Use of Sertraline in Hemodialysis Patients
Abstract
1. Introduction
2. Sertraline—General Properties of the Medication
3. Impact on Cardiovascular System
4. Intradialytic Hypotension
5. Uremic Pruritus
6. Cytokines
7. Sexual Dysfunction
8. Fracture Risk
9. Discussion
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Saran, R.; Robinson, B.; Abbott, K.C.; Bragg-Gresham, J.; Chen, X.; Gipson, D.; Gu, H.; Hirth, R.A.; Hutton, D.; Jin, Y.; et al. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2020, 75 (Suppl. S1), A6–A7. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.; Vecchio, M.; Craig, J.C.; Tonelli, M.; Johnson, D.W.; Nicolucci, A.; Pellegrini, F.; Saglimbene, V.; Logroscino, G.; Fishbane, S.; et al. Prevalence of depression in chronic kidney disease: Systematic review and meta-analysis of observational studies. Kidney Int. 2013, 84, 179. [Google Scholar] [CrossRef] [PubMed]
- King-Wing Ma, T.; Kam-Tao Li, P. Depression in dialysis patients. Nephrology 2016, 21, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Murtagh, F.E.; Addington-Hall, J.; Higginson, I.J. The prevalence of symptoms in end-stage renal disease: A systematic review. Adv. Chronic Kidney Dis. 2007, 14, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Bautovich, A.; Katz, I.; Smith, M.; Loo, C.K.; Harvey, S.B. Depression and chronic kidney disease: A review for clinicians. Aust. N. Z. J. Psychiatry 2014, 48, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, S.S.; Yalamanchili, V.; Finkelstein, F.O. A practical approach to the treatment of depression in patients with chronic kidney disease and end-stage renal disease. Kidney Int. 2012, 81, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Schouten, R.W.; Haverkamp, G.L.; Loosman, W.L.; Chandie Shaw, P.K.; van Ittersum, F.J.; Smets, Y.F.C.; Vleming, L.J.; Dekker, F.W.; Honig, A.; Siegert, C.E.H. Anxiety symptoms, mortality and hospitalization in patients receiving maintenance dialysis: A cohort study. Am. J. Kidney Dis. 2019, 74, 158–166. [Google Scholar] [CrossRef]
- Cohen, S.D.; Cukor, D.; Kimmel, P.L. Anxiety in Patients Treated with Hemodialysis. Clin. J. Am. Soc. Nephrol. 2016, 11, 2250–2255. [Google Scholar] [CrossRef]
- Cohen, S.D.; Norris, L.; Aquaviva, K.; Peterson, R.A.; Kimmel, P.L. Screening, diagnosis and treatment of depression in patients with end-stage renal disease. Clin. J. Am. Soc. Nephrol. 2007, 2, 133–142. [Google Scholar] [CrossRef]
- Fasipe, O.J. Neuropharmacological classification of antidepressant agents based on their mechanisms of action. Arch. Med. Health Sci. 2018, 6, 81–94. [Google Scholar] [CrossRef]
- Hughes, Z.A.; Starr, K.R.; Langmead, C.J.; Hill, M.; Bartoszyk, G.D.; Hagan, J.J.; Middlemiss, D.N.; Dawson, L.A. Neurochemical evaluation of the novel 5-HT1A receptor partial agonist/serotonin reuptake inhibitor, vilazodone. Eur. J. Pharmacol. 2005, 510, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Constantino, J.L.; Fonseca, V.A. Pharmacokinetics of antidepressants in patients undergoing hemodialysis: A narrative literature review. Braz. J. Psychiatry 2019, 41, 441–446. [Google Scholar] [CrossRef]
- Jaber, B.L.; Lee, Y.; Collins, A.J.; Hull, A.R.; Kraus, M.A.; McCarthy, J.; Miller, B.W.; Spry, L.; Finkelstein, F.O. Effect of daily hemodialysis on depressive symptoms and postdialysis recovery time: Interim report from the FREEDOM (Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements) Study. Am J Kidney Dis. 2010, 56, 531–539. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Drug Safety Communication: Revised Recommendations for Celexa (Citalopram Hydrobromide) Related to a Potential Risk of Abnormal Heart Rhythms with High Doses. US Food and Drug Administration (2012). Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-revised-recommendations-celexa-citalopram-hydrobromide-related (accessed on 1 September 2021).
- Health Canada 2012 Health CanadaAntidepressant cipralex (Escitalopram): Updated information Regarding Dose-Related Heart Risk. 2012. Available online: http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2012/13674a-eng.php (accessed on 3 January 2021).
- Crépeau-Gendron, G.; Brown, H.K.; Shorey, C.; Madan, R.; Szabuniewicz, C.; Koh, S.; Veinish, S.; Mah, L. Association between citalopram, escitalopram and QTc prolongation in a real-world geriatric setting. J. Affect. Disord. 2019, 250, 341–345. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research; World Health Organization: Geneva, Switzerland, 1993; p. 248. [Google Scholar]
- American Psychiatric Association. DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Arlington, VA, USA, 2013. Available online: https://www.ncjrs.gov/App/Publications/abstract.aspx?ID=271642 (accessed on 1 September 2021).
