Impact of Periodontal Attachment Loss on the Outcome of Endodontic Microsurgery: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
- Population: teeth with periodontal attachment loss.
- Exposure: EMS.
- Outcome: clinical and radiographic success.
2.1. Searching Criteria
2.2. Searching Method
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
2.6. Meta-Analysis
3. Results
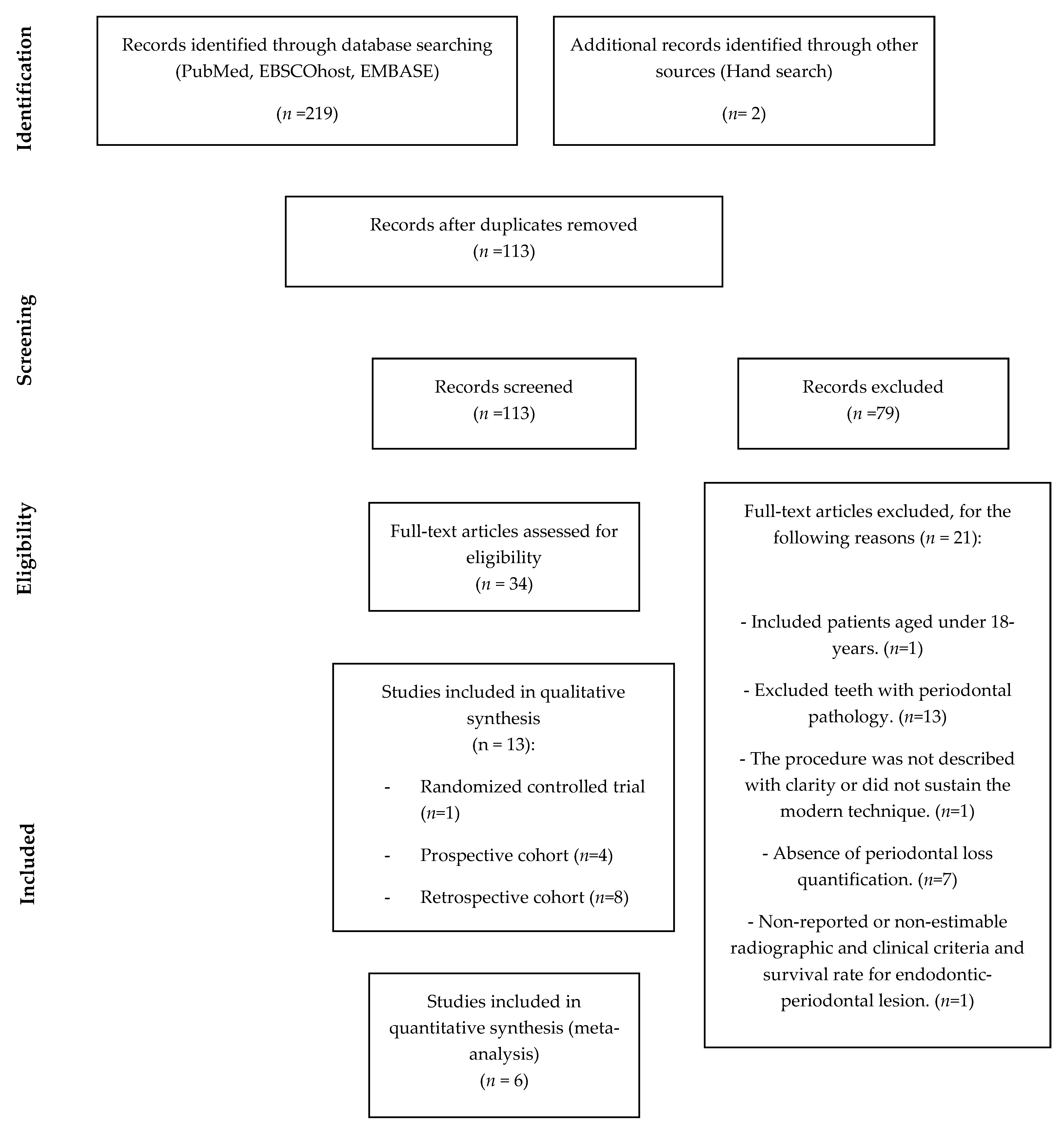
3.1. Study Selection
3.2. Study Characteristics
3.3. Quality Assessment
3.4. Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barone, C.; Dao, T.T.; Basrani, B.B.; Wang, N.; Friedman, S. Treatment outcome in endodontics: The Toronto study-phases 3, 4, and 5: Apical surgery. J. Endod. 2010, 36, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Jakovljevic, A.; Nikolic, N.; Jacimovic, J.; Pavlovic, O.; Milicic, B.; Beljic-Ivanovic, K.; Miletic, M.; Andric, M.; Milasin, J. Prevalence of apical periodontitis and conventional nonsurgical root canal treatment in general adult population: An updated systematic review and meta-analysis of cross-sectional studies published between 2012 and 2020. J. Endod. 2021, 46, 1371–1386. [Google Scholar] [CrossRef]
- Diogo, P.; Palma, P.; Caramelo, F.; Marques dos Santos, J.M. Estudo da prevalência de periodontite apical numa população adulta portuguesa. Rev. Port. Estomat. Med. Dentária Cir. Maxilofac. 2014, 55, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Ran, S.J.; Yang, X.; Sun, Z.; Zhang, Y.; Chen, J.X.; Wang, D.M.; Liu, B. Effect of length of apical root resection on the biomechanical response of a maxillary central incisor in various occlusal relationships. Int. Endod. J. 2020, 53, 111–121. [Google Scholar] [CrossRef]
- Siqueira, J.F. Aetiology of root canal treatment failure: Why well-treated teeth can fail. Int. Endod. J. 2001, 34, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chércoles-Ruiz, A.; Sánchez-Torres, A.; Gay-Escoda, C. Endodontics, endodontic retreatment, and apical surgery versus tooth extraction and implant placement: A systematic review. J Endod. 2017, 43, 679–686. [Google Scholar] [CrossRef]
- Kim, E.; Kim, Y. Endodontic microsurgery: Outcomes and prognostic factors. Curr. Oral Health Rep. 2019, 6, 356–366. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, Q.; Tan, X.; Song, D.; Zhang, L.; Huang, D. Comparison of mineral trioxide aggregate and iroot bp plus root repair material as root-end filling materials in endodontic microsurgery: A prospective randomized controlled study. J. Endod. 2017, 43, 1–6. [Google Scholar] [CrossRef]
