Effects of Pressure Control Device (SensAwake™) on Obstructive Sleep Apnea (OSA) Patients Who Remove the Mask for Unknown Reasons during Automatic Continuous Positive Airway Pressure (Auto-CPAP) Therapy: A Prospective Randomized Crossover Trial
Abstract
1. Introduction
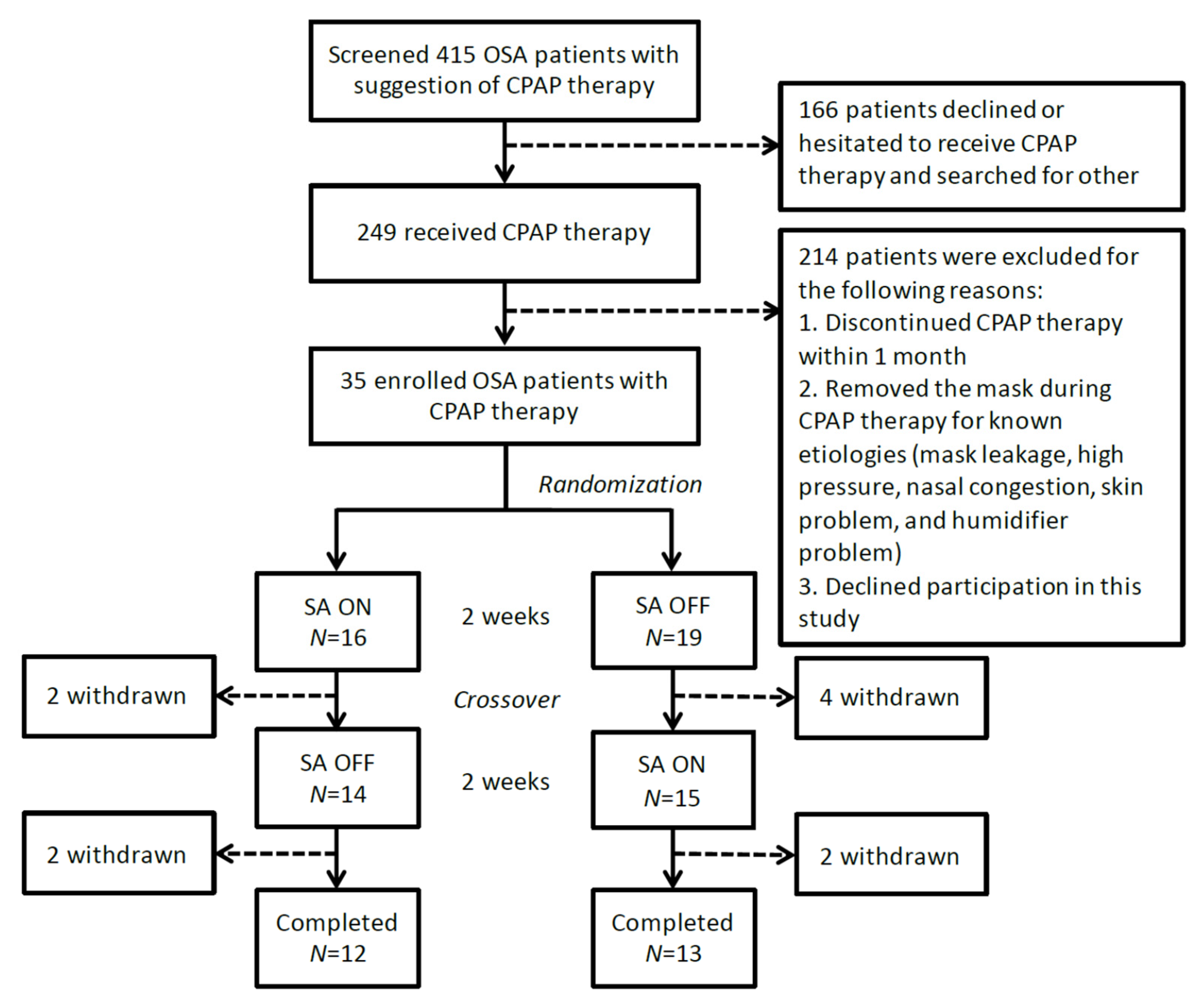
2. Materials and Methods
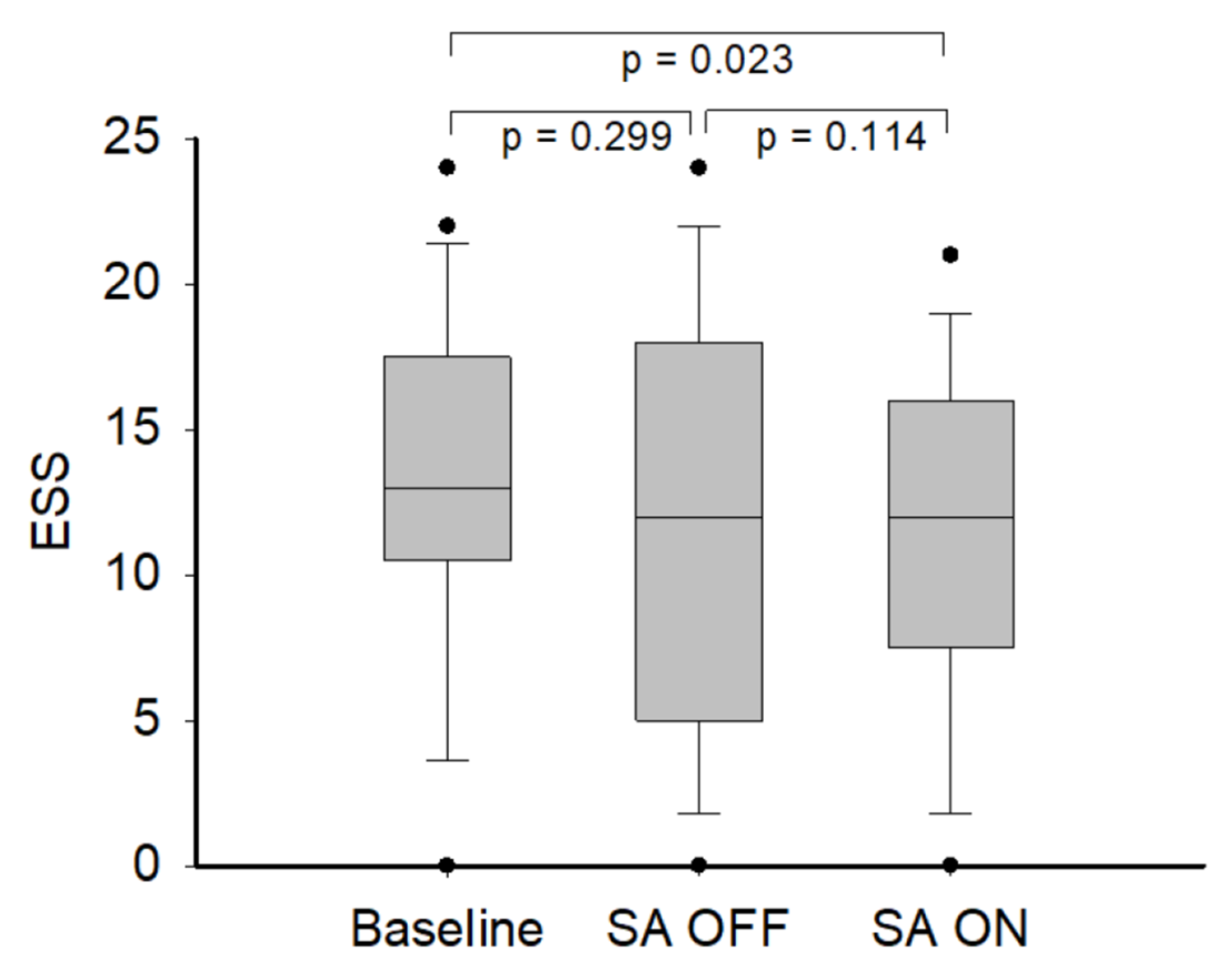
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G.N. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef]
- Young, T.; Finn, L.; Peppard, P.E.; Szklo-Coxe, M.; Austin, D.; Nieto, F.J.; Stubbs, R.; Hla, K.M. Sleep disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008, 31, 1071–1078. [Google Scholar] [PubMed]
- Barnes, M.; Houston, D.; Worsnop, C.J.; Neill, A.M.; Mykytyn, I.J.; Kay, A.; Trinder, J.; Saunders, N.A.; Douglas McEvoy, R.; Pierce, R.J. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2002, 165, 773–780. [Google Scholar] [CrossRef]
- Iftikhar, I.H.; Valentine, C.W.; Bittencourt, L.R.; Cohen, D.L.; Fedson, A.C.; Gislason, T.; Penzel, T.; Phillips, C.L.; Yu-sheng, L.; Pack, A.I.; et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: A meta-analysis. J. Hypertens 2014, 32, 2341–2350; discussion 2350. [Google Scholar] [CrossRef] [PubMed]
- Weaver, T.E.; Maislin, G.; Dinges, D.F.; Bloxham, T.; George, C.F.; Greenberg, H.; Kader, G.; Mahowald, M.; Younger, J.; Pack, A.I. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 2007, 30, 711–719. [Google Scholar] [CrossRef]
- Baltzan, M.A.; Elkholi, O.; Wolkove, N. Evidence of interrelated side effects with reduced compliance in patients treated with nasal continuous positive airway pressure. Sleep Med. 2009, 10, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Lasters, F.; Mallegho, C.; Boudewyns, A.; Vanderveken, O.; Cox, T.; Ketelslagers, K.; Verbraecken, J. Nasal symptoms in patients with obstructive sleep apnea and their impact on therapeutic compliance with continuous positive airway pressure. Acta Clin. Belg. 2014, 69, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.F.; Love, L.; Burt, H.; Fleetham, J.A. A randomized trial of auto-titrating CPAP and fixed CPAP in the treatment of obstructive sleep apnea-hypopnea. Respir. Med. 2004, 98, 330–333. [Google Scholar] [CrossRef]