- Assimon, M.M.; Brookhart, M.A.; Flythe, J.E. Comparative Cardiac Safety of Selective Serotonin Reuptake Inhibitors among Individuals receiving Maintenance Hemodialysis. J. Am. Soc. Nephol. 2019, 30, 611–623. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Chen, H.Z.; Hu, X.J.; Yu, Z.H.; Yang, W.; Deng, M.; Zhang, Y.H.; Ruan, B. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal bleeding: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2015, 13, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Iwagami, M.; Tomlinson, L.A.; Mansfield, K.E.; Douglas, I.J.; Smeeth, L.; Nitsch, D. Gastrointestinal bleeding risk of selective serotonin reuptake inhibitors by level of kidney function: A population-based cohort study. Br. J. Clin. Pharmacol. 2018, 84, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vilchez, I.; Serra-Millas, M.; Navarro, V.; Rosa Hernandez, M.; Villalta, J.; Diaz-Ricart, M.; Gasto, C.; Escolar, G.; Galan, A.M. Prothrombotic platelet phenotype in major depression: Downregulation by antidepressant treatment. J. Affect. Disord. 2014, 159, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.L.; Chiang, M.L.; Lane, H.Y.; Su, K.P.; Lai, Y.C. Selective serotonin reuptake inhibitors reduce P2Y12 receptor-mediated amplification of platelet aggregation. Thromb. Res. 2013, 131, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Acedillo, R.R.; Shah, M.; Devereaux, P.J.; Li, L.; Iansavichus, A.V.; Walsh, M.; Garg, A.X. The risk of perioperative bleeding in patients with chronic kidney disease: A systematic review and meta-analysis. Ann. Surg. 2013, 258, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Molin, C.Z.Z.D.; Sakae, T.M.; Schuelter-Trevisol, F.; Trevisol, D.J. Effects of sertraline in the prevention of low blood pressure in patients undergoing hemodialysis. J. Bras. Nefrol. 2019, 41, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Razeghi, E.; Dashti-Khavidaki, S.; Nassiri, S.; Abolghassemi, R.; Khalili, H.; Nazari, S.S.H.; Mansournia, A.M.; Taraz3, M. A randomized crossover clinical trial of sertraline for intradialytic hypotension. Iran. J. Kidney Dis. 2015, 9, 323–330. [Google Scholar]
- Yalcin, A.U.; Sahin, G.; Erol, M.; Bal, C. Sertraline hydrochloride treatment for patients with hemodialysis hypotension. Blood Purif. 2002, 20, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, A.U.; Kudaiberdieva, G.; Sahin, G.; Gorenek, B.; Akcar, N.; Kuskus, S.; Bayrak, F.; Timuralp, B. Effect of sertraline hydrochloride on cardiac autonomic dysfunction in patients with hemodialysis-induced hypotension. Nephron Physiol. 2003, 93, 21–28. [Google Scholar] [CrossRef]
- Brewster, U.C.; Ciampi, M.A.; Abu-Alfa, A.K.; Perazella, M.A. Addition of sertraline to other therapies to reduce dialysis-associated hypotension. Nephrology 2003, 8, 296–301. [Google Scholar] [CrossRef]
- Shakiba, M.; Sanadgol, H.; Azmoude, H.R.; Mashhadi, M.A.; Sharifi, H. Effect of sertraline on uremic pruritus improvement in ESRD patients. Int. J. Nephrol. 2012, 2012, 363901. [Google Scholar] [CrossRef]
- Chan, K.Y.; Li, C.W.; Wong, H.; Yip, T.; Chan, M.L.; Cheng, H.W.; Sham, M.K. Use of sertraline for antihistamine-refractory uremic pruritus in renal palliative care patients. J. Palliat. Med. 2013, 16, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Pakfetrat, M.; Malekmakan, L.; Hashemi, N.; Tadayon, T. Sertraline can reduce uremic pruritus in hemodialysis patient: A double blind randomized clinical trial from Southern Iran. Hemodial. Int. 2018, 22, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakeim, H.K.; Twayej, A.J.; Al-Dujaili, A.H. Reduction in serum IL-1β IL-6, and IL-18 levels and Beck Depression Inventory-II score by combined sertraline and ketoprofen administration in major depressive disorder: A clinical trial. Neurol. Psychiatry Brain Res. 2018, 30, 148–153. [Google Scholar] [CrossRef]
- Sutcigil, L.; Oktenli, C.; Musabak, U.; Bozkurt, A.; Cansever, A.; Uzun, O.; Sanisoglu, S.Y.; Yesilova, Z.; Ozmenler, N.; Ozsahin, A.; et al. Pro- and anti-inflammatory cytokine balance in major depression: Effect of sertraline therapy. Clin. Dev. Immunol. 2007, 2007, 1–6. [Google Scholar] [CrossRef]
- Taraz, M.; Khatami, M.R.; Dashti-Khavidaki, S.; Akhonzadeh, S.; Noorbala, A.A.; Ghaeli, P.; Taraz, S. Sertraline decreases serum level of interleukin-6 (IL-6) in hemodialysis patients with depression: Results of a randomized double-blind, placebo-controlled clinical trial. Int. Immunopharmacol. 2013, 17, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Khazaie, H.; Rezaie, L.; Rezaei Payam, N.; Najafi, F. Antidepressant-induced sexual dysfunction during treatment with fluoxetine, sertraline and trazodone; a randomized controlled trial. Gen. Hosp. Psychiatry 2015, 37, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Serretti, A.; Chiesa, A. Treatment-emergent sexual dysfunction related to antidepressants: A meta-analysis. J. Clin. Psychopharmacol. 2009, 29, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Natale, P.; Ruospo, M.; Saglimbene, V.M.; Rabindranath, K.S.; Craig, J.C.; Strippoli, G.F. Antidepressants for treating depression in adults with end-stage kidney disease treated with dialysis. Cochrane Database Syst. Rev. 2016, 5, CD004541. [Google Scholar] [CrossRef] [PubMed]
