Is Non-Stimulated C-Peptide at Diagnosis a Good Predictive Value for Insulin Use at Two Years after Diagnosis in Pediatric Diabetic Patients?
Abstract
:1. Introduction
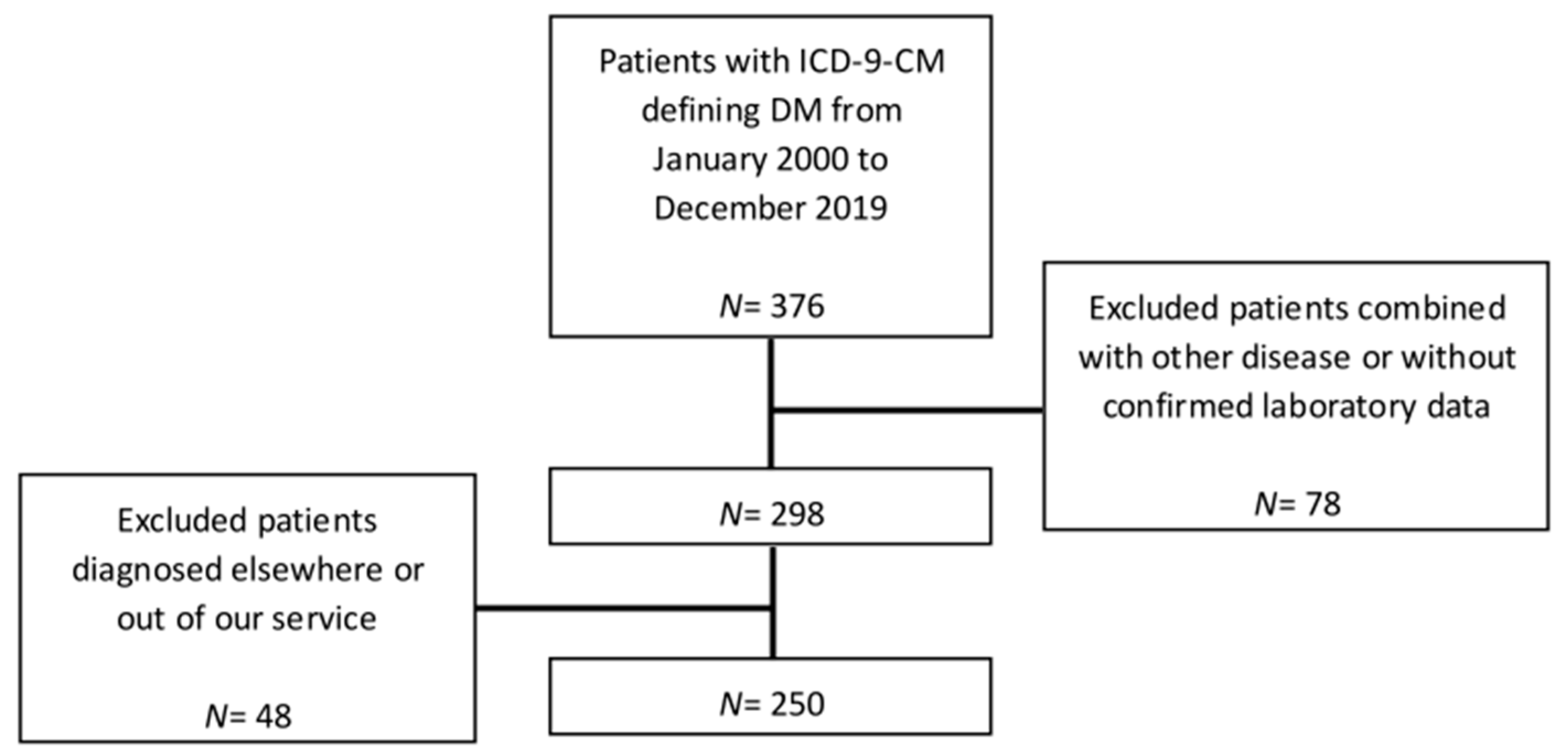
2. Materials and Methods
2.1. Study Subjects
2.2. Collection of Laboratory Data
2.3. Statistical Analysis
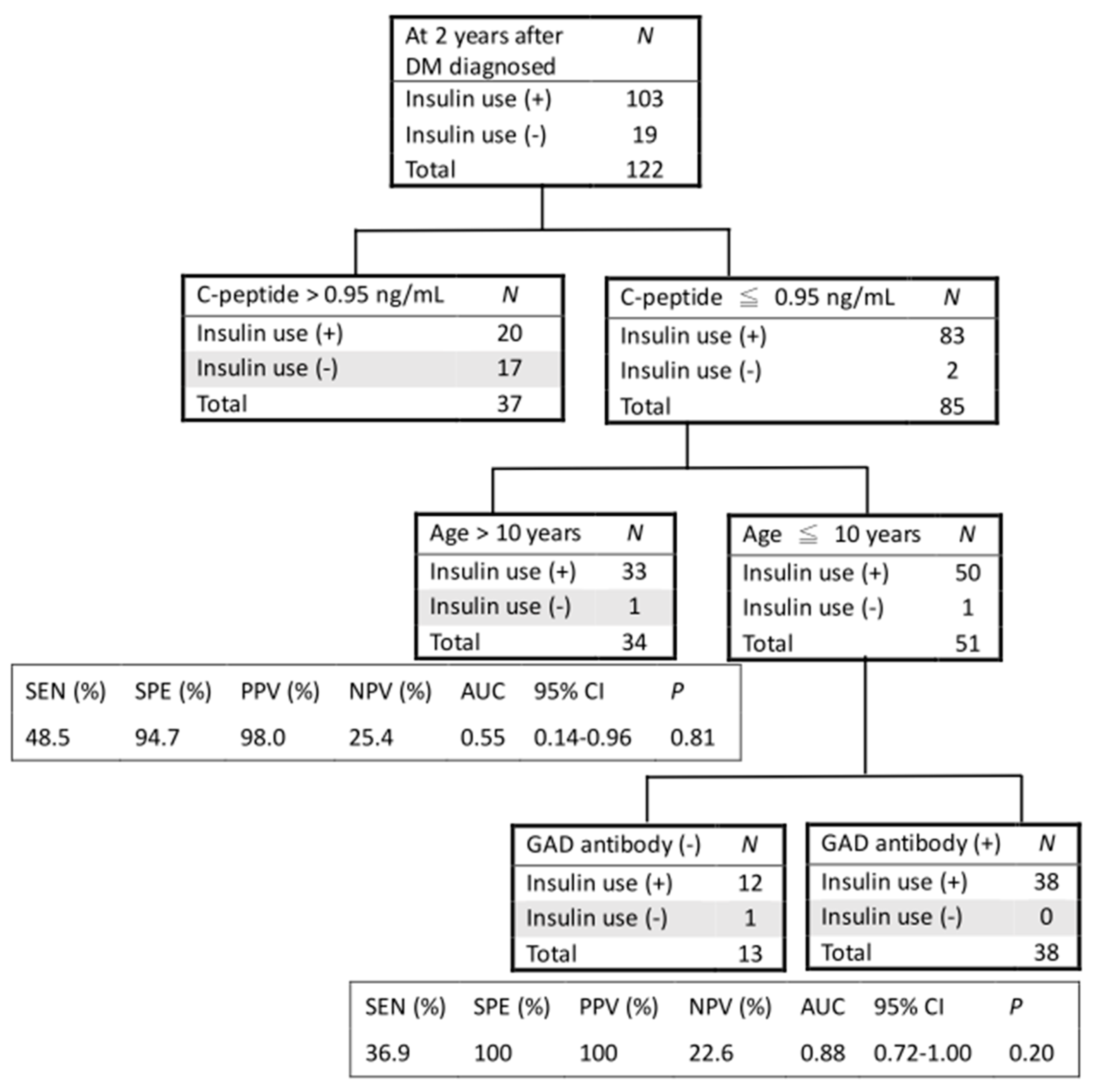
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atkinson, M.A.; Maclaren, N.K. The pathogenesis of insulin-dependent diabetes mellitus. N. Engl. J. Med. 1994, 331, 1428–1436. [Google Scholar] [PubMed]
- Xu, H.; Verre, M.C. Type 2 diabetes mellitus in children. Am. Fam. Physician 2018, 98, 590–594. [Google Scholar] [PubMed]
- Kao, K.T.; Sabin, M.A. Type 2 diabetes mellitus in children and adolescents. Aust. Fam. Physician 2016, 45, 401–406. [Google Scholar] [PubMed]
- McFarlane, S.I.; Chaiken, R.L.; Hirsch, S.; Harrington, P.; Lebovitz, H.E.; Banerji, M.A. Near-Normoglycaemic remission in African-Americans with Type 2 diabetes mellitus is associated with recovery of beta cell function. Diabet. Med. 2001, 18, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.C.; Lee, J.S.; Tsai, W.Y.; Hsiao, P.H. Evaluation of beta-cell function in diabetic Taiwanese children using a 6-min glucagon test. Eur. J. Pediatr. 2008, 167, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.G.; Hattersley, A.T. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet. Med. 2013, 30, 803–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Xin, Y.; Chen, X.; Sood, B.G. Early prediction of moderate to severe bronchopulmonary dysplasia in extremely premature infants. Pediatr. Neonatol. 2020, 61, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chang, M.H. New growth charts for Taiwanese children and adolescents based on World Health Organization standards and health-related physical fitness. Pediatr. Neonatol. 2010, 51, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Goto, A.; Takaichi, M.; Kishimoto, M.; Takahashi, Y.; Kajio, H.; Shimbo, T.; Noda, M. Body mass index, fasting plasma glucose levels, and C-peptide levels as predictors of the future insulin use in Japanese type 2 diabetic patients. Endocr. J. 2010, 57, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madsbad, S.; Krarup, T.; McNair, P.; Christiansen, C.; Faber, O.K.; Transbøl, I.; Binder, C. Practical clinical value of the C-peptide response to glucagon stimulation in the choice of treatment in diabetes mellitus. Acta Med. Scand. 1981, 210, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, P.; Viikari, J.; Irjala, K.; Kaihola, H.L.; Seppälä, P. C-Peptide determination in the choice of treatment in diabetes mellitus. Scand. J. Clin. Lab. Investig. 1985, 45, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.B.; Tong, P.C.; Chow, C.C.; So, W.Y.; Ng, M.C.; Ma, R.C.; Osaki, R.; Cockram, C.S.; Chan, J.C. The associations of body mass index, C-peptide and metabolic status in Chinese Type 2 diabetic patients. Diabet. Med. 2004, 21, 349–353. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 4. Lifestyle Management: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41, S38–S50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Male | Female | Total | |
|---|---|---|---|
| Number, n (%) | 122 (48.8%) | 128 (51.2%) | 250 |
| Age at DM onset | 10.07 (±4.59) | 9.32 (±0.17) | 9.69 (±4.39) |
| BMI at DM onset (Z score) | 1.47 (±3.14) | 0.64 (±2.01) | 1.05 (±2.65) |
| C-peptide (ng/mL) | 1.29 (±1.66) | 1.16 (±1.71) | 1.22 (±1.69) |
| Insulin (uU/mL) | 16.54 (±51.53) | 11.46 (±19.40) | 13.93 (±38.51) |
| GAD antibody-positive, n (%) | 38 (51%) | 50 (65%) | 88 (58%) |
| Ketoacidosis, n (%) | 66 (63%) | 73 (64%) | 139 (64%) |
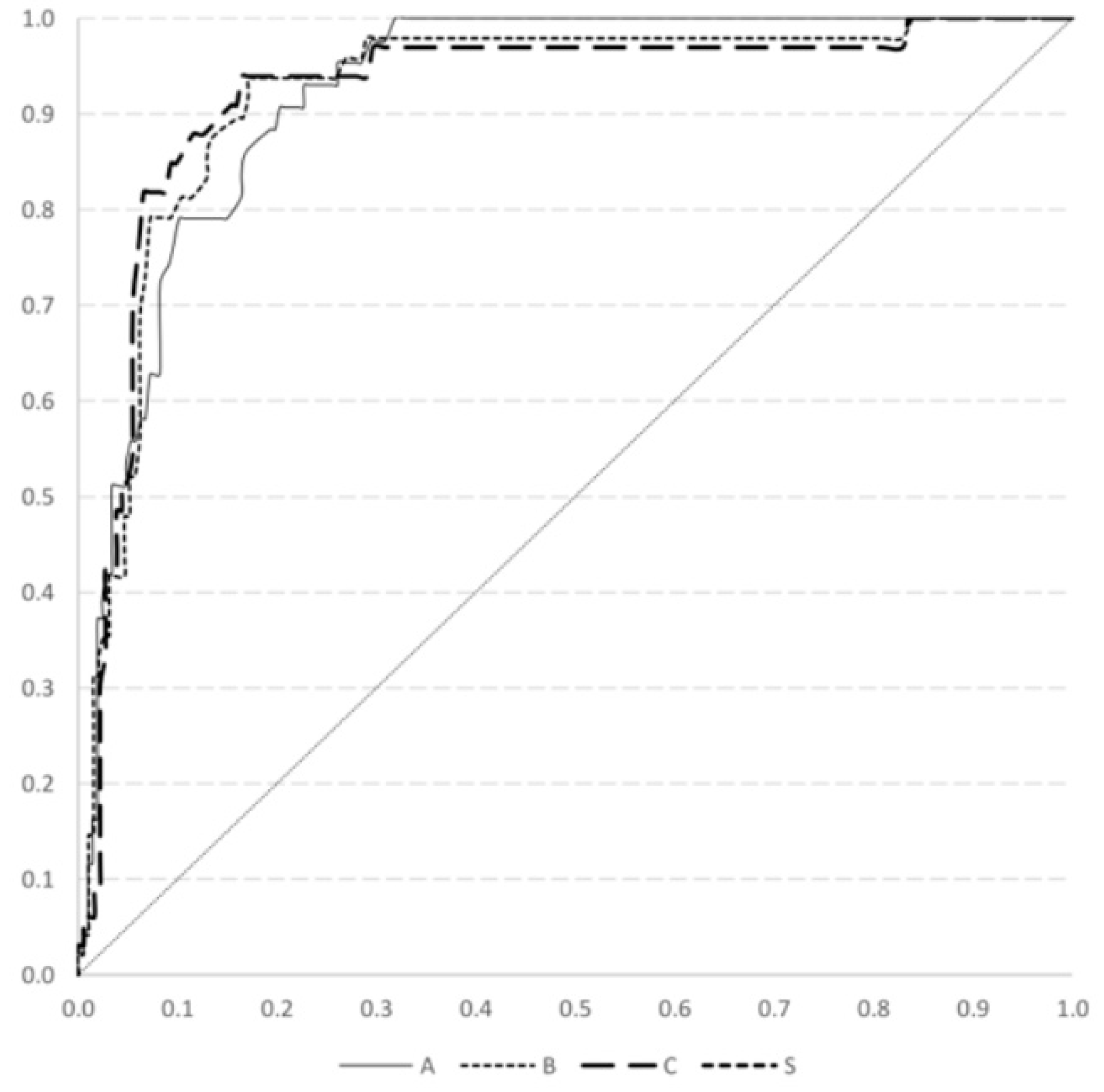
| C-Peptide Cut-Off Point | DM Onset | 1 Year after DM Diagnosis | 2 Years after DM Diagnosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ng/mL | SEN (%) | SPE (%) | PPV (%) | NPV (%) | SEN (%) | SPE (%) | PPV (%) | NPV (%) | SEN (%) | SPE (%) | PPV (%) | NPV (%) |
| ≦0.9 | 78.7 | 90.7 | 97.6 | 47.0 | 82.4 | 93.8 | 98.1 | 57.0 | 82.4 | 93.9 | 98.7 | 49.2 |
