Association of Thyroid Function with Suicidal Behavior: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Study Selection and Characteristics
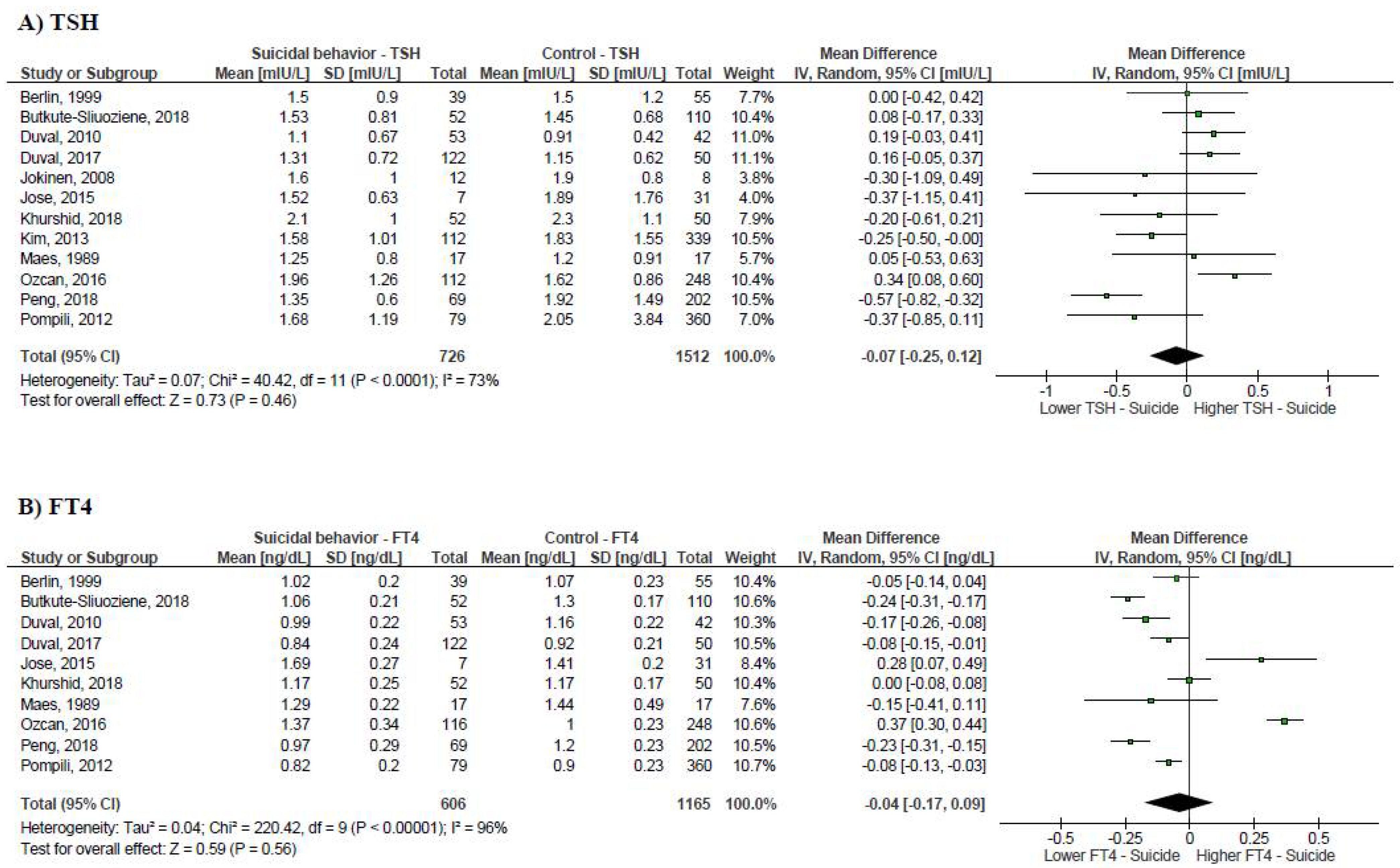
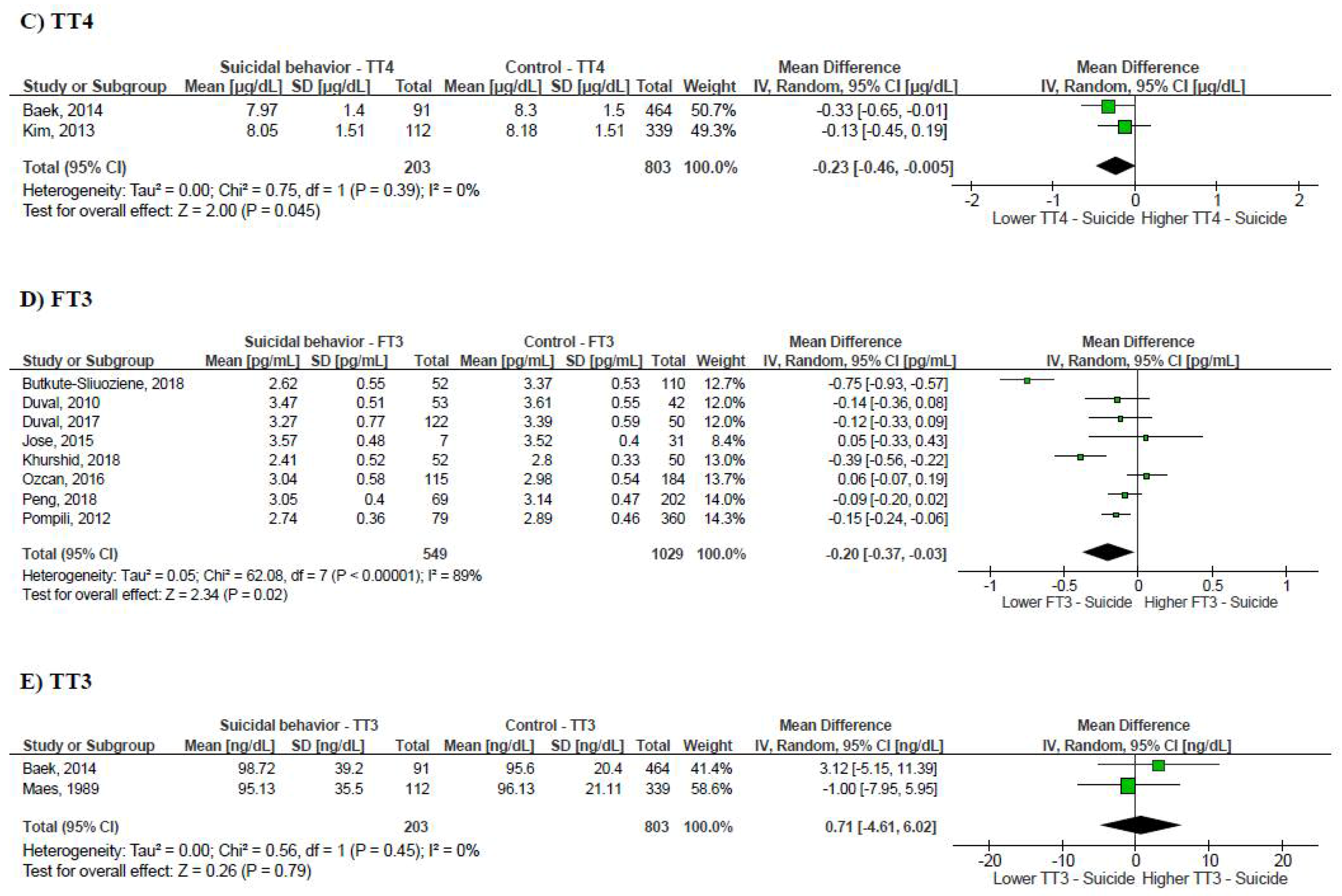
3.2. Suicidal Behavior and Thyroid Function Tests
3.3. Publication Bias and Leave-One-Out Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Suicide. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/suicide (accessed on 10 December 2020).
- Bachmann, S. Epidemiology of Suicide and the Psychiatric Perspective. Int. J. Environ. Res. Public Health 2018, 15, 1425. [Google Scholar] [CrossRef] [PubMed]
- ATA. General Information/Press Room. 2019. Available online: https://www.thyroid.org/media-main/press-room/ (accessed on 10 December 2020).
