Continuous Cold Flow Device Following Total Knee Arthroplasty: Myths and Reality
Abstract
1. Introduction
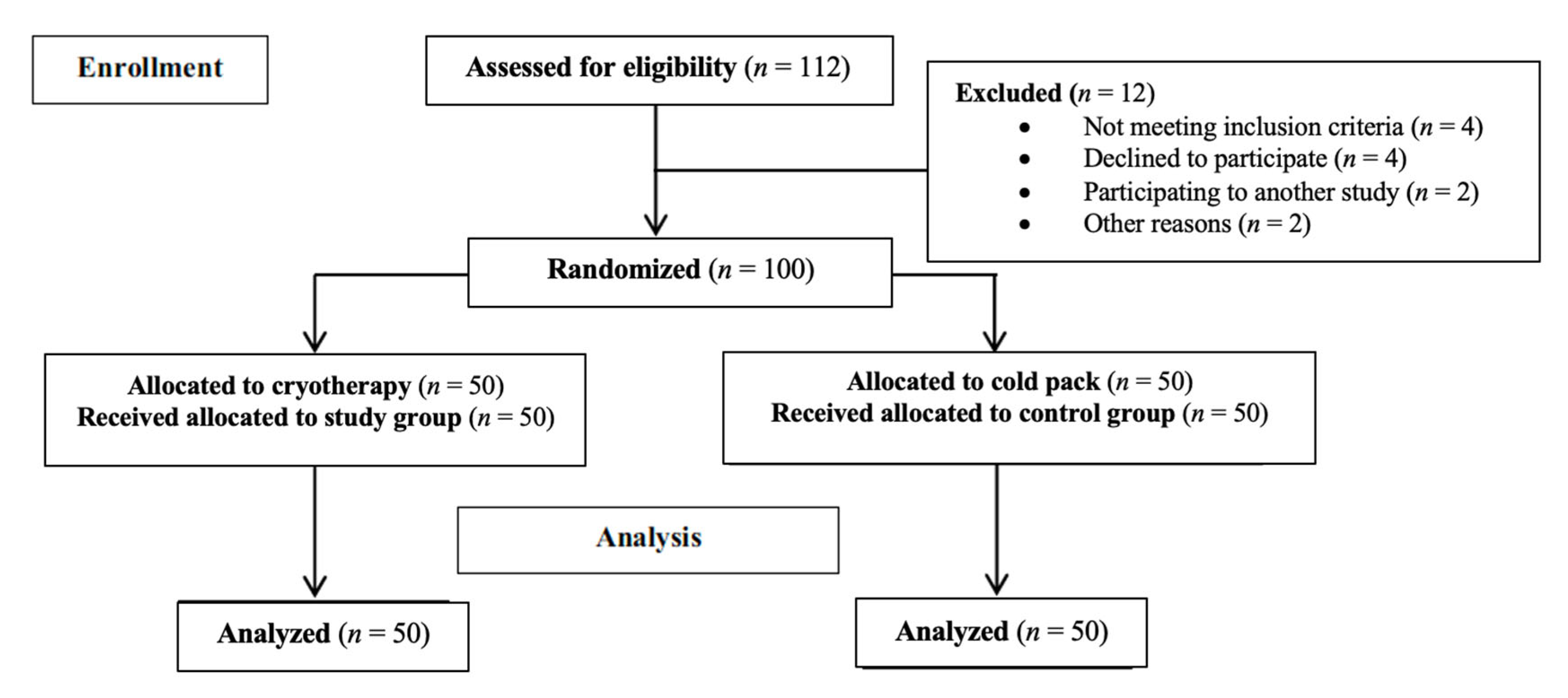
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chesterton, L.S.; Foster, N.E.; Ross, L. Skin temperature response to cryotherapy. Arch. Phys. Med. Rehabil. 2002, 83, 543–549. [Google Scholar] [CrossRef]
- Sehat, K.R.; Evans, R.L.; Newman, J.H. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J. Bone Joint Surg. Br. 2004, 86, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Adie, S.; Naylor, J.M.; Harris, I.A. Cryotherapy after Total Knee Arthroplasty. J. Arthroplast. 2010, 25, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Holm, B.; Husted, H.; Kehlet, H.; Bandholm, T. Effect of knee joint icing on knee extension strength and knee pain early after total knee arthroplasty: A randomized cross-over study. Clin. Rehabil. 2012, 26, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.B.; McCalden, R.W.; MacDonald, S.J.; Mokete, L.; Guerin, J. Influence of Patient Factors on TKA Outcomes at 5 to 11 Years Followup. Clin. Orthop. Relat. Res. 2007, 464, 27–31. [Google Scholar] [CrossRef]
- Barry, S.; Wallace, L.; Lamb, S. Cryotherapy after total knee replacement: A survey of current practice. Physiother. Res. Int. 2003, 8, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Ruffilli, A.; Castagnini, F.; Traina, F.; Corneti, I.; Fenga, D.; Giannini, S.; Faldini, C. Temperature-Controlled Continuous Cold Flow Device after Total Knee Arthroplasty: A Randomized Controlled Trial Study. J. Knee Surg. 2017, 30, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Trueblood, A.; Manning, D.W. Analgesia following total knee arthroplasty. Curr. Opin. Orthop. 2007, 18, 76–80. [Google Scholar] [CrossRef]
- White, P.F. The Changing Role of Non-Opioid Analgesic Techniques in the Management of Postoperative Pain. Anesth. Analg. 2005, 101, S5–S22. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Spindler, K.P.; Tarter, J.W.; Detwiler, K.B. Does Cryotherapy Affect Intraarticular Temperature after Knee Arthroscopy? Clin. Orthop. Relat. Res. 2002, 400, 184–189. [Google Scholar] [CrossRef]
- Algafly, A.A.; George, K.P.; Herrington, L. The effect of cryotherapy on nerve conduction velocity, pain threshold and pain tolerance * Commentary. Br. J. Sports Med. 2007, 41, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Kullenberg, B.; Ylipää, S.; Söderlund, K.; Resch, S. Postoperative Cryotherapy After Total Knee Arthroplasty. J. Arthroplast. 2006, 21, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Horiuchi, H.; Kobayashi, S.; Nawata, M.; Takaoka, K. Continuous local cooling for pain relief following total hip arthroplasty. J. Arthroplast. 