- Song, M.; Chung, W.; Lee, S.J.; Kim, E. Long-term outcome of the cases classified as successes based on short-term follow-up in endodontic microsurgery. J. Endod. 2012, 38, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.; Khin, M.; Krishnaswamy, G.; Chen, N. Prognostic factors relating to the outcome of endodontic. J. Endod. 2014, 40, 1071–1076. [Google Scholar] [CrossRef]
- Song, M.; Kang, M.; Kang, D.R.; Jung, H.I.; Kim, E. Comparison of the effect of endodontic-periodontal combined lesion on the outcome of endodontic microsurgery with that of isolated endodontic lesion: Survival analysis using propensity score analysis. Clin. Oral Invest. 2018, 22, 1717–1724. [Google Scholar] [CrossRef]
- Kim, S.; Kratchman, S. Modern endodontic surgery concepts and practice: A review. J. Endod. 2006, 32, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Kim, S.G.; Lee, S.; Kim, B.; Kim, E. Prognostic factors of clinical outcomes in endodontic microsurgery: A prospective study. J. Endod. 2013, 39, 1491–1497. [Google Scholar] [CrossRef]
- von Arx, T.; Jensen, S.S.; Hänni, S. Clinical and radiographic assessment of various predictors for healing outcome 1 year after periapical surgery. J. Endod. 2007, 33, 123–128. [Google Scholar] [CrossRef]
- Kim, E.; Song, J.S.; Jung, I.Y.; Lee, S.J.; Kim, S. Prospective clinical study evaluating endodontic microsurgery outcomes for cases with lesions of endodontic origin compared with cases with lesions of combined periodontal-endodontic origin. J. Endod. 2008, 34, 546–551. [Google Scholar] [CrossRef]
- Palma, P.J.; Marques, J.A.; Casau, M.; Santos, A.; Caramelo, F.; Falacho, R.I.; Santos, J.M. Evaluation of root-end preparation with two different endodontic microsurgery ultrasonic tips. Biomedicines 2020, 8, 383. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.; Kim, D.; Perinpanayagam, H.; Baek, S.; Zhu, Q.; Safavi, K.; Kum, K. Prognostic factors of long-term outcomes in endodontic microsurgery: A retrospective cohort study over five years. J. Clin. Med. 2020, 9, 2210. [Google Scholar] [CrossRef]
- Setzer, F.C.; Shah, S.B.; Kohli, M.R.; Karabucak, B.; Kim, S. Outcome of endodontic surgery: A meta-analysis of the literature—part 1: Comparison of traditional root-end surgery and endodontic microsurgery. J. Endod. 2010, 36, 1757–1765. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S162–S170. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef] [Green Version]
- European Federation of Periodontology. Dossier on Periodontal Disease 2020 (Periodontal Health for a Better Life), Madrid, Spain. Available online: https://www.efp.org/fileadmin/uploads/efp/Documents/Campaigns/Gum_health_day/Publications/EFP_Dossier_on_Periodontal_Disease_2020.pdf (accessed on 3 May 2021).