- Aoki, Y.; Belin, R.M.; Clickner, R.; Jeffries, R.; Phillips, L.; Mahaffey, K.R. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002). Thyroid 2007, 17, 1211–1223. [Google Scholar] [CrossRef]
- Canaris, G.J.; Manowitz, N.R.; Mayor, G.; Ridgway, E.C. The Colorado Thyroid Disease Prevalence Study. Arch. Intern. Med. 2000, 160, 526–534. [Google Scholar] [CrossRef]
- Haskett, R.F. Diagnostic categorization of psychiatric disturbance in Cushing’s syndrome. Am. J. Psychiatry 1985, 142, 911–916. [Google Scholar]
- Pivonello, R.; Simeoli, C.; De Martino, M.C.; Cozzolino, A.; De Leo, M.; Iacuaniello, D. Neuropsychiatric disorders in Cushing’s syndrome. Front. Neurosci. 2015, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.D.; McMahon, D.J.; Inabnet, W.B.; Lazar, R.M.; Brown, I.; Vardy, S. Neuropsychological features in primary hyperparathyroidism: A prospective study. J. Clin. Endocrinol. Metab. 2009, 94, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Johns, L.E.; Ferguson, K.K.; Cantonwine, D.E.; Mukherjee, B.; Meeker, J.D.; McElrath, T.F. Subclinical Changes in Maternal Thyroid Function Parameters in Pregnancy and Fetal Growth. J. Clin. Endocrinol. Metab. 2018, 103, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Heiberg Brix, T.; Ferlov-Schwensen, C.; Thvilum, M.; Hegedus, L. Death by unnatural causes, mainly suicide, is increased in patients with Hashimoto’s thyroiditis. A nationwide Danish register study. Endocrine 2019, 65, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wu, F.; Zhou, Y.; Ma, Y.; Huang, X.; Ning, Y.; Lang, X.; Luo, X.; Zhang, X. Association of thyroid dysfunction with suicide attempts in first-episode and drug naïve patients with major depressive disorder. J. Affect. Disord. 2019, 259, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Journy, N.M.; Bernier, M.-O.; Doody, M.M.; Alexander, B.H.; Linet, M.S.; Kitahara, C.M. Hyperthyroidism, Hypothyroidism, and Cause-Specific Mortality in a Large Cohort of Women. Thyroid 2017, 27, 1001–1010. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies Inmeta-Analyses. 2019. Available online: www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 10 December 2020).
- Cochrane Training. Cochrane Handbook for Systematic Reviews of Interventions: June 2017: Handbook Editors’ Update. 2019. Available online: http://training.cochrane.org/handbook (accessed on 10 December 2020).
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- CLARITY Group at McMaster University. Tools to Assess Risk of Bias in Cohort and Case Control Studies; Randomized Controlled Trials; and Longitudinal Symptom Research Studies Aimed at the General Population. 2013. Available online: http://www.evidencepartners.com/resources/ (accessed on 10 December 2020).
- Baek, J.H.; Kang, E.S.; Fava, M.; Mischoulon, D.; Nierenberg, A.A.; Yu, B.H. Serum lipids, recent suicide attempt and recent suicide status in patients with major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 51, 113–118. [Google Scholar] [CrossRef]
- Berlin, I.; Payan, C.; Corruble, E.; Puech, A.J. Serum thyroid-stimulating-hormone concentration as an index of severity of major depression. Int. J. Neuropsychopharmacol. 1999, 2, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Berentaitė, B.; Marčiulionienė, A.; Steiblienė, V.; Butkutė-Šliuožienė, K.; Adomaitienė, V. WITHDRAWN: P.2.016 Thyroid axis functioning in patients with high suicide risk. Eur. Neuropsychopharmacol. 2018, 28, S32–S33. [Google Scholar] [CrossRef] [PubMed]
- Duval, F.; Mokrani, M.-C.; Lopera, F.G.; Diep, T.S.; Rabia, H.; Fattah, S. Thyroid axis activity and suicidal behavior in depressed patients. Psychoneuroendocrinology 2010, 35, 1045–1054. [Google Scholar] [CrossRef]
- Duval, F.; Mokrani, M.C.; Erb, A.; Gonzalez Opera, F.; Calleja, C.; Paris, V. Relationship between chronobiological thyrotropin and prolactin responses to protirelin (TRH) and suicidal behavior in depressed patients. Psychoneuroendocrinology 2017, 85, 100–109. [Google Scholar] [CrossRef]