2004, 19, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Thijs, E.; Schotanus, M.G.M.; Bemelmans, Y.F.L.; Kort, N.P. Reduced opiate use after total knee arthroplasty using computer-assisted cryotherapy. Knee Surg. Sport. Traumatol. Arthrosc. 2019, 27, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Karaduman, Z.O.; Turhal, O.; Turhan, Y.; Orhan, Z.; Arican, M.; Uslu, M.; Cangur, S. Evaluation of the Clinical Efficacy of Using Thermal Camera for Cryotherapy in Patients with Total Knee Arthroplasty: A Prospective Study. Medicina 2019, 55, 661. [Google Scholar] [CrossRef]
- Moretti, L.; Maccagnano, G.; Coviello, M.; Cassano, G.D.; Franchini, A.; Laneve, A.; Moretti, B. Platelet Rich Plasma Injections for Knee Osteoarthritis Treatment: A Prospective Clinical Study. J. Clin. Med. 2022, 11, 2640. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ji, X. Sample Size Estimation in Clinical Research. Chest 2020, 158, S12–S20. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.P.; Politzer, C.; Green, C.; Wellman, S.; Bolognesi, M.; Seyler, T. Albumin Versus American Society of Anesthesiologists Score: Which Is More Predictive of Complications Following Total Joint Arthroplasty? Orthopedics 2018, 41, 354–362. [Google Scholar] [CrossRef]
- Moretti, L.; Coviello, M.; Rosso, F.; Calafiore, G.; Monaco, E.; Berruto, M.; Solarino, G. Current Trends in Knee Arthroplasty: Are Italian Surgeons Doing What Is Expected? Medicina 2022, 58, 1164. [Google Scholar] [CrossRef]
- Schotanus, M.G.M.; Bemelmans, Y.F.L.; van der Kuy, P.H.M.; Jansen, J.; Kort, N.P. No advantage of adrenaline in the local infiltration analgesia mixture during total knee arthroplasty. Knee Surg. Sport. Traumatol. Arthrosc. 2017, 25, 2778–2783. [Google Scholar] [CrossRef] [PubMed]
- Kort, N.P.; Bemelmans, Y.; Vos, R.; Schotanus, M.G.M. Low incidence of postoperative urinary retention with the use of a nurse-led bladder scan protocol after hip and knee arthroplasty: A retrospective cohort study. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.N.; Saleem, S.A.; Rehman Rao, S.; Wasim, M.H.; Durrani, N.A.; Naqvi, S.A. Comparison of the Efficacy of Continuous Femoral Nerve Block with Epidural Analgesia for Postoperative Pain Relief after Unilateral Total Knee Replacement. Cureus 2022, 14. [Google Scholar] [CrossRef]
- Vishwanatha, S.; Kalappa, S. Continuous femoral nerve blockade versus epidural analgesia for postoperative pain relief in knee surgeries: A randomized controlled study. Anesth. Essays Res. 2017, 11, 599. [Google Scholar] [CrossRef]
- Danninger, T. Perioperative pain control after total knee arthroplasty: An evidence based review of the role of peripheral nerve blocks. World J. Orthop. 2014, 5, 225. [Google Scholar] [CrossRef]