- Herrera, D.; Retamal-Valdes, B.; Alonso, B.; Feres, M. Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo-periodontal lesions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S78–S94. [Google Scholar] [CrossRef] [Green Version]
- Meng, H.X. Periodontic-endodontic lesions. Ann. Periodontol. 1999, 4, 84–90. [Google Scholar] [CrossRef]
- Jang, Y.; Hong, H.T.; Chun, H.J.; Roh, B.D. Influence of apical root resection on the biomechanical response of a single-rooted tooth—part 2: Apical root resection combined with periodontal bone loss. J. Endod. 2015, 41, 412–416. [Google Scholar] [CrossRef]
- Jang, Y.; Hong, H.T.; Roh, B.D.; Chun, H.J. Influence of apical root resection on the biomechanical response of a single-rooted tooth: A 3-dimensional finite element analysis. J. Endod. 2014, 40, 1489–1493. [Google Scholar] [CrossRef]
- Song, M.; Jung, I.Y.; Lee, S.J.; Lee, C.Y.; Kim, E. Prognostic factors for clinical outcomes in endodontic microsurgery: A retrospective study. J. Endod. 2011, 37, 927–933. [Google Scholar] [CrossRef]
- Pinto, D.; Marques, A.; Pereira, J.F.; Palma, P.J.; Santos, J.M. Long-Term Prognosis of Endodontic Microsurgery-A Systematic Review and Meta-Analysis. Medicina 2020, 56, 447. [Google Scholar] [CrossRef]
- Rud, J.; Andreasen, J.O.; Jensen, J.E. Radiographic criteria for the assessment of healing after endodontic surgery. Int. J. Oral Surg. 1972, 1, 195–214. [Google Scholar] [CrossRef]
- Molven, O.; Halse, A.; Grung, B. Observer strategy and the radiographic classification of healing after endodontic surgery. Int. J. Oral Surg. 1987, 16, 432–439. [Google Scholar] [CrossRef]
- Azim, A.A.; Albanyan, H.; Azim, K.A.; Piasecki, L. The Buffalo study: Outcome and associated predictors in endodontic microsurgery- a cohort study. Int. Endod. J. 2021, 54, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Zhang, M.M.; Wang, J.; Jiang, L.; Liang, Y.H. Outcomes of endodontic microsurgery using a microscope and mineral trioxide aggregate: A prospective cohort study. J. Endod. 2017, 43, 694–698. [Google Scholar] [CrossRef]
- Kim, S.; Song, M.; Shin, S.J.; Kim, E. A randomized controlled study of mineral trioxide aggregate and super ethoxybenzoic acid as root-end filling materials in endodontic microsurgery: Long-term outcomes. J. Endod. 2016, 42, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ku, H.; Nam, T.; Yoon, T.C.; Lee, C.Y.; Kim, E. Influence of size and volume of periapical lesions on the outcome of endodontic microsurgery: 3-dimensional analysis using cone-beam computed tomography. J. Endod. 2016, 42, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Nam, T.; Shin, S.J.; Kim, E. Comparison of clinical outcomes of endodontic microsurgery: 1 year versus long-term follow-up. J. Endod. 2014, 40, 490–494. [Google Scholar] [CrossRef]
- Shinbori, N.; Grama, A.M.; Patel, Y.; Woodmansey, K.; He, J. Clinical outcome of endodontic microsurgery that uses endosequence bc root repair material as the root-end filling material. J. Endod. 2015, 41, 607–612. [Google Scholar] [CrossRef]
- Song, M.; Kim, E. A prospective randomized controlled study of mineral trioxide aggregate and super ethoxy-benzoic acid as root-end filling materials in endodontic microsurgery. J. Endod. 2012, 38, 875–879. [Google Scholar] [CrossRef]