- Khanassov, V.; Hu, J.; Reeves, D.; van Marwijk, H. Selective serotonin reuptake inhibitor and selective serotonin and norepinephrine reuptake inhibitor use and risk of fractures in adults: A systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 2018, 33, 1688–1708. [Google Scholar] [CrossRef]
- Rabenda, V.; Nicolet, D.; Beaudart, C.; Bruyère, O.; Reginster, J.-Y. Relationship between use of antidepressants and risk of fractures: A meta-analysis. Osteoporos. Int. 2013, 24, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Vangala, C.; Niu, J.; Montez-Rath, M.E.; Yan, J.; Navaneethan, S.D.; Winkelmayer, W.C. Selective Serotonin Reuptake Inhibitor Use and Hip Fracture Risk Among Patients on Hemodialysis. Am. J. Kidney Dis. 2020, 75, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Nagler, E.V.; Webster, A.C.; Vanholder, R.; Zoccali, C. Antidepressants for depression in stage 3–5 chronic kidney disease: A systematic review of pharmacokinetics, efficacy and safety with recommendations by European Renal Best Practice (ERBP). Nephrol. Dial. Transplant. 2012, 27, 3736–3745. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, R.M.; Montgomery, S.A.; Aguglia, E.; Amore, M.; Delgado, P.L.; Gastpar, M.; Hawley, C.; Kasper, S.; Linden, M.; Massana, J.; et al. Partial response and nonresponse to antidepressant therapy: Current approaches and treatment options. J. Clin. Psychiatry. 2002, 63, 826–837. [Google Scholar] [CrossRef]
- Fredman, S.J.; Fava, M.; Kienke, A.S.; White, C.N.; Nierenberg, A.A.; Rosenbaum, J.F. Partial response, nonresponse, and relapse with selective serotonin reuptake inhibitors in major depression: A survey of current “next-step” practices. J. Clin. Psychiatry 2000, 61, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Labbate, L.A.; Fva, M.; Rosenbaum, J.F.; Avana, G.W. Drugs for the treatment of depression. In Handbook of Psychiatric Drug Therapy, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010; p. 54. [Google Scholar]
- Schwenk, M.H.; Verga, M.A.; Wagner, J.D. Hemodialyzability of sertraline. Clin. Nephrol. 1995, 44, 121–124. [Google Scholar] [PubMed]
- Brunton, L.; Chabner, B.; Knollman, B. Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 12th ed.; McGraw Hill Professional: New York, NY, USA, 2010; ISBN 9780071769396. [Google Scholar]
- DeVane, C.L.; Liston, H.L.; Markowitz, J.S. Clinical pharmacokinetics of sertraline. Clin. Pharmacokinet. 2002, 41, 1247–1266. [Google Scholar] [CrossRef]
- Hu, X.H.; Bull, S.A.; Hunkeler, E.M.; Ming, E.; Lee, J.Y.; Fireman, B.; Markson, L.E. Incidence and duration of side effects and those rated as bothersome with selective serotonin reuptake inhibitor treatment for depression: Patient report versus physician estimate. J. Clin. Psychiatry 2004, 65, 959. [Google Scholar] [CrossRef]
- Serebruany, V.L.; Glassman, A.H.; Malinin, A.I.; Nemeroff, C.B.; Musselman, D.L.; van Zyl, L.T.; Finkel, M.S.; Krishnan, K.R.; Gaffney, M.; Harrison, W.; et al. Sertraline AntiDepressant Heart Attack Randomized Trial Study Group. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: The Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation 2003, 108, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Labos, C.; Dasgupta, K.; Nedjar, H.; Turecki, G.; Rahme, E. Risk of bleeding associated with combined use of selective serotonin reuptake inhibitors and antiplatelet therapy following acute myocardial infarction. CMAJ 2011, 183, 1835–1843. [Google Scholar] [CrossRef]
- Halperin, D.; Reber, G. Influence of antidepressants on hemostasis. Dialogues Clin. Neurosci. 2007, 9, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Sienaert, P. Managing the Adverse Effects of Antidepressants. Psychiatr. Times 2014, 31. Available online: https://www.psychiatrictimes.com/view/managing-adverse-effects-antidepressants (accessed on 25 January 2021).
- Stryjer, R.; Spivak, B.; Strous, R.D.; Shiloh, R.; Harary, E.; Polak, L.; Birgen, M.; Kotler, M.; Weizman, A. Trazodone for the treatment of sexual dysfunction induced by serotonin reuptake inhibitors: A preliminary open-label study. Clin. Neuropharmacol. 2009, 32, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.B. The long-term treatment of depression. J. Clin. Psychiatry 1999, 60 (Suppl. S17), 41–45. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T.; Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.C.; Bragg-Gresham, J.; Balkrishnan, R.; Bhave, N.; Dietrich, X.; Ding, Z.; Eggers, P.W.; et al. US renal data system 2018 annual data report: Epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2019, 73 (Suppl. S1), A7–A8. [Google Scholar] [CrossRef] [PubMed]
- Charytan, D.; Kuntz, R.E. The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney Int. 2006, 70, 2021–2030. [Google Scholar] [CrossRef]
- Cuijpers, P.; Vogelzangs, N.; Twisk, J.; Kleiboer, A.; Li, J.; Penninx, B.W. Comprehensive meta- analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am. J. Psychiatry 2014, 171, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Yu, C.; Liu, N.; He, M.; Lv, J.; Guo, Y.; Bian, Z.; Yang, L.; Chen, Y.; Zhang, X.; et al. China Kadoorie Biobank Collaborative Group. Association of Depression with All-Cause and Cardiovascular Disease Mortality Among Adults in China. JAMA Netw Open 2020, 3, e1921043. [Google Scholar] [CrossRef] [PubMed]