- Jose, J.; Nandeesha, H.; Kattimani, S.; Meiyappan, K.; Sarkar, S.; Sivasankar, D. Association between prolactin and thyroid hormones with severity of psychopathology and suicide risk in drug free male schizophrenia. Clin. Chim. Acta. 2015, 444, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, J.; Samuelsson, M.; Nordstrom, A.L.; Nordstrom, P. HPT axis, CSF monoamine metabolites, suicide intent and depression severity in male suicide attempters. J. Affect. Disord. 2008, 111, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, S.; Pervaiz, M.; Akhtar, S.; Elahi, S.; Zaidi, A.; Saeed, Z.; Bukhari, S.M. Changes in thyroid function status of suicidal patients. Arch. Clin. Psychiatry 2018, 45, 12–14. [Google Scholar] [CrossRef]
- Kim, B.; Kang, E.-S.; Fava, M.; Mischoulon, D.; Soskin, D.; Yu, B.-H.; Lee, D.; Lee, D.-Y.; Park, H.-D.; Jeon, H.J. Follicle-stimulating hormone (FSH), current suicidal ideation and attempt in female patients with major depressive disorder. Psychiatry Res. 2013, 210, 951–956. [Google Scholar] [CrossRef]
- Maes, M.; Vandewoude, M.; Schotte, C.; Martin, M.; Blockx, P.; Scharpé, S.; Cosyns, P. Hypothalamic-pituitary-adrenal and -thyroid axis dysfunctions and decrements in the availability of L-tryptophan as biological markers of suicidal ideation in major depressed females. Acta Psychiatr. Scand. 1989, 80, 13–17. [Google Scholar] [CrossRef]
- Ozcan, H.; Yucel, A.; Atis, O.; Yucel, N.; Bilen, A.; Emet, M. Thyroxin Levels Associated with Current Suicide Attempts: A Case Control and Follow-Up Study. Klinik Psikofarmakoloji Bülteni-Bulletin of Clinical. Psychopharmacology 2016, 26, 278–286. [Google Scholar]
- Pompili, M.; Gibiino, S.; Innamorati, M.; Serafini, G.; Del Casale, A.; De Risio, L.; Palermo, M.; Montebovi, F.; Campi, S.; De Luca, V.; et al. Prolactin and thyroid hormone levels are associated with suicide attempts in psychiatric patients. Psychiatry Res. 2012, 200, 389–394. [Google Scholar] [CrossRef]
- Peng, R.; Dai, W.; Li, Y. Low serum free thyroxine level is correlated with lipid profile in depressive patients with suicide attempt. Psychiatry Res. 2018, 266, 111–115. [Google Scholar] [CrossRef]
- Barbosa, I.; Ferreira, R.; Huguet, R.; Rocha, F.; Salgado, J.; Teixeira, A. Comorbidades clínicas e psiquiátricas em pacientes com transtorno bipolar do tipo I. J. Bras. Psiquiatria 2011, 60, 271–276. [Google Scholar] [CrossRef][Green Version]
- Sanna, L.; Stuart, A.L.; Pasco, J.A.; Kotowicz, M.; Berk, M.; Girardi, P.; Williams, L. Suicidal ideation and physical illness: Does the link lie with depression? J. Affect. Disord. 2014, 152–154, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Abraham-Nordling, M.; Lonn, S.; Wallin, G.; Yin, L.; Nyren, O.; Tullgren, O. Hyperthyroidism and suicide: A retrospective cohort study in Sweden. Eur. J. Endocrinol. 2009, 160, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Ferløv-Schwensen, C.; Brix, T.H.; Hegedüs, L. Death by Suicide in Graves’ Disease and Graves’ Orbitopathy: A Nationwide Danish Register Study. Thyroid 2017, 27, 1475–1480. [Google Scholar] [CrossRef]
- Wang, B.; An, X.; Shi, X.; Zhang, J.-A. Management of Endocrine Disease: Suicide risk in patients with diabetes: A systematic review and meta-analysis. Eur. J. Endocrinol. 2017, 177, R169–R181. [Google Scholar] [CrossRef]
- Gradus, J.L.; Qin, P.; Lincoln, A.K.; Miller, M.; Lawler, E.; Sorensen, H.T. Inflammatory bowel disease and completed suicide in Danish adults. Inflamm. Bowel. Dis. 2010, 16, 2158–2561. [Google Scholar] [CrossRef]
- Leyhe, T.; Müssig, K. Cognitive and affective dysfunctions in autoimmune thyroiditis. Brain Behav. Immun. 2014, 41, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Siegmann, E.M.; Muller, H.H.O.; Luecke, C.; Philipsen, A.; Kornhuber, J.; Gromer, T.W. Association of Depression and Anxiety Disorders With Autoimmune Thyroiditis: A Systematic Review and Meta-analysis. JAMA Psychiatry 2018, 75, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Loh, H.H.; Lim, L.L.; Yee, A.; Loh, H.S. Association between subclinical hypothyroidism and depression: An updated systematic review and meta-analysis. BMC Psychiatry 2019, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Fountoulakis, K.N.; Iacovides, A.; Grammaticos, P.; St Kaprinis, G.; Bech, P. Thyroid function in clinical subtypes of major depression: An exploratory study. BMC Psychiatry 2004, 4, 6. [Google Scholar] [CrossRef]