- Kuyucu, E.; Bülbül, M.; Kara, A.; Koçyiğit, F.; Erdil, M. Is cold therapy really efficient after knee arthroplasty? Ann. Med. Surg. 2015, 4, 475–478. [Google Scholar] [CrossRef]
- Jamison, R.N.; Ross, M.J.; Hoopman, P.; Griffin, F.; Levy, J.; Daly, M.; Schaffer, J.L. Assessment of Postoperative Pain Management: Patient Satisfaction and Perceived Helpfulness. Clin. J. Pain 1997, 13, 229–236. [Google Scholar] [CrossRef]
- Adie, S.; Kwan, A.; Naylor, J.M.; Harris, I.A.; Mittal, R. Cryotherapy following total knee replacement. Cochrane Database Syst. Rev. 2012, 9. [Google Scholar] [CrossRef]
- Farrar, J.T.; Young, J.P.; LaMoreaux, L.; Werth, J.L.; Poole, M.R. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed]
- Bech, M.; Moorhen, J.; Cho, M.; Lavergne, M.R.; Stothers, K.; Hoens, A.M. Device or Ice: The Effect of Consistent Cooling Using a Device Compared with Intermittent Cooling Using an Ice Bag after Total Knee Arthroplasty. Physiother. Can. 2015, 67, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Duellman, T.J.; Gaffigan, C.; Milbrandt, J.C.; Allan, D.G. Multi-modal, pre-emptive analgesia decreases the length of hospital stay following total joint arthroplasty. Orthopedics 2009, 32, 167. [Google Scholar] [PubMed]
- Su, E.P.; Perna, M.; Boettner, F.; Mayman, D.J.; Gerlinger, T.; Barsoum, W.; Randolph, J.; Lee, G. A prospective, multi-center, randomised trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J. Bone Joint Surg. Br. 2012, 94-B, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Morsi, E. Continuous-flow cold therapy after total knee arthroplasty. J. Arthroplast. 2002, 17, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, K.A.; Saliba, S.A. Should Athletes Return to Activity After Cryotherapy? J. Athl. Train. 2014, 49, 95–96. [Google Scholar] [CrossRef]
- Scharf, H.-P. CORR Insights®: Does Advanced Cryotherapy Reduce Pain and Narcotic Consumption After Knee Arthroplasty? Clin. Orthop. Relat. Res. 2014, 472, 3424–3425. [Google Scholar] [CrossRef]
- Holmström, A.; Härdin, B.C. Cryo/Cuff Compared to Epidural Anesthesia After Knee Unicompartmental Arthroplasty. J. Arthroplast. 2005, 20, 316–321. [Google Scholar]
- Maccagnano, G.; Solarino, G.; Pesce, V.; Vicenti, G.; Coviello, M.; Nappi, V.S.; Giannico, O.V.; Notarnicola, A.; Moretti, B. Plate vs reverse shoulder arthroplasty for proximal humeral fractures: The psychological health influence the choice of device? World J. Orthop. 2022, 13, 297–306. [Google Scholar] [CrossRef]
- Healy, W.L.; Seidman, J.; Pfeifer, B.A.; Brown, D.G. Cold compressive dressing after total knee arthroplasty. Clin. Orthop. Relat. Res. 1994, 143–146. Available online: https://europepmc.org/article/med/7907012 (accessed on 1 September 2022).