- Beach, S.R.; Kostis, W.J.; Celano, C.M.; Januzzi, J.L.; Ruskin, J.N.; Noseworthy, P.A.; Huffman, J.C. Meta-analysis of selective serotonin reuptake inhibitor-associated QTc prolongation. J. Clin. Psychiatry 2014, 75, e441. [Google Scholar] [CrossRef]
- Andrade, C.; Sharma, E. Serotonin Reuptake Inhibitors and Risk of Abnormal Bleeding. Psychiatry Clin. N. Am. 2016, 39, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.; Summers, K.M. Depression in patients with heart failure: Clinical implications and management. Pharmacotherapy 2009, 29, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, G.; Zucchelli, A.; Aloisi, B.; Romanelli, G.; Marengoni, A. Depression and heart failure: An intricate relationship. Monaldi Arch. Chest Dis. 2019, 89. [Google Scholar] [CrossRef] [PubMed]
- Post-Myocardial Infarction Depression Clinical Practice Guideline Panel. AAFP guideline for the detection and management of post-myocardial infarction depression. Ann. Fam. Med. 2009, 7, 71–79. [Google Scholar] [CrossRef]
- Teply, R.M.; Packard, K.A.; White, N.D.; Hilleman, D.E.; DiNicolantonio, J.J. Treatment of Depression in Patients with Concomitant Cardiac Disease. Prog. Cardiovasc. Dis. 2016, 58, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Girardin, F.R.; Gex-Fabry, M.; Berney, P.; Shah, D.; Gaspoz, J.M.; Dayer, P. Drug-induced long QT in adult psychiatric inpatients: The 5-year cross sectional ECG Screening Outcome in Psychiatry study. Am. J. Psychiatry 2013, 170, 1468. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.M.; Clements, C.C.; Murphy, S.N.; Gainer, V.S.; Fava, M.; Weilburg, J.B.; Erb, J.L.; Churchill, S.E.; Kohane, I.S.; Iosifescu, D.V.; et al. QT interval and antidepressant use: A cross sectional study of electronic health records. BMJ 2013, 346, f288. [Google Scholar] [CrossRef] [PubMed]
- Boccardo, P.; Remuzzi, G.; Galbusera, M. Platelet dysfunction in renal failure. Semin. Thromb. Hemost. 2004, 30, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Molnar, A.O.; Bota, S.E.; Garg, A.X.; Harel, Z.; Lam, N.; McArthur, E.; Nesrallah, G.; Perl, J.; Sood, M.M. The Risk of Major Hemorrhage with CKD. J. Am. Soc. Nephrol. 2016, 27, 2825–2832. [Google Scholar] [CrossRef]
- Jain, N.; Li, X.; Adams-Huet, B.; Sarode, R.; Toto, R.D.; Banerjee, S.; Hedayati, S.S. Differences in whole blood platelet aggregation at baseline and in response to aspirin and aspirin plus Clopidogrel in patients with versus without chronic kidney disease. Am. J. Cardiol. 2016, 117, 656–663. [Google Scholar] [CrossRef]
- Pollock, B.G.; Laghrissi-Thode, F.; Wagner, W.R. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. J. Clin. Psychopharmacol. 2000, 20, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Wan, F.; Kothari, M.; Adelodun, A.; Ware, J.; Sarode, R.; Hedayati, S.S. Association of platelet function with depression and its treatment with sertraline in patients with chronic kidney disease: Analysis of a randomized trial. BMC Nephrol. 2019, 20, 395. [Google Scholar] [CrossRef] [PubMed]
- Sands, J.J.; Usvyat, L.A.; Sullivan, T.; Segal, J.H.; Zabetakis, P.; Kotanko, P.; Maddux, F.W.; Diaz-Buxo, J.A. Intradialytic hypotension: Frequency, sources of variation and correlation with clinical outcome. Hemodial. Int. 2014, 18, 415–422. [Google Scholar] [CrossRef]
- Palmer, B.F.; Henrich, W.L. Recent advances in the prevention and management of intradialytic hypotension. J. Am. Soc. Nephrol. 2008, 19, 8–11. [Google Scholar] [CrossRef]
- Diseases, K. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am. J. Kidney Dis. 2005, 45 (Suppl. S3), S1–S53. [Google Scholar] [PubMed]
- Flythe, J.E.; Xue, H.; Lynch, K.E.; Curhan, G.C.; Brunelli, S.M. Association of mortality risk with various definitions of intradialytic hypotension. J. Am. Soc. Nephrol. 2015, 26, 724–734. [Google Scholar] [CrossRef]
- Chou, J.A.; Kalantar-Zadeh, K.; Mathew, A.T. A brief review of intradialytic hypotension with a focus on survival. Semin. Dial. 2017, 30, 473–480. [Google Scholar] [CrossRef]
- Shoji, T.; Tsubakihara, Y.; Fujii, M.; Imai, E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004, 66, 1212–1220. [Google Scholar] [CrossRef]
- Burton, J.O.; Jefferies, H.J.; Selby, N.M.; McIntyre, C.W. Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin. J. Am. Soc. Nephrol. 2009, 4, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Sulowicz, W.; Radziszewski, A. Pathogenesis and treatment of dialysis hypotension. Kidney Int. 2006, 70 (Suppl. S104), S36–S39. [Google Scholar] [CrossRef]
- Grubb, B.P.; Samoil, D.; Kosinski, D.; Kip, K.; Brewster, P. Use of sertraline hydrochloride in the treatment of refractory neurocardiogenic syncope in children and adolescents. J. Am. Coll. Cardiol. 1994, 24, 490–494. [Google Scholar] [CrossRef]
- Georgianos, P.I.; Agarwal, R. Prevention of intradialytic hypotensive episodes: Is setraline an effective pharmacological approach? J. Bras. Nefrol. ’orgao Of. Soc. Bras. Lat.-Am. Nefrologia 2019, 41, 445–447. [Google Scholar] [CrossRef]
- Verduzco, H.A.; Shirazian, S. CKD-Associated Pruritus: New Insights Into Diagnosis, Pathogenesis, and Management. Kidney Int. Rep. 2020, 5, 1387–1402. [Google Scholar] [CrossRef]