- Ip, S.; D’Ambrosio, C.; Patel, K.; Obadan, N.; Kitsios, G.D.; Chung, M.; Balk, E.M. Auto-titrating versus fixed continuous positive airway pressure for the treatment of obstructive sleep apnea: A systematic review with meta-analyses. Syst Rev. 2012, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Ballard, R.D.; Gay, P.C.; Strollo, P.J. Interventions to improve compliance in sleep apnea patients previously non-compliant with continuous positive airway pressure. J. Clin. Sleep Med. 2007, 3, 706–712. [Google Scholar] [CrossRef]
- Antonescu-Turcu, A.; Parthasarathy, S. CPAP and bi-level PAP therapy: New and established roles. Respir. Care 2010, 55, 1216–1229. [Google Scholar]
- Carlucci, A.; Ceriana, P.; Mancini, M.; Cirio, S.; Pierucci, P.; D’Artavilla Lupo, N.; Gadaleta, F.; Morrone, E.; Fanfulla, F. Efficacy of Bilevel-auto Treatment in Patients with Obstructive Sleep Apnea Not Responsive to or Intolerant of Continuous Positive Airway Pressure Ventilation. J. Clin. Sleep Med. 2015, 11, 981–985. [Google Scholar] [CrossRef][Green Version]
- Aloia, M.S.; Stanchina, M.; Arnedt, J.T.; Malhotra, A.; Millman, R.P. Treatment adherence and outcomes in flexible vs standard continuous positive airway pressure therapy. Chest 2005, 127, 2085–2093. [Google Scholar] [CrossRef][Green Version]
- Smith, I.; Lasserson, T.J. Pressure modification for improving usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst. Rev. 2009, 4, CD003531. [Google Scholar] [CrossRef]
- Chihara, Y.; Tsuboi, T.; Hitomi, T.; Azuma, M.; Murase, K.; Toyama, Y.; Harada, Y.; Aihara, K.; Tanizawa, K.; Handa, T.; et al. Flexible positive airway pressure improves treatment adherence compared with auto-adjusting PAP. Sleep 2013, 36, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Donovan, L.M.; Boeder, S.; Malhotra, A.; Patel, S.R. New developments in the use of positive airway pressure for obstructive sleep apnea. J. Thorac. Dis. 2015, 7, 1323–1342. [Google Scholar] [CrossRef]
