Human Stem Cell Transplantation for Retinal Degenerative Diseases: Where Are We Now?
Abstract
:1. Introduction
2. Methods
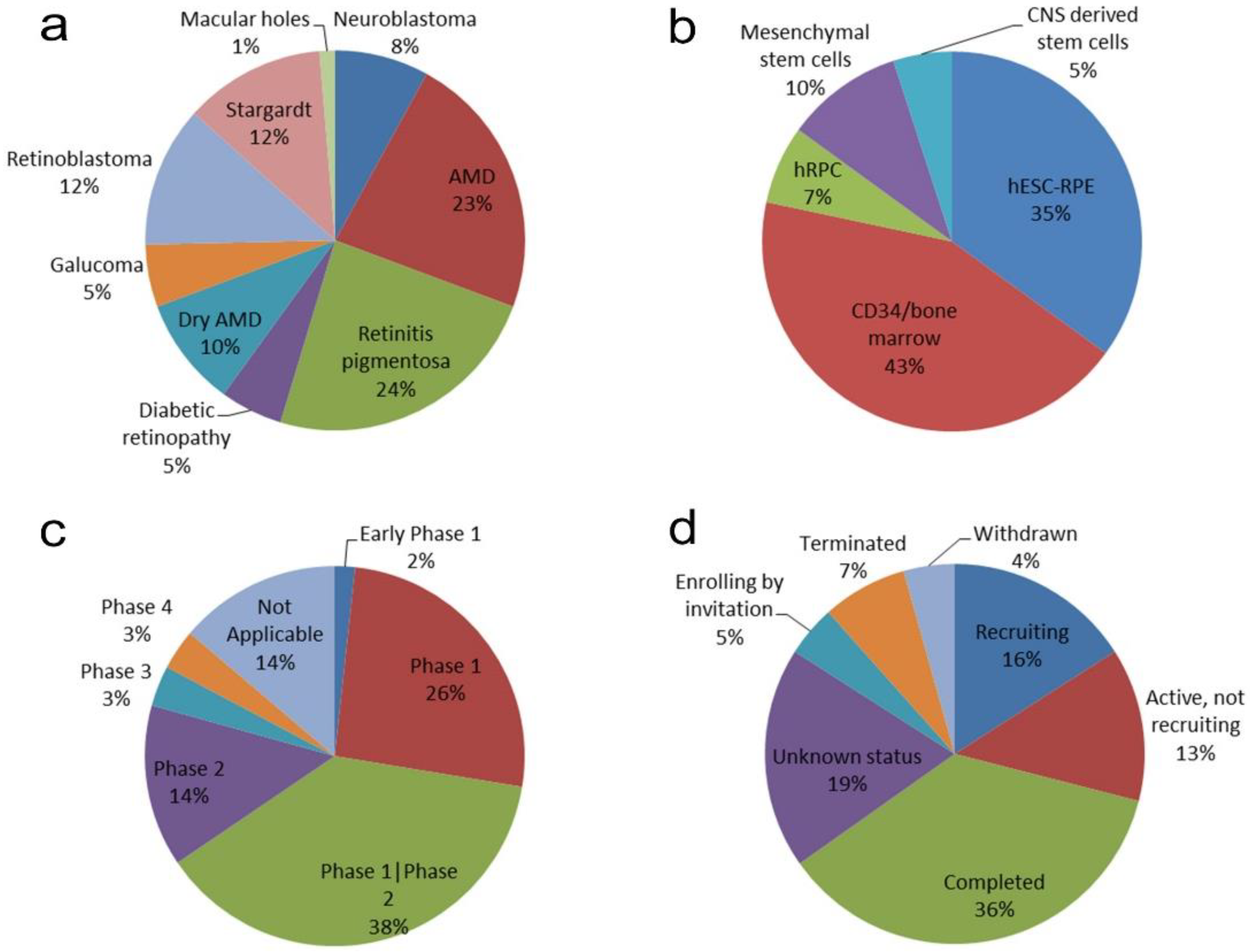
3. Retinal Therapies Using Stem Cells in the Literature Search
4. Clinical Trials Using Stem Cells to Treat the Human Retina
5. Different Administration Routes for Different Types of Stem Cells
6. Current Status of Clinical Trials
7. Future Directions
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzalez-Cordero, A.; Kruczek, K.; Naeem, A.; Fernando, M.; Kloc, M.; Ribeiro, J.; Goh, D.; Duran, Y.; Blackford, S.J.I.; Abelleira-Hervas, L.; et al. Recapitulation of Human Retinal Development from Human Pluripotent Stem Cells Generates Transplantable Populations of Cone Photoreceptors. Stem Cell Rep. 2017, 9, 820–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Jemni-Damer, N.; Guedan-Duran, A.; Fuentes-Andion, M.; Serrano-Bengoechea, N.; Alfageme-Lopez, N.; Armada-Maresca, F.; Guinea, G.V.; Perez-Rigueiro, J.; Rojo, F.; Gonzalez-Nieto, D.; et al. Biotechnology and Biomaterial-Based Therapeutic Strategies for Age-Related Macular Degeneration. Part II: Cell and Tissue Engineering Therapies. Front. Bioeng. Biotechnol. 2020, 8, 1419. [Google Scholar] [CrossRef]
- Jemni-Damer, N.; Guedan-Duran, A.; Fuentes-Andion, M.; Serrano-Bengoechea, N.; Alfageme-Lopez, N.; Armada-Maresca, F.; Guinea, G.V.; Pérez-Rigueiro, J.; Rojo, F.; Gonzalez-Nieto, D.; et al. Biotechnology and Biomaterial-Based Therapeutic Strategies for Age-Related Macular Degeneration. Part I: Biomaterials-Based Drug Delivery Devices. Front. Bioeng. Biotechnol. 2020, 8, 1257. [Google Scholar] [CrossRef]
- Dias, M.F.; Joo, K.; Kemp, J.A.; Fialho, S.L.; da Silva Cunha, A.J.; Woo, S.J.; Kwon, Y.J. Molecular Genetics and Emerging Therapies for Retinitis Pigmentosa: Basic Research and Clinical Perspectives. Prog. Retin. Eye Res. 2018, 63, 107–131. [Google Scholar] [CrossRef]
- Romito, A.; Cobellis, G. Pluripotent Stem Cells: Current Understanding and Future Directions. Stem Cells Int. 2016, 2016, 9451492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.S.; Park, S.S.; Albini, T.A.; Canto-Soler, M.V.; Klassen, H.; MacLaren, R.E.; Takahashi, M.; Nagiel, A.; Schwartz, S.D.; Bharti, K. Retinal Stem Cell Transplantation: Balancing Safety and Potential. Prog. Retin. Eye Res. 2020, 75, 100779. [Google Scholar] [CrossRef]
- da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M.; et al. Phase 1 Clinical Study of an Embryonic Stem Cell-Derived Retinal Pigment Epithelium Patch in Age-Related Macular Degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef] [Green Version]
- Kashani, A.H.; Uang, J.; Mert, M.; Rahhal, F.; Chan, C.; Avery, R.L.; Dugel, P.; Chen, S.; Lebkowski, J.; Clegg, D.O.; et al. Surgical Method for Implantation of a Biosynthetic Retinal Pigment Epithelium Monolayer for Geographic Atrophy: Experience from a Phase 1/2a Study. Ophthalmol. Retin. 2020, 4, 264–273. [Google Scholar] [CrossRef]