- Hayani, K.; Weiss, M.; Weisshaar, E. Clinical Findings and Provision of Care in Haemodialysis Patients with Chronic Itch: New Results from the German Epidemiological Haemodialysis Itch Study. Acta Derm. Venereol. 2016, 96, 361–366. [Google Scholar] [CrossRef]
- Pisoni, R.L.; Wikström, B.; Elder, S.J.; Akizawa, T.; Asano, Y.; Keen, M.L.; Saran, R.; Mendelssohn, D.C.; Young, E.W.; Port, F.K. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol. Dial. Transplant. 2006, 21, 3495–3505. [Google Scholar] [CrossRef] [PubMed]
- Rayner, H.C.; Larkina, M.; Wang, M.; Graham-Brown, M.; van der Veer, S.N.; Ecder, T.; Hasegawa, T.; Kleophas, W.; Bieber, B.A.; Tentori, F.; et al. International Comparisons of Prevalence, Awareness, and Treatment of Pruritus in People on Hemodialysis. Clin. J. Am. Soc. Nephrol. 2017, 12, 2000–2007. [Google Scholar] [CrossRef]
- Mathur, V.S.; Lindberg, J.; Germain, M.; Block, G.; Tumlin, J.; Smith, M.; Grewal, M.; McGuire, D. ITCH National Registry Investigators. A longitudinal study of uremic pruritus in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2010, 5, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Scherer, J.S.; Combs, S.A.; Brennan, F. Sleep Disorders, Restless Legs Syndrome, and Uremic Pruritus: Diagnosis and Treatment of Common Symptoms in Dialysis Patients. Am. J. Kidney Dis. 2017, 69, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Yang, G.; Bai, Y.; Feng, Y.P.; LI, H. The behavioral study on the interactive aggravation between pruritus and depression. Brain Behav. 2018, 8, e00964. [Google Scholar] [CrossRef]
- Weisshaar, E.; Szepietowski, J.C.; Dalgard, F.J.; Garcovich, S.; Gieler, U.; Giménez-Arnau, A.M.; Lambert, J.; Leslie, T.; Mettang, T.; Misery, L.; et al. European S2k Guideline on Chronic Pruritus. Acta Derm. Venereol. 2019, 99, 469–506. [Google Scholar] [CrossRef]
- Kouwenhoven, T.A.; van de Kerkhof, P.C.M.; Kamsteeg, M. Use of oral antidepressants in patients with chronic pruritus: A systematic review. J. Am. Acad. Dermatol. 2017, 77, 1068–1073.e7. [Google Scholar] [CrossRef] [PubMed]
- Zylicz, Z.; Krajnik, M.; Sorge, A.A.; Costantini, M. Paroxetine in the treatment of severe non-dermatological pruritus: A randomized, controlled trial. J. Pain Symptom Manag. 2003, 26, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.P.; Frandsen, J.L.; Walsh, D.; Andresen, S.; Taylor, S. Mirtazapine for pruritus. J. Pain Symptom Manag. 2003, 25, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Gholyaf, M.; Sheikh, V.; Yasrebifar, F.; Mohammadi, Y.; Mirjalili, M.; Mehrpooya, M. Effect of mirtazapine on pruritus in patients on hemodialysis: A cross-over pilot study. Int. Urol. Nephrol. 2020, 52, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Mayo, M.J.; Handem, I.; Saldana, S.; Jacobe, H.; Getachew, Y.; Rush, A.J. Sertraline as a first-line treatment for cholestatic pruritus. Hepatology 2007, 45, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Eusebio-Alpapara, K.M.V.; Castillo, R.L.; Dofitas, B.L. Gabapentin for uremic pruritus: A systematic review of randomized controlled trials. Int. J. Dermatol. 2020, 59, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Fishbane, S.; Jamal, A.; Munera, C.; Wen, W.; Menzaghi, F. KALM-1 Trial Investigators. A Phase 3 Trial of Difelikefalin in Hemodialysis Patients with Pruritus. N. Engl. J. Med. 2020, 382, 222–232. [Google Scholar] [CrossRef] [PubMed]
- den Elzen, W.P.J.; van Manen, J.; Boeschoten, E.W.; Krediet, R.T.; Dekker, F.W. The effect of single and repeatedly high concentrations of C-reactive protein on cardiovascular and non-cardiovascular mortality in patients starting with dialysis. Nephrol. Dial. Transplant. 2006, 21, 1588–1595. [Google Scholar] [CrossRef][Green Version]
- Stenvinkel, P. Inflammation in End-Stage Renal Disease—A Fire that Burns within. Cardiovasc. Disord. Hemodial. 2005, 149, 185–199. [Google Scholar] [CrossRef]
- Zimmermann, J.; Herrlinger, S.; Pruy, A.; Metzger, T.; Wanner, C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999, 55, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Parfrey, P.S.; Sarnak, M.J. Epidemiology of cardiovascular disease in chronic renal disease. J. Am. Soc. Nephrol. JASN 1998, 9 (Suppl. S12), S16–S23. [Google Scholar] [CrossRef]
- Kamińska, J.; Stopiński, M.; Krata, N.; Moszczuk, B.; Foroncewicz, B. Biomarkery uszkodzenia naczyń u pacjentów z przewlekłą chorobą nerek. Forum Nefrol. 2017, 10, 1–9. Available online: https://journals.viamedica.pl/forum_nefrologiczne/article/view/51176 (accessed on 1 September 2021).
- Libetta, C.; Esposito, P.; Martinelli, C.; Grosjean, F.; Gregorini, M.; Rampino, T.; Dal Canton, A. Hepatocyte growth factor (HGF) and hemodialysis: Physiopathology and clinical implications. Clin. Exp. Nephrol. 2016, 20, 371–378. [Google Scholar] [CrossRef]
- Pereira, B.J.G.; Shapiro, L.; King, A.J.; Falagas, M.E.; Strom, J.A.; Dinarello, C.A. Plasma levels of IL-1β, TNFα and their specific inhibitors in undialyzed chronic renal failure, CAPD and hemodialysis patients. Kidney Int. 1994, 45, 890–896. [Google Scholar] [CrossRef]