| ≦0.95 | 79.7 | 90.7 | 97.6 | 48.1 | 82.9 | 91.7 | 97.6 | 57.1 | 83.5 | 93.9 | 98.7 | 50.8 |
| ≦1.0 | 83.1 | 86.0 | 96.6 | 51.4 | 86.5 | 87.5 | 96.5 | 61.8 | 87.4 | 87.9 | 97.5 | 55.8 |
| DM onset | 1 Year after DM diagnosis | 2 Years after DM diagnosis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | Exp (B) | 95% CI | p | Exp (B) | 95% CI | p | Exp (B) | 95% CI | p |
| Gender (male vs. female) | 1.12 | 0.58–2.16 | 0.742 | 0.56 | 0.29–1.07 | 0.078 | 0.51 | 0.24–1.10 | 0.087 |
| Age (≦10 years vs. >10 years) | 8.87 | 3.76–20.90 | <0.001 | 11.53 | 4.90–27.13 | <0.001 | 10.33 | 3.80–28.03 | <0.001 |
| BMI Z score (≦2 SD vs. >2SD) | 7.61 | 3.61–16.01 | <0.001 | 12.45 | 5.88–26.38 | <0.001 | 15.00 | 6.21–36.10 | <0.001 |
| C-peptide (value ≦ 0.95 vs. >0.95) | 38.30 | 12.96–113.17 | <0.001 | 53.33 | 17.93–158.63 | <0.001 | 78.53 | 17.83–345.86 | <0.001 |
| Insulin level | 1.01 | 1.00–1.01 | 0.255 | 1.03 | 1.01–1.05 | 0.008 | 1.02 | 1.00–1.04 | 0.072 |
| GAD antibody (yes vs. no) | 9.55 | 3.07–29.66 | <0.001 | 21.44 | 6.09–75.46 | <0.001 | 38.18 | 4.89–298.31 | 0.001 |
| Ketoacidosis at onset (yes vs. no) | 10.35 | 4.26–25.19 | <0.001 | 6.68 | 3.09–14.47 | <0.001 | 8.82 | 3.32–23.36 | <0.001 |
| Multivariate analysis with stepwise selection | |||||||||
| Age (≦10 years vs. >10 years) | 6.28 | 0.73–54.10 | 0.094 | 4.49 | 0.81–24.97 | 0.086 | |||