- Forsyth, A.L.; Zourikian, N.; Valentino, L.A.; Rivard, G.E. The effect of cooling on coagulation and haemostasis: Should “Ice” be part of treatment of acute haemarthrosis in haemophilia? Haemophilia 2012, 18, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.F.; Hendricks, B.D.; Edwards, D.R.; Maclean, K.M.; Bastawrous, S.S.; Middleton, J.F. Total hip and knee replacement surgery results in changes in leukocyte and endothelial markers. J. Inflamm. 2010, 7, 2. [Google Scholar] [CrossRef]
- Gao, F.-Q.; Li, Z.-J.; Zhang, K.; Sun, W.; Zhang, H. Four Methods for Calculating Blood-loss after Total Knee Arthroplasty. Chin. Med. J. 2015, 128, 2856–2860. [Google Scholar] [CrossRef] [PubMed]
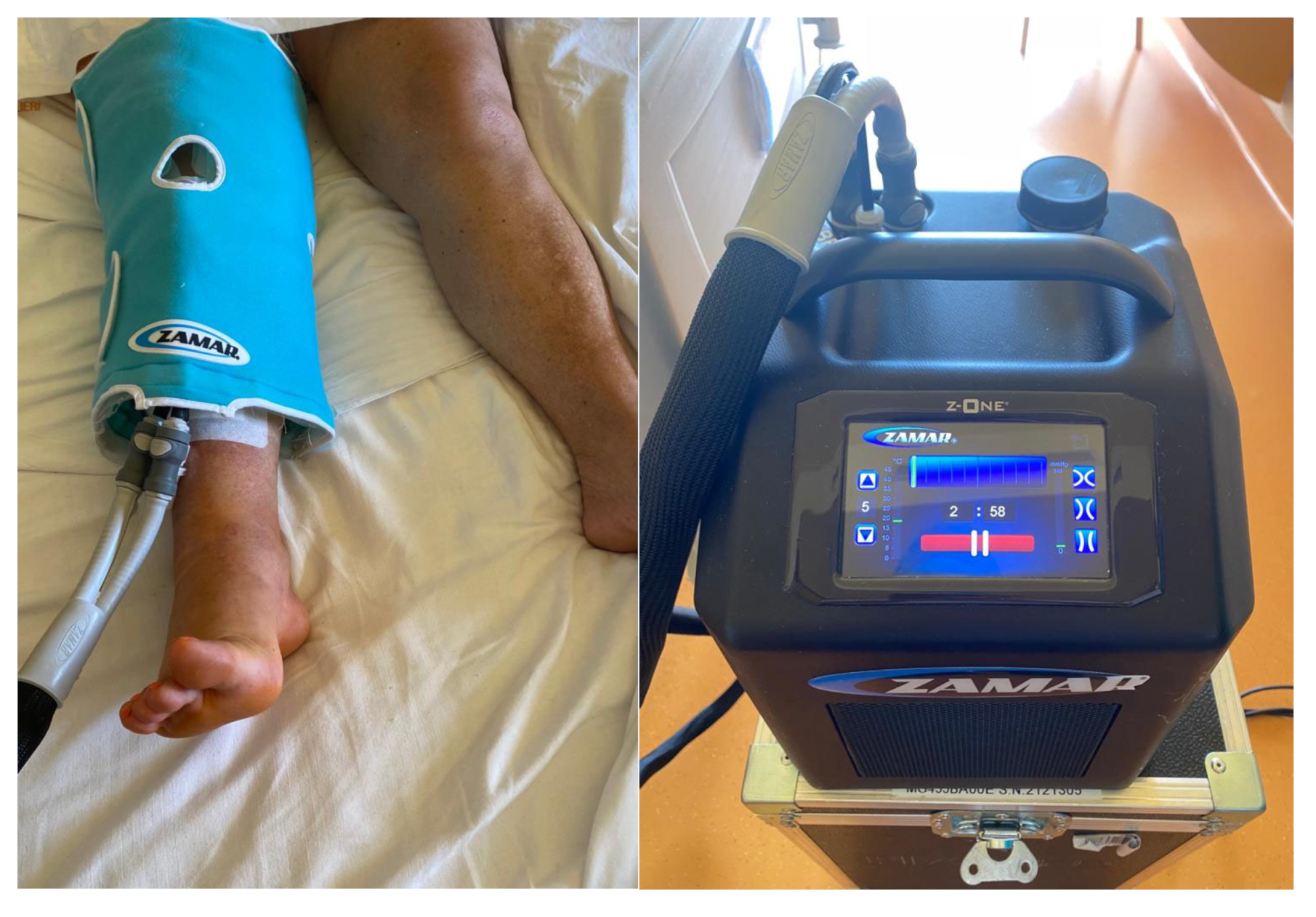
| Preoperative Features | Study Group | Control Group | p-Value |
|---|---|---|---|
| Age (year) | 66.56 ± 6.78 | 65.76 ± 6.23 | 0.63 |
| Gender (female) | 27 (54%) | 25 (50%) | 0.84 |
| BMI (Kg/m2) | 28.50 ± 4.32 | 27.82 ± 3.47 | 0.56 |
| Side (left) | 23 (46%) | 21 (42%) | 0.84 |
| ASA Classification | 0.86 | ||
| I | 20 (40%) | 18 (36%) | |
| II | 16 (32%) | 14 (28%) | |