- Park, S.S.; Bauer, G.; Abedi, M.; Pontow, S.; Panorgias, A.; Jonnal, R.; Zawadzki, R.J.; Werner, J.S.; Nolta, J. Intravitreal Autologous Bone Marrow CD34+ Cell Therapy for Ischemic and Degenerative Retinal Disorders: Preliminary Phase 1 Clinical Trial Findings. Investig. Ophthalmol. Vis. Sci. 2014, 56, 81–89. [Google Scholar] [CrossRef]
- Song, W.K.; Park, K.-M.; Kim, H.-J.; Lee, J.H.; Choi, J.; Chong, S.Y.; Shim, S.H.; Del Priore, L.V.; Lanza, R. Treatment of Macular Degeneration Using Embryonic Stem Cell-Derived Retinal Pigment Epithelium: Preliminary Results in Asian Patients. Stem Cell Rep. 2015, 4, 860–872. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.-P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium in Patients with Age-Related Macular Degeneration and Stargardt’s Macular Dystrophy: Follow-up of Two Open-Label Phase 1/2 Studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef]
- Mehat, M.S.; Sundaram, V.; Ripamonti, C.; Robson, A.G.; Smith, A.J.; Borooah, S.; Robinson, M.; Rosenthal, A.N.; Innes, W.; Weleber, R.G.; et al. Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells in Macular Degeneration. Ophthalmology 2018, 125, 1765–1775. [Google Scholar] [CrossRef] [Green Version]
- Sung, Y.; Lee, M.J.; Choi, J.; Jung, S.Y.; Chong, S.Y.; Sung, J.H.; Shim, S.H.; Song, W.K. Long-Term Safety and Tolerability of Subretinal Transplantation of Embryonic Stem Cell-Derived Retinal Pigment Epithelium in Asian Stargardt Disease Patients. Br. J. Ophthalmol. 2021, 105, 829–837. [Google Scholar] [CrossRef]
- Oner, A.; Gonen, Z.B.; Sinim, N.; Cetin, M.; Ozkul, Y. Subretinal Adipose Tissue-Derived Mesenchymal Stem Cell Implantation in Advanced Stage Retinitis Pigmentosa: A Phase I Clinical Safety Study. Stem Cell Res. Ther. 2016, 7, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Chen, S.J.; Li, S.Y.; Qu, L.H.; Meng, X.H.; Wang, Y.; Xu, H.W.; Liang, Z.Q.; Yin, Z.Q. Long-Term Safety of Human Retinal Progenitor Cell Transplantation in Retinitis Pigmentosa Patients. Stem Cell Res. Ther. 2017, 8, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.-Y.; Liu, Y.; Wang, L.; Wang, F.; Zhao, T.-T.; Li, Q.-Y.; Xu, H.-W.; Meng, X.-H.; Hao, J.; Zhou, Q.; et al. A Phase I Clinical Trial of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells for Early-Stage Stargardt Macular Degeneration: 5-Years’ Follow-Up. Cell Prolif. 2021, 54, e13100. [Google Scholar] [CrossRef]
- Decembrini, S.; Koch, U.; Radtke, F.; Moulin, A.; Arsenijevic, Y. Derivation of Traceable and Transplantable Photoreceptors from Mouse Embryonic Stem Cells. Stem Cell Rep. 2014, 2, 853–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- del Cerro, M.; Ison, J.R.; Bowen, G.P.; Lazar, E.; del Cerro, C. Intraretinal Grafting Restores Visual Function in Light-Blinded Rats. Neuroreport 1991, 2, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Gouras, P.; Du, J.; Kjeldbye, H.; Kwun, R.; Lopez, R.; Zack, D.J. Transplanted Photoreceptors Identified in Dystrophic Mouse Retina by a Transgenic Reporter Gene. Investig. Ophthalmol. Vis. Sci. 1991, 32, 3167–3174. [Google Scholar]
