Sustained Long-Term Retention Rates of Abatacept in Combination with Conventional Synthetic Disease-Modifying Antirheumatic Drugs in Elderly Patients with Rheumatoid Arthritis
Abstract
1. Introduction
2. Materials and Methods
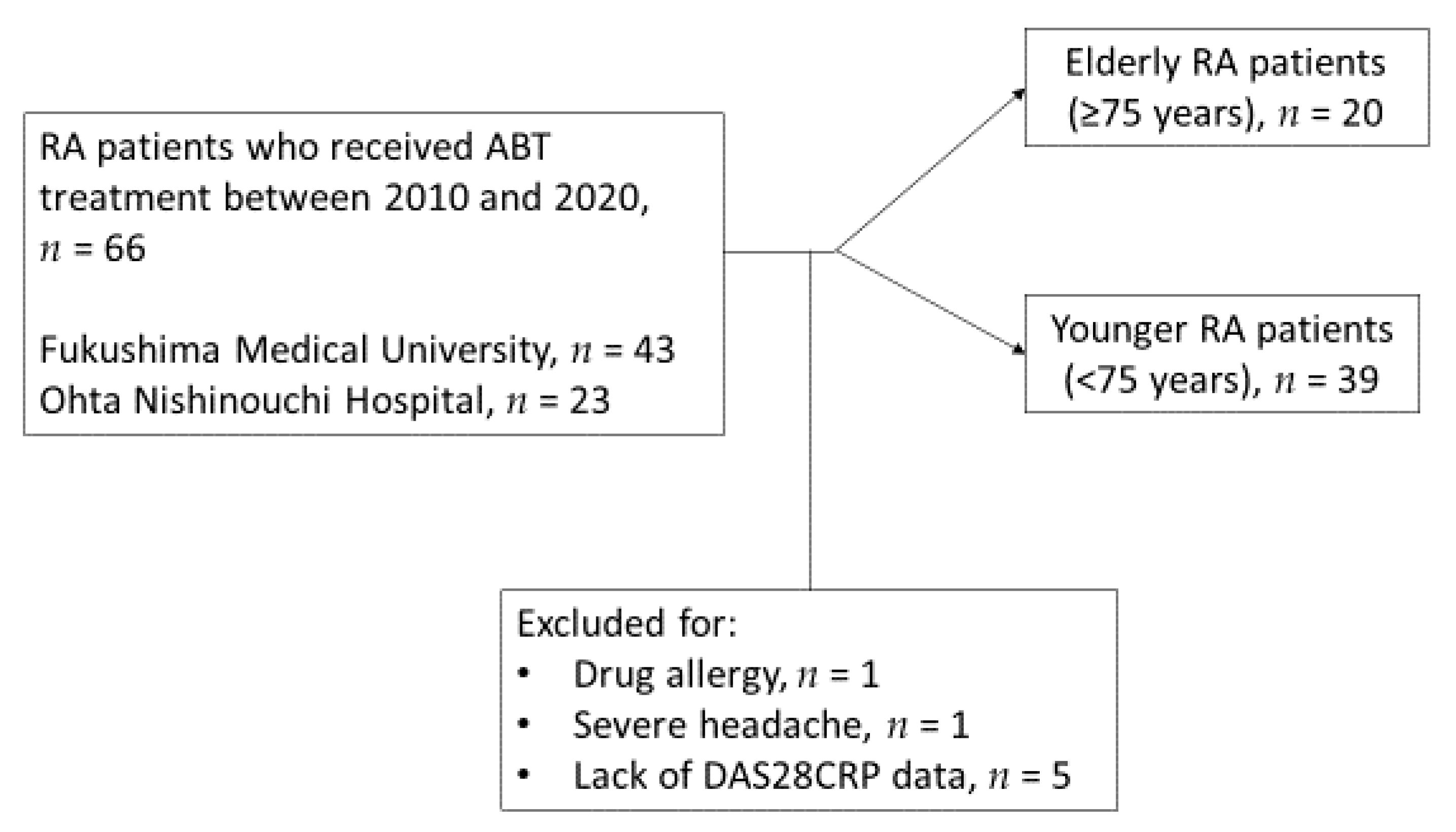
2.1. Study Participants, Clinical Investigations and Treatment
2.2. Statistical Analysis
3. Results
3.1. General Characteristics of RA Patients Treated with ABT in This Study
3.2. Clinical Features Complications and the Choice of Concomitant Therapy in Elderly RA Patients Treated with ABT
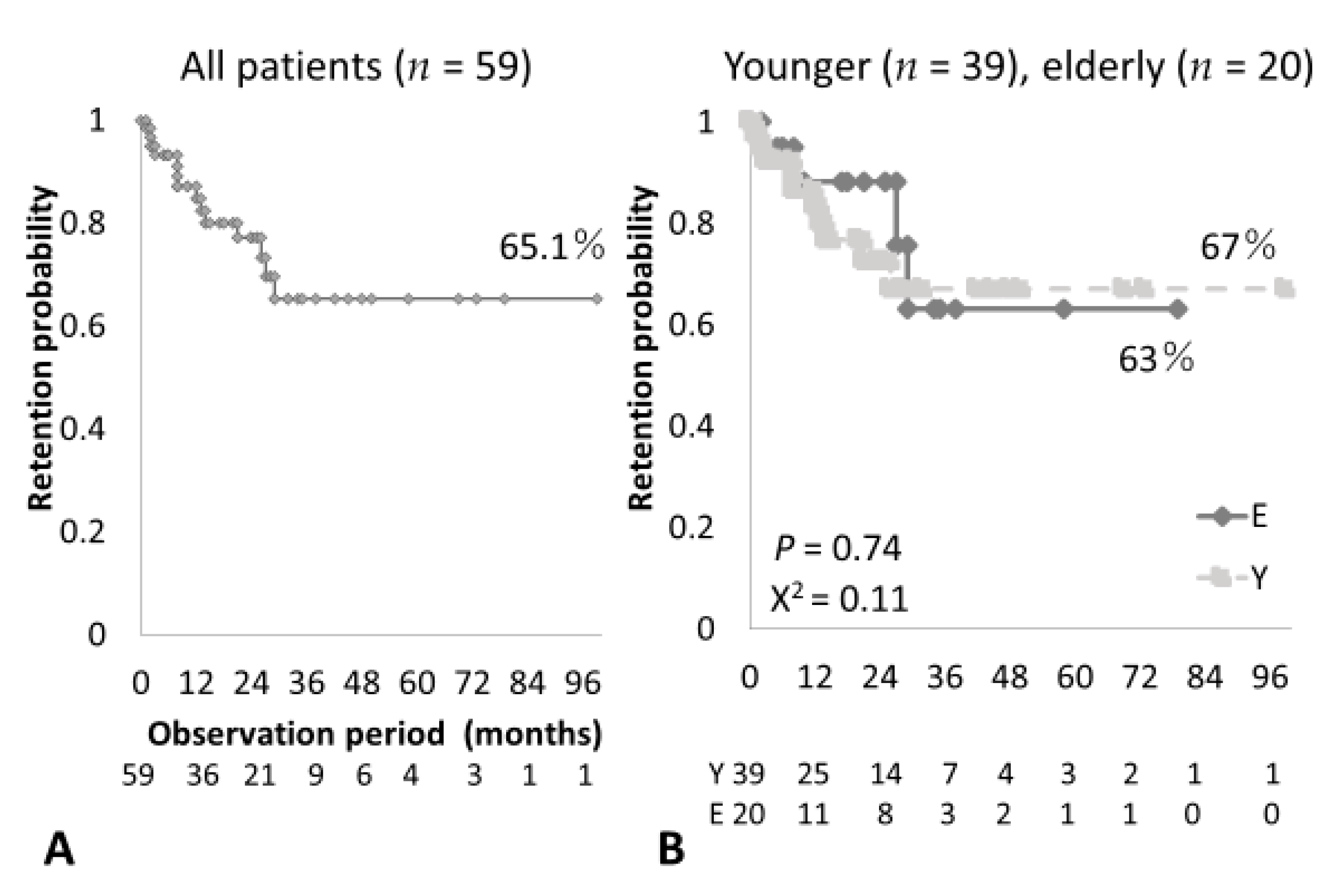
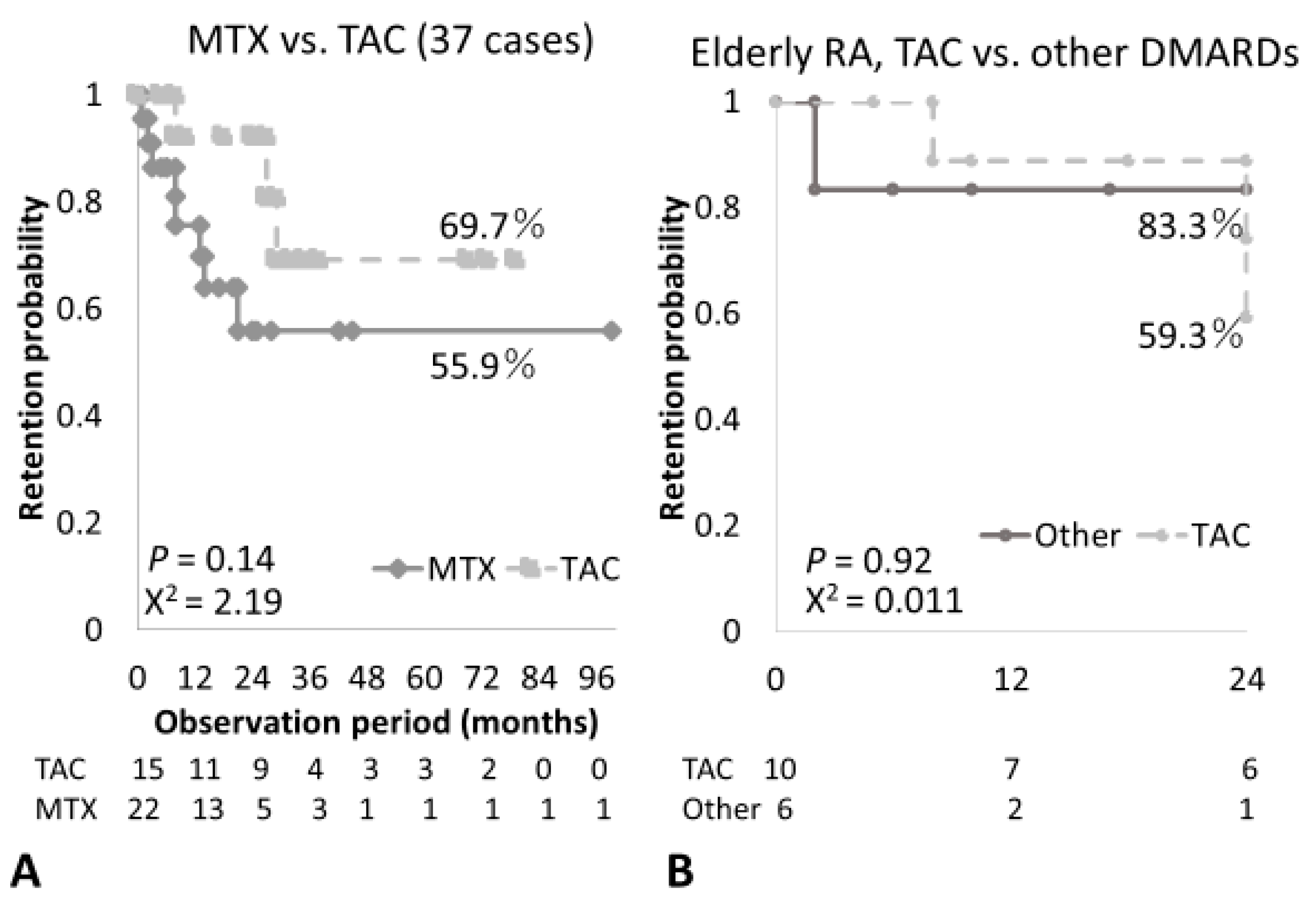
3.3. Clinical Efficacy, Treatment and Overall Retention Rates of ABT in Elderly RA Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kato, E.; Sawada, T.; Tahara, K.; Hayashi, H.; Tago, M.; Mori, H.; Nishino, J.; Matsui, T.; Tohma, S. The age at onset of rheumatoid arthritis is increasing in Japan: A nationwide database study. Int. J. Rheum. Dis. 2017, 20, 839–845. [Google Scholar] [CrossRef]
- Kohn, R.R. Human aging and disease. J. Chronic. Dis. 1963, 16, 5–21. [Google Scholar] [CrossRef]
- Lahaye, C.; Soubrier, M.; Mulliez, A.; Bardin, T.; Cantagrel, A.; Combe, B.; Dougados, M.; Flipo, R.M.; Le, L.X.; Shaeverbeke, T.; et al. Effectiveness and safety of abatacept in elderly patients with rheumatoid arthritis enrolled in the French Society of Rheumatology’s ORA registry. Rheumatology 2016, 55, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Harigai, M.; Ishiguro, N.; Inokuma, S.; Mimori, T.; Ryu, J.; Takei, S.; Takeuchi, T.; Tanaka, Y.; Takasaki, Y.; Yamanaka, H.; et al. Safety and effectiveness of abatacept in Japanese non-elderly and elderly patients with rheumatoid arthritis in an all-cases post-marketing surveillance. Mod. Rheumatol. 2019, 29, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Harigai, M.; Ishiguro, N.; Inokuma, S.; Mimori, T.; Ryu, J.; Takei, S.; Takeuchi, T.; Tanaka, Y.; Takasaki, Y.; Yamanaka, H.; et al. Postmarketing surveillance of the safety and effectiveness of abatacept in Japanese patients with rheumatoid arthritis. Mod. Rheumatol. 2016, 26, 491–498. [Google Scholar] [CrossRef]
- Ozen, G.; Pedro, S.; Schumacher, R.; Simon, T.A.; Michaud, K. Safety of abatacept compared with other biologic and conventional synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: Data from an observational study. Arthritis Res. Ther. 2019, 21, 141. [Google Scholar] [CrossRef] [PubMed]
- Ebina, K.; Hashimoto, M.; Yamamoto, W.; Ohnishi, A.; Kabata, D.; Hirano, T.; Hara, R.; Katayama, M.; Yoshida, S.; Nagai, K.; et al. Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis–The ANSWER cohort study. PLoS ONE 2018, 13, e0194130. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, A.; Nakano, K.; Kubo, S.; Asakawa, T.; Tanaka, Y. Differential long-term retention of biological disease-modifying antirheumatic drugs in patients with rheumatoid arthritis by age group from the FIRST registry. Arthritis Res. Ther. 2020, 22, 136. [Google Scholar] [CrossRef]