- Ayappa, I.; Norman, R.G.; Whiting, D.; Tsai, A.H.; Anderson, F.; Donnely, E.; Silberstein, D.J.; Rapoport, D.M. Irregular respiration as a marker of wakefulness during titration of CPAP. Sleep 2009, 32, 99–104. [Google Scholar]
- Killick, R.; Marshall, N.S. The Impact of Device Modifications and Pressure Delivery on Adherence. Sleep Med. Clin. 2021, 16, 75–84. [Google Scholar] [CrossRef]
- Dungan, G.C., 2nd; Marshall, N.S.; Hoyos, C.M.; Yee, B.J.; Grunstein, R.R. A randomized crossover trial of the effect of a novel method of pressure control (SensAwake) in automatic continuous positive airway pressure therapy to treat sleep disordered breathing. J. Clin. Sleep Med. 2011, 7, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Pepin, J.L.; Gagnadoux, F.; Foote, A.; Vicars, R.; Ogra, B.; Viot-Blanc, V.; Benmerad, M.; D’Ortho, M.P.; Tamisier, R. Combination of obstructive sleep apnoea and insomnia treated by continuous positive airway pressure with the SensAwake pressure relief technology to assist sleep: A randomised cross-over trial protocol. BMJ Open 2017, 7, e015836. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Stewart, M.G.; Witsell, D.L.; Smith, T.L.; Weaver, E.M.; Yueh, B.; Hannley, M.T. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol. Head Neck Surg. 2004, 130, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Hollandt, J.H.; Mahlerwein, M. Nasal breathing and continuous positive airway pressure (CPAP) in patients with obstructive sleep apnea (OSA). Sleep Breath 2003, 7, 87–94. [Google Scholar] [CrossRef]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann. Intern. Med. 1999, 131, 485–491. [Google Scholar] [CrossRef]
- Gliklich, R.E.; Wang, P.C. Validation of the snore outcomes survey for patients with sleep-disordered breathing. Arch. Otolaryngol. Head Neck Surg 2002, 128, 819–824. [Google Scholar] [CrossRef]