- Gouras, P.; Du, J.; Gelanze, M.; Lopez, R.; Kwun, R.; Kjeldbye, H.; Krebs, W. Survival and Synapse Formation of Transplanted Rat Rods. J. Neural Transpl. Plast. 1991, 2, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Lamba, D.A.; Gust, J.; Reh, T.A. Transplantation of Human Embryonic Stem Cell-Derived Photoreceptors Restores Some Visual Function in Crx-Deficient Mice. Cell Stem Cell 2009, 4, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Jha, B.S.; Bharti, K. Regenerating Retinal Pigment Epithelial Cells to Cure Blindness: A Road Towards Personalized Artificial Tissue. Curr. Stem Cell Rep. 2015, 1, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Radtke, N.D.; Aramant, R.B.; Petry, H.M.; Green, P.T.; Pidwell, D.J.; Seiler, M.J. Vision Improvement in Retinal Degeneration Patients by Implantation of Retina Together with Retinal Pigment Epithelium. Am. J. Ophthalmol. 2008, 146, 172–182. [Google Scholar] [CrossRef] [Green Version]
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [Green Version]
- Gasparini, S.J.; Llonch, S.; Borsch, O.; Ader, M. Transplantation of Photoreceptors into the Degenerative Retina: Current State and Future Perspectives. Prog. Retin. Eye Res. 2019, 69, 1–37. [Google Scholar] [CrossRef]
- Barber, A.C.; Hippert, C.; Duran, Y.; West, E.L.; Bainbridge, J.W.B.; Warre-Cornish, K.; Luhmann, U.F.O.; Lakowski, J.; Sowden, J.C.; Ali, R.R.; et al. Repair of the Degenerate Retina by Photoreceptor Transplantation. Proc. Natl. Acad. Sci. USA 2013, 110, 354–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, R.A.; Barber, A.C.; Rizzi, M.; Hippert, C.; Xue, T.; West, E.L.; Duran, Y.; Smith, A.J.; Chuang, J.Z.; Azam, S.A.; et al. Restoration of Vision after Transplantation of Photoreceptors. Nature 2012, 485, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Malcuit, C.; Wang, S.; Girman, S.; Francis, P.; Lemieux, L.; Lanza, R.; Lund, R. Long-Term Safety and Function of RPE from Human Embryonic Stem Cells in Preclinical Models of Macular Degeneration. Stem Cells 2009, 27, 2126–2135. [Google Scholar] [CrossRef]
- Ben M’Barek, K.; Habeler, W.; Plancheron, A.; Jarraya, M.; Regent, F.; Terray, A.; Yang, Y.; Chatrousse, L.; Domingues, S.; Masson, Y.; et al. Human ESC-Derived Retinal Epithelial Cell Sheets Potentiate Rescue of Photoreceptor Cell Loss in Rats with Retinal Degeneration. Sci. Transl. Med. 2017, 9, eaai7471. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Jin, Z.-B.; Okamoto, S.; Mandai, M.; Takahashi, M. Induced Pluripotent Stem Cells for Retinal Degenerative Diseases: A New Perspective on the Challenges. J. Genet. 2009, 88, 417–424. [Google Scholar] [CrossRef]
- Jin, Z.-B.; Takahashi, M. Generation of Retinal Cells from Pluripotent Stem Cells. Prog. Brain Res. 2012, 201, 171–181. [Google Scholar] [CrossRef]