- Pianta, T.J.; Peake, P.W.; Pickering, J.W.; Kelleher, M.; Buckley, N.A.; Endre, Z.H. Evaluation of biomarkers of cell cycle arrest and inflammation in prediction of dialysis or recovery after kidney transplantation. Transpl. Int. 2015, 28, 1392–1404. [Google Scholar] [CrossRef]
- Taraz, M.; Taraz, S.; Dashti-Khavidaki, S. Association between depression and inflammatory/anti-inflammatory cytokines in chronic kidney disease and end-stage renal disease patients: A review of literature. Hemodial. Int. 2015, 19, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Yirmyia, R.; Noraberg, J.; Brene, S.; Hibbeln, J.; Perini, G.; Kubera, M.; Bob, P.; Lerer, B.; Maj, M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: Leads for future research and new drug developments in depression. Metab. Brain Dis. 2009, 24, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Hocaoglu, C.; Kural, B.; Aliyazıcıoglu, R.; Deger, O.; Cengiz, S. IL-1β, IL-6, IL-8, IL-10, IFN-γ, TNF-α and its relationship with lipid parameters in patients with major depression. Metab. Brain Dis. 2012, 27, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Hasegawa, T.; Takeda, M. Serum level of soluble interleukin 6 receptor is a useful biomarker for identification of treatment-resistant major depressive disorder. Neuropsychopharmacol. Rep. 2020, 40, 130–137. [Google Scholar] [CrossRef]
- Soskin, D.P.; Cassiello, C.; Isacoff, O.; Fava, M. The Inflammatory Hypothesis of Depression. FOCUS 2012, 10, 413–421. [Google Scholar] [CrossRef]
- Kroenke, K.; West, S.L.; Swindle, R.; Gilsenan, A.; Eckert, G.J.; Dolor, R.; Stang, P.; Zhou, X.H.; Hays, R.; Weinberger, M. Similar effectiveness of paroxetine, fluoxetine, and sertraline in primary care: A randomized trial. J. Am. Med. Assoc. 2001, 286, 2947–2955. [Google Scholar] [CrossRef]
- Sanchez, C.; Reines, E.H.; Montgomery, S.A. A comparative review of escitalopram, paroxetine, and sertraline: Are they all alike? Int. Clin. Psychopharmacol. 2014, 29, 185–196. [Google Scholar] [CrossRef]
- Glassman, A.H.; O’Connor, C.M.; Califf, R.M.; Swedberg, K.; Schwartz, P.; Bigger, J.T.; Krishnan, K.R.R.; van Zyl, L.T.; Swenson, J.R.; Finkel, M.S.; et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. J. Am. Med. Assoc. 2002, 288, 701–709. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Jiang, W.; Kuchibhatla, M.; Silva, S.G.; Cuffe, M.S.; Callwood, D.D.; Zakhary, B.; Stough, W.G.; Arias, R.M.; Rivelli, S.K.; et al. Safety and efficacy of sertraline for depression in patients with heart failure: Results of the SADHART-CHF (Sertraline against depression and heart disease in chronic heart failure) trial. J. Am. Coll. Cardiol. 2010, 56, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Farrokhi, F.; Abedi, N.; Beyene, J.; Kurdyak, P.; Jassal, S.V. Association between depression and mortality in patients receiving long-term dialysis: A systematic review and meta-analysis. Am. J. Kidney Dis. 2014, 63, 623–635. [Google Scholar] [CrossRef]
- Friedli, K.; Guirguis, A.; Almond, M.; Day, C.; Chilcot, J.; da Silva-Gane, M.; Davenport, A.; Fineberg, N.A.; Spencer, B.; Wellsted, D.; et al. Sertraline versus placebo in patients with major depressive disorder undergoing hemodialysis: A randomized, controlled feasibility trial. Clin. J. Am. Soc. Nephrol. 2017, 12, 280–286. [Google Scholar] [CrossRef]
- Hedayati, S.S.; Gregg, L.P.; Carmody, T.; Jain, N.; Toups, M.; Rush, A.J.; Toto, R.D.; Trivedi, M.H. Effect of sertraline on depressive symptoms in patients with chronic kidney disease without dialysis dependence: The CAST randomized clinical trial. JAMA J. Am. Med. Assoc. 2017, 318, 1876–1890. [Google Scholar] [CrossRef]
- Weisbord, S.D.; Shields, A.M.; Mor, M.K.; Sevick, M.A.; Homer, M.; Peternel, J.; Porter, P.; Rollman, B.L.; Palevsky, P.M.; Arnold, R.M.; et al. Methodology of a randomized clinical trial of symptom management strategies in patients receiving chronic hemodialysis: The SMILE study. Contemp. Clin. Trials 2010, 31, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Weisbord, S.D.; Mor, M.K.; Green, J.A.; Sevick, M.A.; Shields, A.M.; Zhao, X.; Rollman, B.L.; Palevsky, P.M.; Arnold, R.M.; Fine, M.J. Comparison of symptom management strategies for pain, erectile dysfunction, and depression in patients receiving chronic hemodialysis: A cluster randomized effectiveness trial. Clin. J. Am. Soc. Nephrol. 2013, 8, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Mor, M.K.; Sevick, M.A.; Shields, A.M.; Green, J.A.; Palevsky, P.M.; Arnold, R.M.; Fine, M.J.; Weisbord, S.D. Sexual function, activity, and satisfaction among women receiving maintenance hemodialysis. Clin. J. Am. Soc. Nephrol. 2014, 9, 128–134. [Google Scholar] [CrossRef]
- Montejo-Gonzalez, A.L.; Liorca, G.; Izquierdo, J.A.; Ledesma, A.; Bousono, M.; Calcedo, A.; Carrasco, J.L.; Ciudad, J.; Daniel, E.; de la Gandara, J.; et al. SSRI-induced sexual dysfunction: Fluoxetine, paroxetine, sertraline, and fluvoxamine in a prospective, multicenter, and descriptive clinical study of 344 patients. J. Sex Marital. Ther. 1997, 23, 176–194. [Google Scholar] [CrossRef]
- Navaneethan, S.D.; Vecchio, M.; Johnson, D.W.; Saglimbene, V.; Graziano, G.; Pellegrini, F.; Lucisano, G.; Craig, J.C.; Ruospo, M.; Gentile, G.; et al. Prevalence and correlates of self-reported sexual dysfunction in CKD: A meta-analysis of observational studies. Am. J. Kidney Dis. 2010, 56, 670–685. [Google Scholar] [CrossRef]