- Albanyan, H.; Aksel, H.; Azim, A.A. Soft and hard tissue remodeling after endodontic microsurgery: A cohort study. J. Endod. 2020, 46, 1824–1831. [Google Scholar] [CrossRef]
- Taha, N.A.; Aboyounes, F.B.; Tamimi, Z.Z. Root-end microsurgery using a premixed tricalcium silicate putty as root-end filling material: A prospective study. Clin. Oral Investig. 2021, 25, 311–317. [Google Scholar] [CrossRef]
- Chan, S.; Glickman, G.N.; Woodmansey, K.F.; He, J. Retrospective analysis of root-end microsurgery outcomes in a postgraduate program in endodontics using calcium silicate– based cements as root-end filling materials. J. Endod. 2020, 46, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Karan, N.B.; Aricioğlu, B. Assessment of bone healing after mineral trioxide aggregate and platelet-rich fibrin application in periapical lesions using cone-beam computed tomographic imaging. Clin. Oral Investig. 2020, 24, 1065–1072. [Google Scholar] [CrossRef]
- Tawil, P.Z.; Saraiya, V.M.; Galicia, J.C.; Duggan, D.J. Periapical microsurgery: The effect of root dentinal defects on short- and long-term outcome. J. Endod. 2015, 41, 22–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taschieri, S.; del Fabbro, M. Endoscopic endodontic microsurgery: 2-year evaluation of healing and functionality. Braz. Oral Res. 2009, 23, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Shin, S.J.; Kim, E. Outcomes of endodontic micro-resurgery: A prospective clinical study. J. Endod. 2011, 37, 316–320. [Google Scholar] [CrossRef]
- Schloss, T.; Sonntag, D.; Kohli, M.R.; Setzer, F.C. A comparison of 2- and 3-dimensional healing assessment after endodontic surgery using cone-beam computed tomographic volumes or periapical radiographs. J. Endod. 2017, 43, 1072–1079. [Google Scholar] [CrossRef]
- Li, H.; Zhai, F.; Zhang, R.; Hou, B. Evaluation of microsurgery with supereba as root-end filling material for treating post-treatment endodontic disease: A 2-year retrospective study. J. Endod. 2014, 40, 345–350. [Google Scholar] [CrossRef]
- Pallarés-Serrano, A.; Glera-Suarez, P.; Tarazona-Alvarez, B.; Peñarrocha-Diago, M.; Peñarrocha-Diago, M.; Peñarrocha-Oltra, D. Prognostic factors after endodontic microsurgery: A retrospective study of 111 cases with 5 to 9 years of follow-up. J. Endod. 2021, 47, 397–403. [Google Scholar] [CrossRef]
- Kang, S.; Ha, S.W.; Kim, U.; Kim, S.; Kim, E. A one-year radiographic healing assessment after endodontic microsurgery using cone-beam computed tomographic scans. J. Clin. Med. 2020, 11, 3714. [Google Scholar] [CrossRef]
- Safi, C.; Kohli, M.R.; Kratchman, S.I.; Setzer, F.C.; Karabucak, B. Outcome of endodontic microsurgery using mineral trioxide aggregate or root repair material as root-end filling material: A randomized controlled trial with cone-beam computed tomographic evaluation. J. Endod. 2019, 45, 831–839. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, H.; Gao, J.; Du, X.; Chen, Y.; Han, L. Short-term observation of clinical and radiographic results of periapical microsurgery: A prospective study. Biomed. Res. 2016, 27, 923–928. [Google Scholar]
- Kim, D.; Kim, S.; Song, M.; Kang, D.R.; Kohli, M.R.; Kim, E. Outcome of endodontic micro-resurgery: A retrospective study based on propensity score–matched survival analysis. J. Endod. 2018, 44, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Kim, S.G.; Shin, S.J.; Kim, H.C.; Kim, E. The influence of bone tissue deficiency on the outcome of endodontic microsurgery: A prospective study. J. Endod. 2013, 39, 1341–1345. [Google Scholar] [CrossRef]