- Zhong, X.; Gutierrez, C.; Xue, T.; Hampton, C.; Vergara, M.N.; Cao, L.-H.; Peters, A.; Park, T.S.; Zambidis, E.T.; Meyer, J.S.; et al. Generation of Three-Dimensional Retinal Tissue with Functional Photoreceptors from Human IPSCs. Nat. Commun. 2014, 5, 4047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Reynolds, J.; Garcia, T.; Cifuentes, H.; Chew, S.; Zeng, X.; Lamba, D.A. Generation of Transplantable Retinal Photoreceptors from a Current Good Manufacturing Practice-Manufactured Human Induced Pluripotent Stem Cell Line. Stem Cells Transl. Med. 2018, 7, 210–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.M.; Vetter, M.L. From Retina to Stem Cell and Back Again: Memories of a Chromatin Journey. Cell Rep. 2018, 22, 2519–2520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, H.-Y.; Watanabe, T.; Shirai, H.; Yamasaki, S.; Kinoshita, M.; Matsushita, K.; Hashiguchi, T.; Onoe, H.; Matsuyama, T.; Kuwahara, A.; et al. Medium- to Long-Term Survival and Functional Examination of Human IPSC-Derived Retinas in Rat and Primate Models of Retinal Degeneration. EBioMedicine 2019, 39, 562–574. [Google Scholar] [CrossRef] [Green Version]
| Title | Disease | Intervention | Results |
|---|---|---|---|
| Human embryonic stem cell (hESC)-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt macular dystrophy: follow-up of two open-label phase 1/2 studies (NCT01345006 and NCT01344993) [14] | Stargardt macular dystrophy. | Subretinal transplantation of hESC-derived retinal pigment epithelium | Evidence of the medium-term to long-term safety, graft survival, and possible biological activity of pluripotent stem cell progeny in individuals with any disease. |
| Atrophic age-related macular degeneration. | |||
| Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration (NCT01691261) [10] | Severe exudative AMD. | Subretinal implant of engineered human embryonic stem cell (hESC)-derived RPE patch. | Successful delivery and survival of the RPE patch. |
| data | Visual acuity gain of 29 and 21 letters. | ||
| Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells in Macular Degeneration (NCT01469832) [15] | Stargardt disease (STGD1). | Subretinal transplantation of up to 200,000 hESC-derived RPE cells with systemic immunosuppressive therapy for 13 weeks | Survival of viable transplanted hESC-derived RPE cells. |
| Long-term safety and tolerability of subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium in Asian Stargardt disease patients (NCT01625559) [16] | Stargardt macular dystrophy (SMD). | Subretinal transplantation of hESC-retinal pigment epithelium (RPE) cells | No serious adverse events. Long-term safety, tolerability and feasibility. |
| Favorable functional and anatomical results. | |||
| Surgical Method for Implantation of a Biosynthetic Retinal Pigment Epithelium Monolayer for Geographic Atrophy: Experience from a Phase 1/2a Study (NCT02590692) [11] | Advanced non-neovascular age-related macular degeneration (NNAMD). | Subretinal implantation of a human embryonic stem cell-derived retinal pigment epithelium (RPE) monolayer seeded on a synthetic substrate. | No unanticipated serious adverse events related to the implant or surgery were reported. |
| Surgical implantation of CPCB-RPE1 targeted to the area of GA in subjects with advanced NNAMD is feasible in an outpatient setting. | |||
| Subretinal adipose tissue-derived mesenchymal stem cell implantation in advanced stage retinitis pigmentosa: a phase I clinical safety study (Ministry of Health of Turkey approval: 56733164/203) [17] | Retinitis pigmentosa. | Subretinal adipose tissue-derived mesenchymal stem cell (ADMSC) implantation. | Ocular complications. |