| III | 11 (22%) | 13 (26%) | |
| IV | 3 (6%) | 5 (10%) | |
| Preoperative hematocrit (%) | 38.82 ± 3.67 | 39.73 ± 3.87 | 0.16 |
| Surgical time (min) | 57.09 ± 15.04 | 58.16 ± 15.46 | 0.70 |
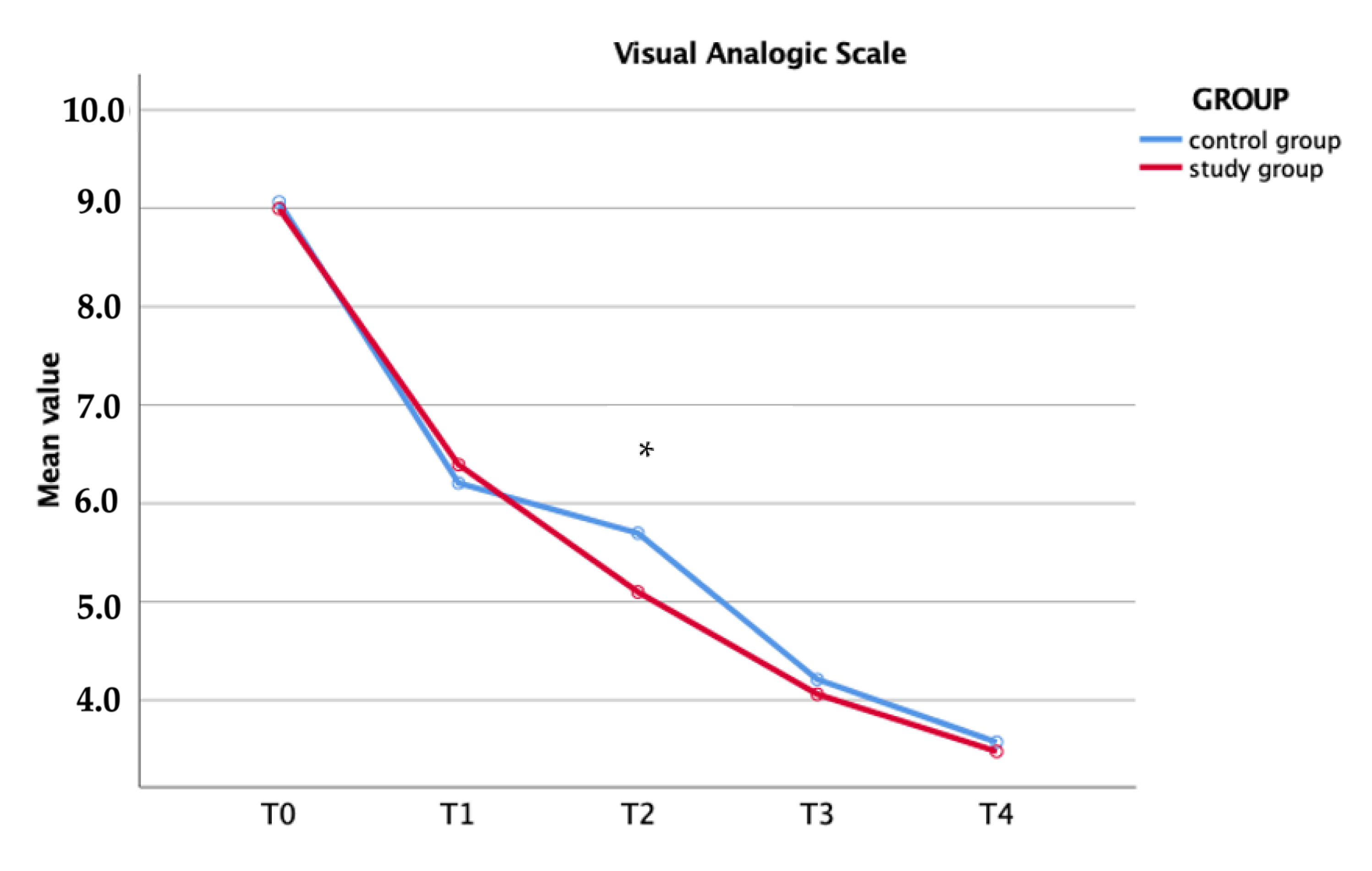
| VAS | Study Group | Control Group | p-Value | |
|---|---|---|---|---|
| T0 | 9.00 ± 0.47 | 9.06 ± 0.49 | 0.52 | |
| T1 | 6.39 ± 1.23 | 6.20 ± 1.12 | 0.46 | |
| T2 | 5.09 ± 0.94 | 5.69 ± 1.08 | 0.01 | |
| T3 | 4.06 ± 0.89 | 4.21 ± 0.72 | 0.51 | |
| T4 | 3.48 ± 0.68 | 3.57 ± 0.64 | 0.52 | |
| Opioid consumption | ||||
| T1 (mg) | 8 (400) | 9 (450) | 1.00 | |
| T2 (mg) | 17 (850) | 32 (1600) | 0.01 | |
| T3 (mg) | 9 (450) | 19 (950) | 0.05 | |
| T4 (mg) | 11 (550) | 14 (700) | 0.64 | |
| Total (mg) | 45 (2250) | 74 (3700) | 0.02 |
| Passive ROM (°) | Study Group | Control Group | p-Value | |
|---|---|---|---|---|
| T0 | 84.40 ± 7.14 | 83.48 ± 7.02 | 0.55 | |
| T2 | 111.57 ± 7.04 | 105.49 ± 11.24 | 0.01 | |
| T3 | 110.94 ± 7.52 | 107.39 ± 7.89 | 0.01 | |
| T4 | 108.84 ± 6.07 | 108.22 ± 6.61 | 0.64 | |
| Total blood loss (Gross formula) | ||||
| T2 | 1.03 ± 0.42 | 1.06 ± 0.55 | 0.86 | |
