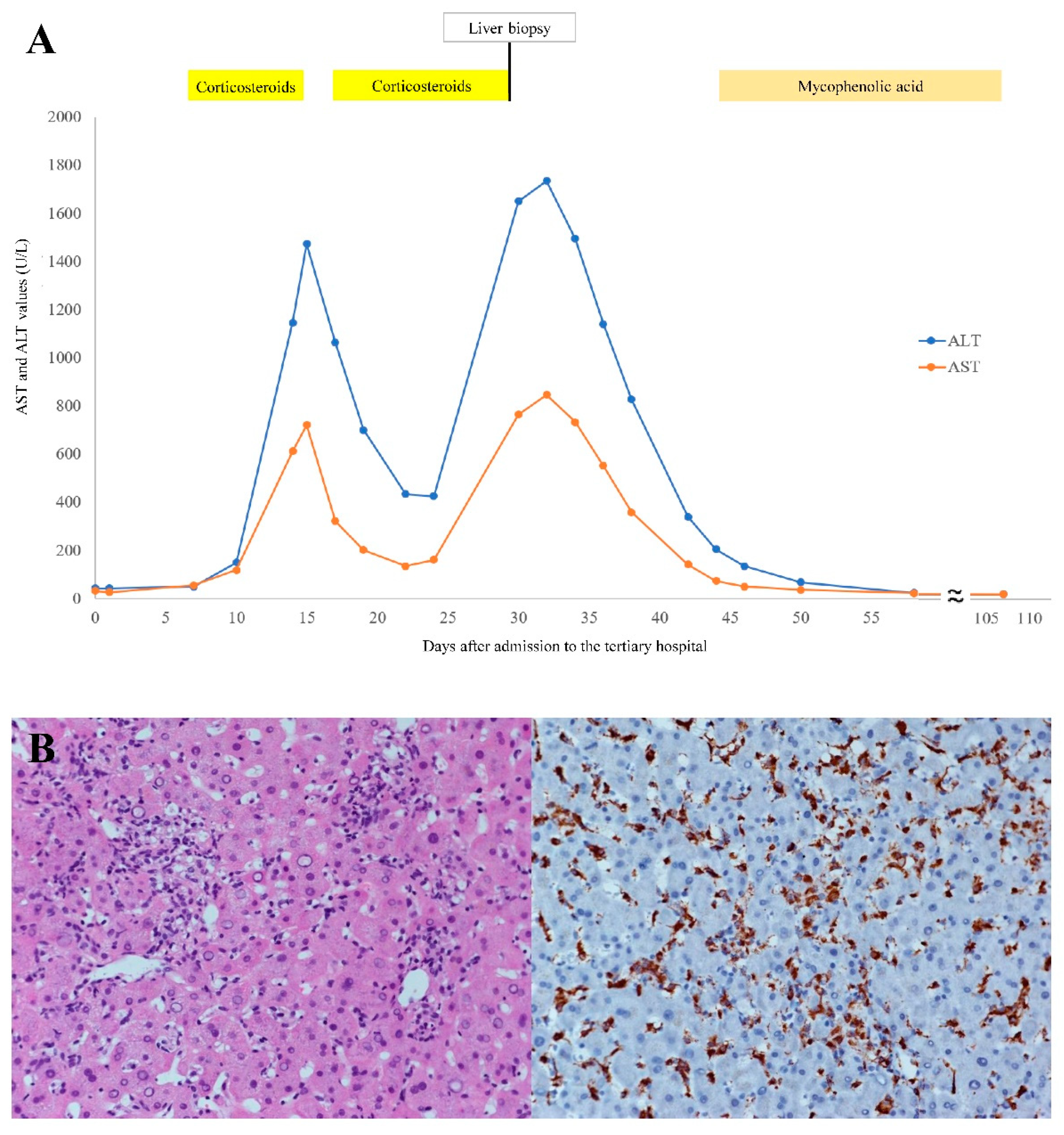
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamaguchi, M.; Ohta, A.; Tsunematsu, T.; Kasukawa, R.; Mizushima, Y.; Kashiwagi, H.; Kashiwazaki, S.; Tanimoto, K.; Matsumoto, Y.; Ota, T. Preliminary criteria for classification of adult Still’s disease. J. Rheumatol. 1992, 19, 424–430. [Google Scholar] [PubMed]
- Seve, P.; Jamilloux, Y.; Gerfaud-Valentin, M.; Henry, T. Treatment of adult-onset Still’s disease: A review. Ther. Clin. Risk Manag. 2014, 11, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Zoubek, M.E.; Bandera, P.; Ortega-Alonso, A.; Hernández, N.; Crespo, J.; Contreras, F.; Medina-Caliz, I.; Sanabria-Cabrera, J.; Sanjuan-Jiménez, R.; González-Jiménez, A.; et al. Liver injury after methylprednisolone pulses: A disputable cause of hepatotoxicity. A case series and literature review. United Eur. Gastroenterol. J. 2019, 7, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Emad, Y.; Ragab, Y.; EL Shaarawy, N. Lichen planus in association with adult-onset still’s disease successfully treated with mycophenolate mofetil. J. Rheumatol. 2012, 39, 1305–1306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, G.; Liu, G.; Liu, Y.; Xie, Q.; Shi, G. Liver abnormalities in adult onset Still’s disease: A retrospective study of 77 Chinese patients. J. Clin. Rheumatol. 2009, 15, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.; Gerbes, A.L. Acute liver injury in a patient with adult-onset Still’s disease—the challenge of differential diagnosis. Oxf. Med Case Rep. 2020, 2020, omaa102. [Google Scholar] [CrossRef]
- Aly, L.; Iking-Konert, C.; Quaas, A.; Benten, D. Subacute liver failure following anakinra treatment for adult-onset still disease. J. Rheumatol. 2013, 40, 1775–1777. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Drepper, M.; Rubbia-Brandt, L.; Spahr, L. Tocilizumab-Induced Acute Liver Injury in Adult Onset Still’s Disease. Case Rep. Hepatol. 2013, 2013, 964828. [Google Scholar] [CrossRef] [PubMed]
- Gutkowski, K.; Chwist, A.; Hartleb, M. Liver Injury Induced by High-Dose Methylprednisolone Therapy: A Case Report and Brief Review of the Literature. Hepat. Mon. 2011, 11, 656–661. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-C.; Peng, Y.-J.; Lu, C.-C.; Chen, H.-C.; Yeh, F.-C. Corticosteroid-Induced Liver Injury in Adult-Onset Still’s Disease. Medicina 2022, 58, 191. https://doi.org/10.3390/medicina58020191
Lee C-C, Peng Y-J, Lu C-C, Chen H-C, Yeh F-C. Corticosteroid-Induced Liver Injury in Adult-Onset Still’s Disease. Medicina. 2022; 58(2):191. https://doi.org/10.3390/medicina58020191
Chicago/Turabian StyleLee, Chin-Chi, Yi-Jen Peng, Chun-Chi Lu, Hsiang-Cheng Chen, and Fu-Chiang Yeh. 2022. "Corticosteroid-Induced Liver Injury in Adult-Onset Still’s Disease" Medicina 58, no. 2: 191. https://doi.org/10.3390/medicina58020191
APA StyleLee, C.-C., Peng, Y.-J., Lu, C.-C., Chen, H.-C., & Yeh, F.-C. (2022). Corticosteroid-Induced Liver Injury in Adult-Onset Still’s Disease. Medicina, 58(2), 191. https://doi.org/10.3390/medicina58020191