- Samsoon, G.L.; Young, J.R. Difficult tracheal intubation: A retrospective study. Anaesthesia 1987, 42, 487–490. [Google Scholar] [CrossRef]
- Dalewski, B.; Kamińska, A.; Syrico, A.; Kałdunska, A.; Pałka, Ł.; Sobolewska, E. The Usefulness of Modified Mallampati Score and CT Upper Airway Volume Measurements in Diagnosing OSA among Patients with Breathing-Related Sleep Disorders. Appl. Sci. 2021, 11, 3764. [Google Scholar] [CrossRef]
- Bogan, R.K.; Wells, C. A Randomized Crossover Trial of a Pressure Relief Technology (SensAwake) in Continuous Positive Airway Pressure to Treat Obstructive Sleep Apnea. Sleep Disord. 2017, 2017, 3978073. [Google Scholar] [CrossRef]
| Arm 1 (n = 12) SA ON First | Arm 2 (n = 13) SA OFF First | p Value | |
|---|---|---|---|
| General data | |||
| Gender: Female (%) | 0 (0.0) | 3 (23.1) | 0.220 |
| Age | 47.6 ± 10.9 | 41.5 ± 12.0 | 0.207 |
| BMI | 31.8 ± 4.3 | 32.9 ± 6.9 | 0.642 |
| NC | 42.3 ± 2.1 | 41.0 ± 2.2 | 0.167 |
| Polysomnography | |||
| AHI | 55.3 ± 30.8 | 54.0 ± 35.3 | 0.923 |
| Sleep efficiency (%) | 82.0 ± 8.9 | 73.0 ± 21.3 | 0.183 |
| Total sleep time (min) | 301.0 ± 34.2 | 276.2 ± 79.2 | 0.347 |
| Deep sleep—Stage 3 (%) | 6.1 ± 10.4 | 4.4 ± 5.6 | 0.624 |
| CPAP device recorded data | |||
| Average used time (min) | 187.4 ± 104.6 | 227.9 ± 104.1 | 0.342 |
| Compliance (%) | 31.9 ± 31.9 | 46.2 ± 30.6 | 0.266 |
| Residual AHI | 2.9 ± 1.3 | 4.4 ± 2.8 | 0.101 |
| Self-administered questionnaire | |||
| PSQI | 7.4 ± 2.3 | 10.0 ± 3.8 | 0.053 |
| ESS | 13.3 ± 5.5 | 13.1 ± 6.7 | 0.918 |
| NOSE | 13.5 ± 6.8 | 15.6 ± 8.4 | 0.498 |
| SA ON | SA OFF | p Value | |
|---|---|---|---|
| CPAP device recorded data | |||
| Average SA detections (/h) | 1.9 (1.5–2.6) | N/A | - |
| Pressure | 6.0 (5.0–8.5) | 6.5 (5.0–9.0) | 0.063 |
| 90% pressure | 8.0 (6.3–10.0) | 8.5 (6.8–10.5) | 0.004 * |
| Leak | 31.0 (27.5–54.5) | 43.0 (26.0–54.5) | 0.367 |
| 90% leak | 57.0 (35.5–79.0) | 49.0 (34.0–80.0) | 0.626 |
| Residual AHI | 3.9 (3.3–6.3) | 3.8 (2.4–6.5) | 0.017 * |
| Average use (min) | 236.0 (111.0–293.0) | 193.0 (124.0–285.5) | 0.904 |