| Number of transfusions | 4 (8%) | 5 (10%) | 0.50 | |
| Patient satisfaction (cm) | 8.55 ± 0.36 | 6.05 ± 0.58 | 0.01 | |
| Patient who recommended “yes” | 43 (86%) | 31 (62%) | 0.01 |
| VAS | Opioid Consumption | |||||||
|---|---|---|---|---|---|---|---|---|
| B | 95% CI | p-Value | B | 95% CI | p-Value | |||
| Intercept | 7.50 | <0.01 | 0.915 | 0.05 | ||||
| Group (cryotherapy) | −0.62 | −1.04 | −0.20 | <0.01 | −0.31 | −0.50 | −0.11 | <0.01 |
| Age | −0.01 | −0.04 | 0.02 | 0.57 | 0.01 | −0.01 | 0.03 | 0.15 |
| Sex (female) | −0.25 | −0.93 | 0.41 | 0.45 | 0.12 | −0.19 | 0.44 | 0.44 |
| Left knee | 0.53 | −0.14 | 1.20 | 0.12 | −0.21 | −0.52 | 0.10 | 0.18 |
| BMI | −0.01 | −0.06 | 0.05 | 0.93 | −0.02 | −0.05 | 0.01 | 0.12 |
| ASA Classification | −0.01 | −0.24 | 0.22 | 0.96 | −0.07 | −0.17 | 0.04 | 0.22 |
| Preoperative hematocrit | −0.01 | −0.07 | 0.04 | 0.69 | −0.01 | −0.03 | 0.02 | 0.72 |
| Surgical time | −0.01 | −0.02 | 0.01 | 0.32 | −0.01 | −0.01 | 0.01 | 0.69 |