- Huang, S.; Chen, N.; Yu, V.S.H.; Lim, H.A.; Lui, J.N. Long-term success and survival of endodontic microsurgery. J. Endod. 2020, 46, 149–157. [Google Scholar] [CrossRef]
- von Arx, T.; Jensen, S.S.; Hänni, S.; Friedman, S. Five-year longitudinal assessment of the prognosis of apical microsurgery. J. Endod. 2012, 38, 570–579. [Google Scholar] [CrossRef]
- Kim, D.; Lee, H.; Chung, M.; Kim, S.; Song, M.; Kim, E. Effects of fast- and slow-setting calcium silicate—based root-end filling materials on the outcome of endodontic microsurgery: A retrospective study up to 6 years. Clin. Oral Investig. 2020, 24, 247–255. [Google Scholar] [CrossRef]
- Kvist, T.; Reit, C. Results of endodontic retreatment: A randomized clinical study comparing surgical and nonsurgical procedures. J. Endod. 1999, 25, 814–817. [Google Scholar] [CrossRef]
- Nowzari, H.; MacDonald, E.S.; Flynn, J.; London, R.M.; Morrison, J.L.; Slots, J. The dynamics of microbial colonization of barrier membranes for guided tissue regeneration. J. Periodontol. 1996, 67, 694–702. [Google Scholar] [CrossRef]
- von Arx, T.; Hänni, S.; Jensen, S.S. Correlation of bone defect dimensions with healing outcome one year after apical surgery. J. Endod. 2007, 33, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S. Outcome of endodontic surgery: A meta-analysis of the literature—Part 1: Comparison of traditional root-end surgery and endodontic microsurgery. J. Endod. 2011, 37, 577–578. [Google Scholar] [CrossRef]

| Inclusion criteria | 1. Clinical studies in humans |
| 2. Randomized clinical trials (RCT) | |
| 3. Prospective clinical studies | |
| 4. Retrospective clinical studies | |
| 5. Teeth with indication for EMS (periapical lesion, post-treatment apical periodontitis, extrusion of root canal filling material resulting from primary endodontic treatment, persistent extra-radicular infection) | |
| 6. Studies in which the surgical procedure was detailed or sustained the modern technique by using magnification devices (microscope and endoscope) and ultrasonic root-end preparation | |
| 7. Clinical and radiographic success following Rud and Molven’s criteria [28,29] | |
| 8. Reported or estimable clinical and radiographic success rate for both isolated endodontic and endodontic-periodontal groups | |
| 9. Minimum follow-up period of one year | |
| 10. Quantified periodontal attachment loss | |
| Exclusion criteria | 1. Patients aged under 18 years |
| 2. Exclusion of teeth with periodontal attachment loss | |
| 3. Systematic review | |
| 4. Case series | |
| 5. Case report | |
| 6. The surgical procedure was not detailed or did not sustain the modern technique | |
| 7. Unclear clinical and radiographic success criteria | |
| 8. Non-reported or non-estimable endodontic-periodontal lesion success rate | |
| 9. < One-year follow-up | |
| 10. Absence of periodontal attachment loss quantification |
| Study | Study Design | Sample Size (Teeth) | Diagnostic Criteria of Periodontal Attachment Loss | Root Filling Material | Follow-Up Period (Years) | Recall Rate (%) | Success Rate (%) | Regeneration | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n Initial | n Final | n EP | Endo-Group | EP-Group | Yes/No | Material | ||||||