- Westhovens, R.; Connolly, S.E.; Margaux, J.; Vanden Berghe, M.; Maertens, M.; Van den Berghe, M.; Elbez, Y.; Chartier, M.; Baeke, F.; Rober, T.S.; et al. Up to 5-year retention of abatacept in Belgian patients with moderate-to-severe rheumatoid arthritis: A sub-analysis of the international, observational ACTION study. Rheumatol. Int. 2020, 40, 1409–1421. [Google Scholar] [CrossRef]
- Takahashi, N.; Kojima, T.; Terabe, K.; Kaneko, A.; Kida, D.; Hirano, Y.; Fujibayashi, T.; Yabe, Y.; Takagi, H.; Oguchi, T.; et al. Clinical efficacy of abatacept in Japanese rheumatoid arthritis patients. Mod. Rheumatol. 2013, 23, 904–912. [Google Scholar] [CrossRef]
- Pascart, T.; Philippe, P.; Drumez, E.; Deprez, X.; Cortet, B.; Duhamel, A.; Houvenagel, E.; Flipo, R.M. Abatacept Monotherapy Versus Abatacept Plus Methotrexate for Treatment-Refractory Rheumatoid Arthritis. Am. J. Ther. 2019, 26, e358–e363. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Hidaka, M.; Kawakita, T.; Hidaka, T.; Tsuda, H.; Yoshitama, T.; Migita, K.; Ueki, Y. Factors Associated with Myelosuppression Related to Low-Dose Methotrexate Therapy for Inflammatory Rheumatic Diseases. PLoS ONE 2016, 11, e0154744. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Oh, J.S.; Kim, Y.G.; Lee, C.K.; Yoo, B.; Hong, S. Methotrexate-related toxicity in patients with rheumatoid arthritis and renal dysfunction. Rheumatol. Int. 2020, 40, 765–770. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; Nikiphorou, E.; Larsen, J.; Korsten, P.; Conway, R. Methotrexate-Associated Pneumonitis and Rheumatoid Arthritis-Interstitial Lung Disease: Current Concepts for the Diagnosis and Treatment. Front. Med. (Lausanne) 2019, 6, 238. [Google Scholar] [CrossRef]
- Juge, P.A.; Lee, J.S.; Lau, J.; Kawano-Dourado, L.; Rojas Serrano, J.; Sebastiani, M.; Koduri, G.; Matteson, E.; Bonfiglioli, K.; Sawamura, M.; et al. Methotrexate and rheumatoid arthritis associated interstitial lung disease. Eur. Respir. J. 2021, 57, 2000337. [Google Scholar] [CrossRef]
- Nakashita, T.; Ando, K.; Kaneko, N.; Takahashi, K.; Motojima, S. Potential risk of TNF inhibitors on the progression of interstitial lung disease in patients with rheumatoid arthritis. BMJ Open 2014, 4, e005615. [Google Scholar] [CrossRef]
- Fernández-Díaz, C.; Loricera, J.; Castañeda, S.; López-Mejías, R.; Ojeda-García, C.; Olivé, A.; Rodríguez-Muguruza, S.; Carreira, P.E.; Pérez-Sandoval, T.; Retuerto, M.; et al. Abatacept in patients with rheumatoid arthritis and interstitial lung disease: A national multicenter study of 63 patients. Semin. Arthritis Rheum. 2018, 48, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Bristol-Myers Squibb. Orencia (Abatacept) Prescribing Information. 2017. Available online: http://packageinserts.bms.com/pi/pi_orencia.pdf (accessed on 28 May 2021).