| Total blood loss | −0.20 | −0.63 | 0.25 | 0.39 | −0.01 | −0.21 | 0.20 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coviello, M.; Abate, A.; Ippolito, F.; Nappi, V.; Maddalena, R.; Maccagnano, G.; Noia, G.; Caiaffa, V. Continuous Cold Flow Device Following Total Knee Arthroplasty: Myths and Reality. Medicina 2022, 58, 1537. https://doi.org/10.3390/medicina58111537
Coviello M, Abate A, Ippolito F, Nappi V, Maddalena R, Maccagnano G, Noia G, Caiaffa V. Continuous Cold Flow Device Following Total Knee Arthroplasty: Myths and Reality. Medicina. 2022; 58(11):1537. https://doi.org/10.3390/medicina58111537
Chicago/Turabian StyleCoviello, Michele, Antonella Abate, Francesco Ippolito, Vittorio Nappi, Roberto Maddalena, Giuseppe Maccagnano, Giovanni Noia, and Vincenzo Caiaffa. 2022. "Continuous Cold Flow Device Following Total Knee Arthroplasty: Myths and Reality" Medicina 58, no. 11: 1537. https://doi.org/10.3390/medicina58111537
APA StyleCoviello, M., Abate, A., Ippolito, F., Nappi, V., Maddalena, R., Maccagnano, G., Noia, G., & Caiaffa, V. (2022). Continuous Cold Flow Device Following Total Knee Arthroplasty: Myths and Reality. Medicina, 58(11), 1537. https://doi.org/10.3390/medicina58111537