- Carta, M.G.; Loviselli, A.; Hardoy, M.C.; Massa, S.; Cadeddu, M.; Sardu, C. The link between thyroid autoimmunity (antithyroid peroxidase autoantibodies) with anxiety and mood disorders in the community: A field of interest for public health in the future. BMC Psychiatry 2004, 4, 25. [Google Scholar] [CrossRef]
- Leyhe, T.; Hugle, M.; Gallwitz, B.; Saur, R.; Eschweiler, G.W. Increased occurrence of severe episodes in elderly depressed patients with elevated anti-thyroid antibody levels. Int. J. Geriatr. Psychiatry 2009, 24, 779–781. [Google Scholar] [CrossRef]
- Kupka, R.W.; Nolen, W.A.; Post, R.M.; McElroy, S.L.; Altshuler, L.L.; Denicoff, K.D. High rate of autoimmune thyroiditis in bipolar disorder: Lack of association with lithium exposure. Biol. Psychiatry 2002, 51, 305–311. [Google Scholar] [CrossRef]
- Vandoolaeghe, E.; Maes, M.; Vandevyvere, J.; Neels, H. Hypothalamic-pituitary-thyroid-axis function in treatment resistant depression. J. Affect. Disord. 1997, 43, 143–150. [Google Scholar] [CrossRef]
- Loosen, P.T. Hormones of the hypothalamic-pituitary-thyroid axis: A psychoneuroendocrine perspective. Pharmacopsychiatry 1986, 19, 401–415. [Google Scholar] [CrossRef]
- Hage, M.P.; Azar, S.T. The Link between Thyroid Function and Depression. J. Thyroid. Res. 2012, 2012, 590648. [Google Scholar] [CrossRef]
- Garlow, S.J.; Dunlop, B.W.; Ninan, P.T.; Nemeroff, C.B. The combination of triiodothyronine (T3) and sertraline is not superior to sertraline monotherapy in the treatment of major depressive disorder. J. Psychiatr. Res. 2012, 46, 1406–1413. [Google Scholar] [CrossRef]
- Kelly, T.F.; Lieberman, D.Z. Long term augmentation with T3 in refractory major depression. J. Affect. Disord. 2009, 115, 230–233. [Google Scholar] [CrossRef]
- Touma, K.T.B.; Zoucha, A.M.; Scarff, J.R. Liothyronine for Depression: A Review and Guidance for Safety Monitoring. Innov. Clin. Neurosci. 2017, 14, 24–29. [Google Scholar] [PubMed]
- Cooper-Kazaz, R.; Apter, J.T.; Cohen, R.; Karagichev, L.; Muhammed-Moussa, S.; Grupper, D. Combined treatment with sertraline and liothyronine in major depression: A randomized, double-blind, placebo-controlled trial. Arch. Gen. Psychiatry 2007, 64, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Nestler, E.J. Linking molecules to mood: New insight into the biology of depression. Am. J. Psychiatry 2010, 167, 1305–1320. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, J.P.; Appelhof, B.C.; Hoogendijk, W.J.; Huyser, J.; Endert, E.; Zuketto, C. Thyroid and adrenal axis in major depression: A controlled study in outpatients. Eur. J. Endocrinol. 2005, 152, 185–191. [Google Scholar] [CrossRef]
- Duval, F.; Mokrani, M.-C.; Bailey, P.; Correa, H.; Diep, T.-S.; Crocq, M.-A.; Macher, J.-P. Thyroid axis activity and serotonin function in major depressive episode. Psychoneuroendocrinology 1999, 24, 695–712. [Google Scholar] [CrossRef]
- Jackson, I.M. The Thyroid Axis and Depression. Thyroid 1998, 8, 951–956. [Google Scholar] [CrossRef]

| First Author | Publication Year | Country | Study Type | Suicidal Behavior Group | Control Group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Female (%) | Mean Age (SD) | Group Description | Suicidal Behavior Assessment | N | Female (%) | Mean Age (SD) | Group Description | ||||
| Baek | 2014 | South Korea | Cross-sectional | 91 | 78.0 | 40.1 (NR) | Patients newly diagnosed with major depressive disorder [Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV criteria]-Recent and lifetime suicide attempters | The Korean version of the Mini International Neuropsychiatric Interview (MINI) suicidality module. Three groups: recent suicide attempters (within the last month), lifetime attempters (those who have attempted suicide in their lifetime except for the past month) and never attempters | 464 | 72 | 47.5 (15.6) | Patients newly diagnosed with major depressive disorder (DSM-IV criteria)-Never suicide attempters |