- Simon, T.A.; Soule, B.P.; Hochberg, M.; Fleming, D.; Torbeyns, A.; Banerjee, S.; Boers, M. Safety of Abatacept Versus Placebo in Rheumatoid Arthritis: Integrated Data Analysis of Nine Clinical Trials. ACR Open Rheumatol. 2019, 1, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Inoue, H.; Nakajima, T.; Tanimura, K.; Sagawa, A.; Sato, Y.; Osano, K.; Nagano, S.; Ueki, Y.; Hanyu, T.; et al. Abatacept in combination with methotrexate in Japanese biologic-naive patients with active rheumatoid arthritis: A randomised placebo-controlled phase IV study. RMD Open 2018, 4, e000813. [Google Scholar] [CrossRef]
- Miyata, M.; Asano, T.; Satoh, S. Effect of additional administration of tacrolimus in patients with rheumatoid arthritis treated with biologics. Fukushima J. Med. Sci. 2011, 57, 54–59. [Google Scholar] [CrossRef][Green Version]
- Ishida, K.; Shiraki, K.; Yoshiyasu, T. Evaluation of the Safety and Effectiveness of Add-On Tacrolimus in Patients with Rheumatoid Arthritis Who Failed to Show an Adequate Response to Biological DMARDs: The Interim Results of a Specific Drug Use-Results Survey of Tacrolimus. Drugs R D 2015, 15, 307–317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inui, K.; Koike, T. Combination therapy with biologic agents in rheumatic diseases: Current and future prospects. Ther. Adv. Musculoskelet. Dis. 2016, 8, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Yamamoto, K. Safety of tacrolimus, an immunosuppressive agent, in the treatment of rheumatoid arthritis in elderly patients. Rheumatology 2006, 45, 441–444. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, Y.H.; Woo, J.H.; Choi, S.J.; Ji, J.D.; Bae, S.C.; Song, G.G. Tacrolimus for the treatment of active rheumatoid arthritis: A systematic review and meta-analysis of randomized controlled trials. Scand. J. Rheumatol. 2010, 39, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Park, E.Y.; Lee, S.G.; Park, E.K.; Koo, D.W.; Park, J.H.; Kim, G.T.; Tag, H.S.; Kim, H.O.; Suh, Y.S. Drug survival and the associated predictors in South Korean patients with rheumatoid arthritis receiving tacrolimus. Korean J. Intern. Med. 2018, 33, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Ishida, K.; Shiraki, K.; Yoshiyasu, T. Safety and effectiveness of tacrolimus add-on therapy for rheumatoid arthritis patients without an adequate response to biological disease-modifying anti-rheumatic drugs (DMARDs): Post-marketing surveillance in Japan. Mod. Rheumatol. 2018, 28, 48–57. [Google Scholar] [CrossRef]
- Fujibayashi, T.; Takahashi, N.; Kida, D.; Kaneko, A.; Hirano, Y.; Fukaya, N.; Yabe, Y.; Oguchi, T.; Tsuboi, S.; Miyake, H.; et al. Comparison of efficacy and safety of tacrolimus and methotrexate in combination with abatacept in patients with rheumatoid arthritis; a retrospective observational study in the TBC Registry. Mod. Rheumatol. 2015, 25, 825–830. [Google Scholar] [CrossRef]