| Evidence of the short-term safety of ADMSCs in humans | |||
| Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients (NCT01625559 and NCT01674829) [13] | Dry age-related macular degeneration. | Subretinal transplantation of human embryonic-stem-cell (hESC)-derived retinal pigment epithelium. | No evidence of adverse serious safety issues. |
| Stargardt macular dystrophy. | Visual acuity improvement. | ||
| Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: preliminary phase 1 clinical trial findings (NCT01736059) [12] | Irreversible vision loss from retinal vascular occlusion, hereditary or nonexudative age-related macular degeneration, or retinitis pigmentosa. | Intravitreal human bone marrow (BM) CD34+ stem cell injection. | No intraocular inflammation or hyperproliferation. |
| Mild progression of geographic atrophy in AMD patients. | |||
| A phase I clinical trial of human embryonic stem cell-derived retinal pigment epithelial cells for early-stage Stargardt macular degeneration: 5-years’ follow-up (NCT02749734) [19] | Early-stage of Stargardt macular degeneration (STGD1). | Subretinal transplantation of human embryonic stem cell-derived retinal pigment epithelial (hESC-RPE) cells. | Safe and tolerable. Increased visual function. |
| Visual function loss in two patients. | |||
| Long-term safety of human retinal progenitor cell transplantation in retinitis pigmentosa patients (ChiCTR-TNRC-08000193) [18] | Advanced retinitis pigmentosa. | Transplantation of purified human fetal-derived retinal progenitor cells (RPCs) | No immunological rejection or tumorigenesis. Long-term safety and feasibility. |
| Significant improvement in visual acuity and increase in retinal sensitivity. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcalde, I.; Sánchez-Fernández, C.; Martín, C.; De Pablo, N.; Jemni-Damer, N.; Guinea, G.V.; Merayo-Lloves, J.; Del Olmo-Aguado, S. Human Stem Cell Transplantation for Retinal Degenerative Diseases: Where Are We Now? Medicina 2022, 58, 102. https://doi.org/10.3390/medicina58010102
Alcalde I, Sánchez-Fernández C, Martín C, De Pablo N, Jemni-Damer N, Guinea GV, Merayo-Lloves J, Del Olmo-Aguado S. Human Stem Cell Transplantation for Retinal Degenerative Diseases: Where Are We Now? Medicina. 2022; 58(1):102. https://doi.org/10.3390/medicina58010102
Chicago/Turabian StyleAlcalde, Ignacio, Cristina Sánchez-Fernández, Carla Martín, Nagore De Pablo, Nahla Jemni-Damer, Gustavo V. Guinea, Jesús Merayo-Lloves, and Susana Del Olmo-Aguado. 2022. "Human Stem Cell Transplantation for Retinal Degenerative Diseases: Where Are We Now?" Medicina 58, no. 1: 102. https://doi.org/10.3390/medicina58010102
APA StyleAlcalde, I., Sánchez-Fernández, C., Martín, C., De Pablo, N., Jemni-Damer, N., Guinea, G. V., Merayo-Lloves, J., & Del Olmo-Aguado, S. (2022). Human Stem Cell Transplantation for Retinal Degenerative Diseases: Where Are We Now? Medicina, 58(1), 102. https://doi.org/10.3390/medicina58010102