| Zhou et al., 2017 [8] | Randomized Controlled Trial | 240 | 158 | 17 | Alveolar dehiscence | ProRoot MTA BP-RRM | 1 | 65.8 | 94.3 | 88.2 | Yes | Resorbable collagen membrane |
| von Arx et al., 2007 [14] | Prospective Cohort | 194 | 191 | 43 | Marginal bone level >3 mm | SuperEBA ProRoot MTA Retroplast | 1 | 98.5 | 83.1 | 86.1 | No | - |
| Kim E et al., 2008 [15] | Prospective Cohort | 263 | 192 | 40 | Kim and Kratchman | IRM SuperEBA ProRoot MTA | 2 | 73.0 | 95.3 | 77.5 | Yes | Calcium sulfate + resorbable collagen membrane (CollaTape) |
| Song et al., 2013 [51] | Prospective Cohort | 199 | 135 | 33 | Marginal bone loss >3 mm | SuperEBA ProRoot MTA | 1–7 | 67.8 | 89.3 | 87.9 | No | - |
| Song et al., 2013 [13] | Prospective Cohort | 584 | 431 | 87 | Kim and Kratchman | IRM SuperEBA ProRoot MTA | 1–10 | 73.8 | 88.4 | 74.7 | No | - |
| Song et al., 2011 [26] | Retrospective cohort | 907 | 491 | 50 | Kim and Kratchman | IRM SuperEBA ProRoot MTA | ≥1 | 54.1 | 84.8 | 70.0 | No | - |
| von Arx et al., 2012 [53] | Retrospective cohort | 194 | 170 | 37 | Crestal bone level >3mm | SuperEBA ProRoot MTA Retroplast | 5 | 87.6 | 78.2 | 67.6 | No | - |
| Song et al., 2012 [9] | Retrospective cohort | 172 | 104 | 23 | Kim and Kratchman | IRM SuperEBA ProRoot MTA | 6–10 | 60.5 | 92.6 | 95.7 | Yes | Resorbable collagen membrane (CollaTape) |
| Lui et al., 2014 [10] | Retrospective cohort | 243 | 93 | 14 | PD > 3 mm | IRM MTA | 1–2 | 38 | 95.2 | 73.0 | Yes | Resorbable collagen membrane (BioMend) + bone substitute (BioOss) |
| Song et al., 2018 [11] | Retrospective cohort | 249 | 249 | 83 | Kim and Kratchman | IRM SuperEBA ProRoot MTA | 1 | 100 | 87.3 | 72.3 | No | - |
| Kim D et al., 2020 [54] | Retrospective cohort | 244 | 244 | 56 | Kim and Kratchman | SCSM group: gray or white ProRoot MTA; FCSM group: RetroMTA or EndoCem MTA | 1–6 | 100 | 94.7 | 71.4 | No | - |
| Huang et al., 2020 [52] | Retrospective cohort | 191 | 92 95 * | 4 * | Preoperative PD >3 mm | IRM ProRoot MTA | 5–9 | 48.2 | 80.8 | 50.0 | Yes | Resorbable collagen membrane |
| Yoo et al., 2020 [17] | Retrospective cohort | 652 | 225 | 9 | Periodontal involvement | ProRoot MTA | 5 | 34.5 | 82.4 | 33.3 | Yes | BioOss |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarnadas, M.; Marques, J.A.; Baptista, I.P.; Santos, J.M. Impact of Periodontal Attachment Loss on the Outcome of Endodontic Microsurgery: A Systematic Review and Meta-Analysis. Medicina 2021, 57, 922. https://doi.org/10.3390/medicina57090922
Sarnadas M, Marques JA, Baptista IP, Santos JM. Impact of Periodontal Attachment Loss on the Outcome of Endodontic Microsurgery: A Systematic Review and Meta-Analysis. Medicina. 2021; 57(9):922. https://doi.org/10.3390/medicina57090922
Chicago/Turabian StyleSarnadas, Margarida, Joana A. Marques, Isabel Poiares Baptista, and João Miguel Santos. 2021. "Impact of Periodontal Attachment Loss on the Outcome of Endodontic Microsurgery: A Systematic Review and Meta-Analysis" Medicina 57, no. 9: 922. https://doi.org/10.3390/medicina57090922
APA StyleSarnadas, M., Marques, J. A., Baptista, I. P., & Santos, J. M. (2021). Impact of Periodontal Attachment Loss on the Outcome of Endodontic Microsurgery: A Systematic Review and Meta-Analysis. Medicina, 57(9), 922. https://doi.org/10.3390/medicina57090922