- Anantharaman, P.; Schmidt, R.J. Sexual Function in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2007, 14, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. Gonadal dysfunction in chronic kidney disease. Rev. Endocr. Metab. Disord. 2017, 18, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F. Sexual Dysfunction in Uremia. J. Am. Soc. Nephrol. 1999, 10, 1381–1388. Available online: https://jasn.asnjournals.org/content/10/6/1381 (accessed on 1 September 2021). [CrossRef]
- Pizzol, D.; Xiao, T.; Yang, L.; Demurtas, J.; McDermott, D.; Garolla, A.; Nardelotto, A.; Grabovac, I.; Soysal, P.; Kazancioglu, R.T.; et al. Prevalence of erectile dysfunction in patients with chronic kidney disease: A systematic review and meta-analysis. Int. J. Impot. Res. 2020, 33, 508–515. [Google Scholar] [CrossRef]
- Atlantis, E.; Sullivan, T. Bidirectional association between depression and sexual dysfunction: A systematic review and meta-analysis. J. Sex. Med. 2012, 9, 1497–1507. [Google Scholar] [CrossRef]
- Kennedy, S.H.; Rizvi, S. Sexual dysfunction, depression, and the impact of antidepressants. J. Clin. Psychopharmacol. 2009, 29, 157–164. [Google Scholar] [CrossRef]
- Theofilou, P.A. Sexual functioning in chronic kidney disease: The association with depression and anxiety. Hemodial. Int. 2012, 16, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, P.L.; Mahableshwarkar, A.R.; Chen, Y.; Chrones, L.; Clayton, A.H. Effect of Vortioxetine vs. Escitalopram on Sexual Functioning in Adults with Well-Treated Major Depressive Disorder Experiencing SSRI-Induced Sexual Dysfunction. J. Sex. Med. 2015, 12, 2036–2048. [Google Scholar] [CrossRef] [PubMed]
- Safarinejad, M.R. Reversal of SSRI-induced female sexual dysfunction by adjunctive bupropion in menstruating women: A double-blind, placebo-controlled and randomized study. J. Psychopharmacol. 2011, 25, 370–378. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Sharma, M.S.; Brunoni, A.R.; Vieta, E.; Fava, G.A. The Safety, Tolerability and Risks Associated with the Use of Newer Generation Antidepressant Drugs: A Critical Review of the Literature. Psychother Psychosom. 2016, 85, 270–288. [Google Scholar] [CrossRef] [PubMed]
- Jing, E.; Straw-Wilson, K. Sexual dysfunction in selective serotonin reuptake inhibitors (SSRIs) and potential solutions: A narrative literature review. Ment. Health Clin. 2016, 6, 191–196. [Google Scholar] [CrossRef]
- Chokka, P.R.; Hankey, J.R. Assessment and management of sexual dysfunction in the context of depression. Ther. Adv. Psychopharmacol. 2018, 8, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Jasiak, N.M.; Bostwick, J.R. Risk of QT/QTc Prolongation Among Newer Non-SSRI Antidepressants. Ann. Pharmacother. 2014, 48, 1620–1628. [Google Scholar] [CrossRef]
- Nair, S.S.; Mitani, A.A.; Goldstein, B.A.; Chertow, G.M.; Lowenberg, D.W.; Winkelmayer, W.C. Temporal trends in the incidence, treatment, and outcomes of hip fracture in older patients initiating dialysis in the United States. Clin. J. Am. Soc. Nephrol. 2013, 8, 1336–1342. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arneson, T.J.; Li, S.; Liu, J.; Kilpatrick, R.D.; Newsome, B.B.; St Peter, W.L. Trends in hip fracture rates in US hemodialysis patients, 1993-2010. Am. J. Kidney Dis. 2013, 62, 747–754. [Google Scholar] [CrossRef]
- Erken, E.; Ozelsancak, R.; Sahin, S.; Yılmaz, E.E.; Torun, D.; Leblebici, B.; Kuyucu, Y.E.; Sezer, S. The effect of hemodialysis on balance measurements and risk of fall. Int. Urol. Nephrol. 2016, 48, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Desmet, C.; Beguin, C.; Swine, C.; Jadoul, M.; Université Catholique de Louvain Collaborative Group. Falls in hemodialysis patients: Prospective study of incidence, risk factors, and complications. Am. J. Kidney Dis. 2005, 45, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Briggs, R.; Kennelly, S.P.; Kenny, R.A. Does baseline depression increase the risk of unexplained and accidental falls in a cohort of community-dwelling older people? Data from The Irish Longitudinal Study on Ageing (TILDA). Int. J. Geriatr. Psychiatry 2018, 33, e205–e211. [Google Scholar] [CrossRef]
- Deandrea, S.; Bravi, F.; Turati, F.; Lucenteforte, E.; La Vecchia, C.; Negri, E. Risk factors for falls in older people in nursing homes and hospitals. A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2013, 56, 407–415. [Google Scholar] [CrossRef]
- Lam, K.; Lee, D.-C.A.; Lalor, A.F.; Stolwyk, R.; Russell, G.; Brown, T.; McDermott, F.; Haines, T.P. The relationship between discharge medications and falls in post-hospitalised older adults: A 6-month follow-up. Australas. J. Ageing 2019, 38, 190–198. [Google Scholar] [CrossRef]
- Lee, D.-C.A.; Lalor, A.F.; Russell, G.; Stolwyk, R.; Brown, T.; McDermott, F.; Haines, T.P. Understanding temporal relationships between depression, falls, and physical activity in a cohort of post-hospitalized older adults—A breakthrough or a conundrum? Int. Psychogeriatr. 2017, 29, 1681–1692. [Google Scholar] [CrossRef]
- Stubbs, B. Falls in older adult psychiatric patients: Equipping nurses with knowledge to make a difference. J. Psychiatr. Ment. Health Nurs. 2011, 18, 457–462. [Google Scholar] [CrossRef]