| Days used (%) | 93.0 (74.0–100.0) | 92.0 (79.5–100.0) | 0.681 |
| Compliance (%) | 36.0 (4.0–81.0) | 31.0 (9.5–69.0) | 0.972 |
| Self-administered questionnaire | |||
| PSQI | 8.0 (6.0–10.8) | 8.0 (5.5–11.0) | 0.924 |
| ESS | 12.0 (7.5–16.0) | 12.0 (5.0–18.0) | 0.121 |
| NOSE | 12.0 (7.5–23.5) | 12.0 (8.5–18.5) | 0.245 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-L.; Chuang, L.-P.; Lin, S.-W.; Huang, H.-Y.; Liu, G.-H.; Hsu, H.-F.; Chen, N.-H. Effects of Pressure Control Device (SensAwake™) on Obstructive Sleep Apnea (OSA) Patients Who Remove the Mask for Unknown Reasons during Automatic Continuous Positive Airway Pressure (Auto-CPAP) Therapy: A Prospective Randomized Crossover Trial. Medicina 2021, 57, 915. https://doi.org/10.3390/medicina57090915
Chen Y-L, Chuang L-P, Lin S-W, Huang H-Y, Liu G-H, Hsu H-F, Chen N-H. Effects of Pressure Control Device (SensAwake™) on Obstructive Sleep Apnea (OSA) Patients Who Remove the Mask for Unknown Reasons during Automatic Continuous Positive Airway Pressure (Auto-CPAP) Therapy: A Prospective Randomized Crossover Trial. Medicina. 2021; 57(9):915. https://doi.org/10.3390/medicina57090915
Chicago/Turabian StyleChen, Yen-Lung, Li-Pang Chuang, Shih-Wei Lin, Hung-Yu Huang, Geng-Hao Liu, Hung-Fu Hsu, and Ning-Hung Chen. 2021. "Effects of Pressure Control Device (SensAwake™) on Obstructive Sleep Apnea (OSA) Patients Who Remove the Mask for Unknown Reasons during Automatic Continuous Positive Airway Pressure (Auto-CPAP) Therapy: A Prospective Randomized Crossover Trial" Medicina 57, no. 9: 915. https://doi.org/10.3390/medicina57090915
APA StyleChen, Y.-L., Chuang, L.-P., Lin, S.-W., Huang, H.-Y., Liu, G.-H., Hsu, H.-F., & Chen, N.-H. (2021). Effects of Pressure Control Device (SensAwake™) on Obstructive Sleep Apnea (OSA) Patients Who Remove the Mask for Unknown Reasons during Automatic Continuous Positive Airway Pressure (Auto-CPAP) Therapy: A Prospective Randomized Crossover Trial. Medicina, 57(9), 915. https://doi.org/10.3390/medicina57090915