- Suzuki, M.; Takahashi, N.; Kida, D.; Hirano, Y.; Kato, T.; Yabe, Y.; Oguchi, T.; Fujibayashi, T.; Hayashi, M.; Asai, S.; et al. Clinical effectiveness and safety of additional administration of tacrolimus in rheumatoid arthritis patients with an inadequate response to abatacept: A retrospective cohort study. Int. J. Rheum. Dis. 2019, 22, 2199–2205. [Google Scholar] [CrossRef]
- Magari, K.; Miyata, S.; Ohkubo, Y.; Mutoh, S. Inflammatory cytokine levels in paw tissues during development of rat collagen-induced arthritis: Effect of FK506, an inhibitor of T cell activation. Inflamm. Res. 2004, 53, 469–474. [Google Scholar] [CrossRef]
- Sakuma, S.; Kato, Y.; Nishigaki, F.; Sasakawa, T.; Magari, K.; Miyata, S.; Ohkubo, Y.; Goto, T. FK506 potently inhibits T cell activation induced TNF-alpha and IL-1beta production in vitro by human peripheral blood mononuclear cells. Br. J. Pharmacol. 2000, 130, 1655–1663. [Google Scholar] [CrossRef]
- de Germay, S.; Bagheri, H.; Despas, F.; Rousseau, V.; Montastruc, F. Abatacept in rheumatoid arthritis and the risk of cancer: A world observational post-marketing study. Rheumatology 2020, 59, 2360–2367. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.A.; Boers, M.; Hochberg, M.; Baker, N.; Skovron, M.L.; Ray, N.; Singhal, S.; Suissa, S.; Gomez-Caminero, A. Comparative risk of malignancies and infections in patients with rheumatoid arthritis initiating abatacept versus other biologics: A multi-database real-world study. Arthritis Res. Ther. 2019, 21, 228. [Google Scholar] [CrossRef] [PubMed]
| General Characteristics (n = 59) | Average ± Standard Deviation |
|---|---|
| Age (years) Male/female (number), female (%) | 67.5 ± 12.6 20/39 (66.1) |
| Disease duration (months) | 126 ± 133 |
| Steinbrocker Stage (III/IV) | 33/59 (55.9) |
| Steinbrocker Class (III/IV) | 9/59 (15.3) |
| Swollen joint count | 3.03 ± 3.58 |
| Tender joint count | 3.22 ± 4.44 |
| CRP (mg/dL) | 2.01 ± 2.22 |
| ESR (mm/h) | 37.4 ± 27.2 |
| RF- or anti-CCP antibody-positive (%) | 55/59 (93.2) |
| Methotrexate, n (%), (mean mg/week) | 24/59 (40.7%), 7.08 mg/week |
| Tacrolimus. n (%), (mean mg/day) | 15/59 (25.4%), 1.6 mg/day |
| Observation period in months (range) | 23.1 ± 20.7 months (1–99) |
| Clinical Items | Elderly (75≤) | Younger (75>) | p |
|---|---|---|---|
| Number | 20 | 39 | - |
| Age (years) Male/female (female, %) | 78.4 ± 2.56 4/16 (80%) | 62.0 ± 12.1 8/31 (79.5%) | - NS |
| Disease duration (month) | 149 ± 135 | 114 ± 132 | NS |
| Steinbrocker Stage (III/IV) Class (III/IV) | 14/20 (70%) 6/20 (30%) | 19/39 (48.7%) 3/39 (7.7%) | NS 0.03 |
| Swollen joint count (SJC) | 1.8 ± 1.58 | 3.67 ± 4.14 | NS |
| Tender joint count (TJC) | 1.5 ± 2.26 | 4.1 ± 5.02 | 0.02 |
| CRP (mg/dL) | 1.83 ± 2.07 | 2.1 ± 2.32 | NS |
| ESR (mm/1hour) | 41.4 ± 31.1 | 35.2 ± 25.0 | NS |
| DAS28CRP(3) | 2.79 ± 0.71 | 3.35± 1.15 | NS |
| Retention period (months) | 21.6± 20.2 | 23.9± 21.2 | NS |
| Treatment | |||
| Methotrexate (%) | 3/20 (15.0) | 21/39 (53.8) | <0.01 |
| Tacrolimus | 10/20 (50.0) | 5/39 (16.7) | <0.01 |
| Salazosulfapyridine | 8/20 (40.0) | 4/39 (10.3) | 0.01 |
| Prednisolone Doses (mg/day) | 12/20 (60.0) 6.71± 2.58 | 24/39 (61.5) 4.98± 2.78 | NS NS |