- Kistler, B.M.; Khubchandani, J.; Bennett, P.; Wilund, K.R.; Sosnoff, J. Depressive Disorders Are Associated with Risk of Falls in People with Chronic Kidney Disease. J. Am. Psychiatr. Nurses Assoc. 2020. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kistler, B.M.; Khubchandani, J.; Wiblishauser, M.; Wilund, K.R.; Sosnoff, J.J. Epidemiology of falls and fall-related injuries among middle-aged adults with kidney disease. Int. Urol. Nephrol. 2019, 51, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Kistler, B.M.; Khubchandani, J.; Jakubowicz, G.; Wilund, K.; Sosnoff, J. Falls and Fall-Related Injuries Among US Adults Aged 65 or Older with Chronic Kidney Disease. Prev. Chronic Dis. 2018, 15, E82. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Thomas, F.; Nagy, K.; Arogundade, F.; Avesani, C.M.; Chan, M.; Chmielewski, M.; Cordeiro, A.C.; Espinosa-Cuevas, A.; Fiaccadori, E.; et al. Global Prevalence of Protein-Energy Wasting in Kidney Disease: A Meta-analysis of Contemporary Observational Studies from the International Society of Renal Nutrition and Metabolism. J. Ren. Nutr. 2018, 28, 380–392. [Google Scholar] [CrossRef] [PubMed]
- López-Soto, P.J.; De Giorgi, A.; Senno, E.; Tiseo, R.; Ferraresi, A.; Canella, C.; Rodríguez-Borrego, M.A.; Manfredini, R.; Fabbian, F. Renal disease and accidental falls: A review of published evidence. BMC Nephrol. 2015, 16, 176. [Google Scholar] [CrossRef]
- Moe, S.M.; Nickolas, T.L. Fractures in Patients with CKD: Time for Action. Clin. J. Am. Soc. Nephrol. CJASN 2016, 11, 1929–1931. [Google Scholar] [CrossRef]
- Moe, S.M.; Radcliffe, J.S.; White, K.E.; Gattone, V.H., 2nd; Seifert, M.F.; Chen, X.; Aldridge, B.; Chen, N.X. The pathophysiology of early-stage chronic kidney disease-mineral bone disorder (CKD-MBD) and response to phosphate binders in the rat. J. Bone Miner Res. 2011, 26, 2672–2681. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Hodge, J.M.; Pasco, J.A.; Berk, M.; Williams, L.J. Effects of Depression and Serotonergic Antidepressants on Bone: Mechanisms and Implications for the Treatment of Depression. Drugs Aging 2016, 33, 21–25. [Google Scholar] [CrossRef] [PubMed]
| Subject of Comparison | ICD-10 Classification | DSM-5 Classification |
|---|---|---|
| Nomenclature | Depressive Episode [18] | Major depressive disorder (MDD) [19] |
| Main symptoms |
|
|
| Additional symptoms |
|
|
| Diagnostic criteria | At least two main symptoms and additional symptoms in a total number of at least four [18] | At least one main symptom and additional symptoms in a total number of at least |
| Duration of symptoms | At least two weeks [18] | At least two weeks [19] |
| Severity of symptoms | Clinical differentiation:
| The symptoms ought to cause significant impairment in social, occupational or another important area of functioning [19] |
| Exclusion criteria |
|
|
| SSRI | SNRI | SARI | SNRISA | SMS | TCA |
|---|---|---|---|---|---|
| Citalopram | Desvenlafaxine | Nefazodone | Amoxapine | Vilazodone | Amitriptyline |
| Escitalopram | Duloxetine | Trazodone | Vortioxetine | Clomipramine | |
| Fluoxetine | Levomilnacipram | Desipramine | |||
| Fluvoxamine | Milnacipran | Doxepin | |||
| Paroxetine | Venlafaxine | Imipramine | |||
| Sertraline | Nortriptyline | ||||
| Protriptyline | |||||
| Trimipramine |
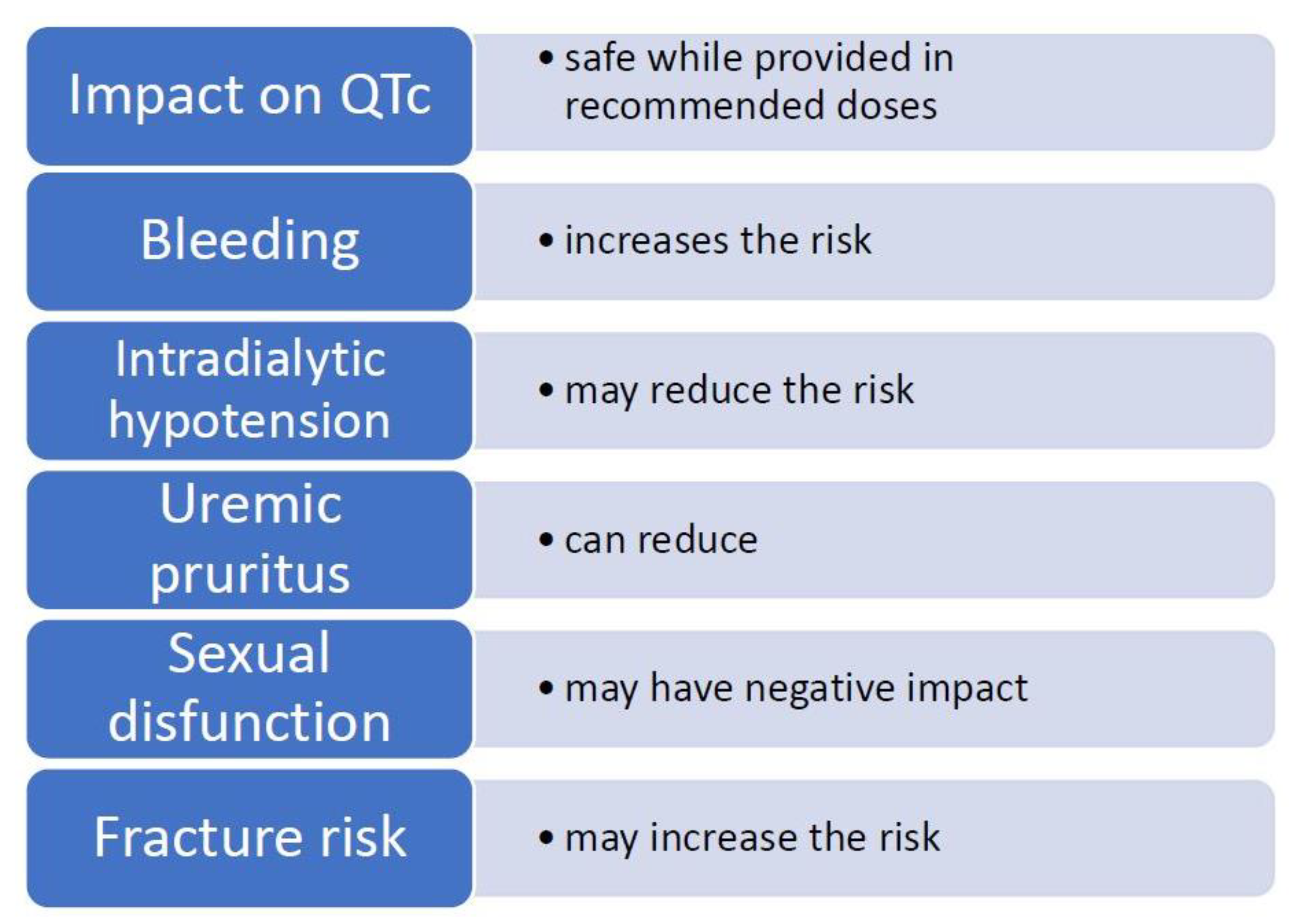
| Impact on QTc Prolongation | Safe While Provided in Recommended Doses [20] |
|---|---|
| Bleeding | Increases the risk of bleeding [21,22] |
| Platelet reactivity | May reduce platelet activation [23,24,25] |
| Intradialytic hypotension (IDH) | Inconsistent study results, may reduce the risk of IDH [26,27,28,29,30] |
| Chronic kidney disease-associated pruritus (CKD-aP) | Can reduce pruritus in cases caused by CKD [31,32,33] |
| Cytokines | Reduces the concentration of pro-inflammatory and increases the levels of anti-inflammatory cytokines, insufficient data in HD population [34,35,36] |
| Sexual disfunction (SD) | Negative impact on SD in general population [37,38] and in HD population [39] |
| Fracture risk and osteoporosis | Is associated with hip fracture risk and may decrease bone mass in general population [40,41], may increase fracture risk in ESRD population [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubanek, A.; Paul, P.; Przybylak, M.; Kanclerz, K.; Rojek, J.J.; Renke, M.; Bidzan, L.; Grabowski, J. Use of Sertraline in Hemodialysis Patients. Medicina 2021, 57, 949. https://doi.org/10.3390/medicina57090949
Kubanek A, Paul P, Przybylak M, Kanclerz K, Rojek JJ, Renke M, Bidzan L, Grabowski J. Use of Sertraline in Hemodialysis Patients. Medicina. 2021; 57(9):949. https://doi.org/10.3390/medicina57090949
Chicago/Turabian StyleKubanek, Alicja, Przemysław Paul, Mateusz Przybylak, Katarzyna Kanclerz, Jakub Jan Rojek, Marcin Renke, Leszek Bidzan, and Jakub Grabowski. 2021. "Use of Sertraline in Hemodialysis Patients" Medicina 57, no. 9: 949. https://doi.org/10.3390/medicina57090949
APA StyleKubanek, A., Paul, P., Przybylak, M., Kanclerz, K., Rojek, J. J., Renke, M., Bidzan, L., & Grabowski, J. (2021). Use of Sertraline in Hemodialysis Patients. Medicina, 57(9), 949. https://doi.org/10.3390/medicina57090949