| C-peptide (value ≦ 0.95 vs. >0.95) | 15.37 | 3.19–73.98 | 0.001 | 12.93 | 2.46–67.78 | 0.002 | 26.13 | 2.97–229.84 | 0.003 |
| GAD antibody (yes vs. no) | 7.19 | 1.33–38.87 | 0.022 | 8.49 | 0.91–78.87 | 0.060 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, W.-C.; Chou, Y.-Y.; Pan, Y.-W.; Tsai, M.-C. Is Non-Stimulated C-Peptide at Diagnosis a Good Predictive Value for Insulin Use at Two Years after Diagnosis in Pediatric Diabetic Patients? Medicina 2021, 57, 902. https://doi.org/10.3390/medicina57090902
Chou W-C, Chou Y-Y, Pan Y-W, Tsai M-C. Is Non-Stimulated C-Peptide at Diagnosis a Good Predictive Value for Insulin Use at Two Years after Diagnosis in Pediatric Diabetic Patients? Medicina. 2021; 57(9):902. https://doi.org/10.3390/medicina57090902
Chicago/Turabian StyleChou, Wei-Chih, Yen-Yin Chou, Yu-Wen Pan, and Meng-Che Tsai. 2021. "Is Non-Stimulated C-Peptide at Diagnosis a Good Predictive Value for Insulin Use at Two Years after Diagnosis in Pediatric Diabetic Patients?" Medicina 57, no. 9: 902. https://doi.org/10.3390/medicina57090902
APA StyleChou, W.-C., Chou, Y.-Y., Pan, Y.-W., & Tsai, M.-C. (2021). Is Non-Stimulated C-Peptide at Diagnosis a Good Predictive Value for Insulin Use at Two Years after Diagnosis in Pediatric Diabetic Patients? Medicina, 57(9), 902. https://doi.org/10.3390/medicina57090902