| Timing of ABT use for 1st Biologics (%) | 8/20 (40.0) | 15/39 (38.5) | NS |
| 2nd Biologics (%) | 11/20 (55.0) | 12/39 (29.7) | NS |
| 3rd or more (%) | 1/20 (5.0) | 12/39 (33.3) | 0.02 |
| Complication | |||
| Interstitial pneumonia | 5/20 (25%) | 6/39 (15.4%) | NS |
| Osteoporotic fracture | 2/20 (10%) | 4/37 (10.2%) | NS |
| Cardio/cerebrovascular disease | 3/20 (15%) | 2/39 (5.1%) | NS |
| Malignancy | 4/20 (20%) Prostate cancer, Myelodysplastic syndrome, IPMN SCC (lower leg) | 1/39 (2.6%) Lung cancer | 0.04 |
| Infections | 3/20 (15%) Pyelonephritis Pneumonia 2 | 4/39 (10.3%) Phlegmon 2 Pneumonia, Lung abscess VZV infection | NS |
| ABT cessation Reason | 4/20 (20%) Ineffective 2 Infection 1 Brain hemorrhage 1 | 9/39 (23.1%) Ineffective 6 Lung cancer 1 Kidney dysfunction 1 Infection 1 | NS |
| Clinical Characteristics | Abatacept/Tacrolimus | Abatacept/Methotrexate | p-Value |
|---|---|---|---|
| Number of patients treated | 15 | 22 | - |
| Age (years) Male/female (female, %) | 75.1 ± 7.93 2/13 (86.7) | 62.0 ± 14.8 6/16 (72.7) | <0.01 NS |
| Disease duration (month) | 140 ± 121 | 114 ± 136 | NS |
| Steinbrocker Stage (III/IV) | 12/15 (80.0) | 12/22 (54.5) | NS |
| Steinbrocker Class (III/IV) | 2/15 (13.3%) | 2/22 (9.1%) | NS |
| Retention period (months) | 31.8 ± 24.2 | 19.7 ± 25.0 | 0.054 |
| DAS28CRP(3) | |||
| Before treatment | 3.27 ± 1.33 | 3.31 ± 1.13 | NS |
| Six months after treatment | 2.16 ± 0.78 | 2.26 ± 0.92 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, S.; Matsumoto, H.; Temmoku, J.; Fujita, Y.; Matsuoka, N.; Yashiro-Furuya, M.; Asano, T.; Suzuki, E.; Watanabe, H.; Kanno, T.; et al. Sustained Long-Term Retention Rates of Abatacept in Combination with Conventional Synthetic Disease-Modifying Antirheumatic Drugs in Elderly Patients with Rheumatoid Arthritis. Medicina 2021, 57, 914. https://doi.org/10.3390/medicina57090914
Sato S, Matsumoto H, Temmoku J, Fujita Y, Matsuoka N, Yashiro-Furuya M, Asano T, Suzuki E, Watanabe H, Kanno T, et al. Sustained Long-Term Retention Rates of Abatacept in Combination with Conventional Synthetic Disease-Modifying Antirheumatic Drugs in Elderly Patients with Rheumatoid Arthritis. Medicina. 2021; 57(9):914. https://doi.org/10.3390/medicina57090914
Chicago/Turabian StyleSato, Shuzo, Haruki Matsumoto, Jumpei Temmoku, Yuya Fujita, Naoki Matsuoka, Makiko Yashiro-Furuya, Tomoyuki Asano, Eiji Suzuki, Hiroshi Watanabe, Takashi Kanno, and et al. 2021. "Sustained Long-Term Retention Rates of Abatacept in Combination with Conventional Synthetic Disease-Modifying Antirheumatic Drugs in Elderly Patients with Rheumatoid Arthritis" Medicina 57, no. 9: 914. https://doi.org/10.3390/medicina57090914
APA StyleSato, S., Matsumoto, H., Temmoku, J., Fujita, Y., Matsuoka, N., Yashiro-Furuya, M., Asano, T., Suzuki, E., Watanabe, H., Kanno, T., & Migita, K. (2021). Sustained Long-Term Retention Rates of Abatacept in Combination with Conventional Synthetic Disease-Modifying Antirheumatic Drugs in Elderly Patients with Rheumatoid Arthritis. Medicina, 57(9), 914. https://doi.org/10.3390/medicina57090914