| Berlin | 1999 | France | Cross-sectional | 39 | NR | NR | Patients with major depressive disorder (DSM-III-R criteria) and necessitating hospitalization-Previous suicide attempt | NR | 55 | NR | NR | Patients with major depressive disorder (DSM-III-R criteria) and necessitating hospitalization-No previous suicide attempt |
| Butkute-Sliuoziene | 2018 | Lithuania | Cross-sectional | 56 | 66.1 | 36.5 (13.1) | Adult non-psychotic patients, without organic brain disorder, hospitalized due to high suicide risk (Suicide ideation or attempt) | An authors’ composed socio-demographic questionnaire including questions about history and type of suicidal ideations or behavior. The majority of patients presented after a suicidal attempt (83.9%) and a small percentage with severe suicidal ideations and high risk of suicide (16.1%) | 120 | 42.5 | 34.3 (13.0) | Healthy volunteers (without a history of mental disorders or suicide attempts) |
| Duval | 2010 | France | Case/Control | 53 | 58.5 | 38.1 (10.7) | Inpatients with current major depressive episode (DSM-IV criteria) and recent suicidal attempt or history of suicidal attempt | Semi-structured interview with an experienced psychiatrist and a review of medical records. Recent suicide attempters: if the suicidal act occurred during the current depressive episode. Past suicide attempters, if the most recent suicide attempt had not occurred during the current depressive episode | 42 | 45.2 | 40.9 (11.1) | Inpatients with current major depressive episode (DSM-IV criteria) and no history of suicidal attempt |
| Duval | 2017 | France | Case/Control | 122 | 68 | 39.4 (11.8) | Adults with major depressive episode (DSM-5 criteria) (95 with major depressive disorder; 18 with bipolar I disorder, and 9 with bipolar II disorder) with a suicide attempt that occurred within the last twp years | Semi-structured interview with an experienced psychiatrist. Current/recent suicide attempt (occurred within the last year) and in early remission/history of suicide attempt (the last suicide attempt occurred 12–24 months prior to evaluation) | 50 | 62 | 40.2 (8.3) | Hospitalized volunteers free of concomitant psychiatric and medical illness |
| Jokinen | 2008 | Sweden | Case/Control | 12 | 0 | 32 (7.2) | Patients not receiving any antidepressant treatment admitted to the hospital after a suicide attempt | The Suicide Intent Scale performed by a psychiatrist within 48 h after admission. This 15-item instrument was designed to measure the factual aspects of a suicide attempt | 8 | 0 | 24 (1.8) | Healthy volunteers without psychiatric history |
| Jose | 2015 | India | Case/Control | 7 | 0 | 23.3 (3.8) | Male patients with schizophrenia (DSM IV TR criteria) aged 18 to 45 years and who were not on any treatment for schizophrenia for at least 4 weeks-Suicidal ideation present in the last three months | The Columbia Suicide Severity Rating Scale assessing suicidal ideations and behaviors for recent past (three months) was used | 31 | 0 | 30.3 (7.1) | Male patients with schizophrenia (DSM IV TR criteria) aged 18 to 45 years and who were not on any treatment for schizophrenia for at least four weeks-Suicidal ideation absent in the last three months |
| Khurshid | 2018 | Pakistan | Cross-sectional | 54 | 39 | 30.5 (10.1) | Consecutive patients with past history of suicide attempt/ideation | NR | 50 | 44 | NR | Psychiatric patients without suicide attempt or ideation |
| Kim | 2013 | South Korea | Cross-sectional | 112 | 100 | 41.7 (13.9) | Female patients with major depressive disorder from the outpatient clinic-Suicide attempt/ideation within the last month | The Korean version of the MINI suicidality module assessing suicidal behavior within the last month | 339 | 100 | 49.1 (14.7) | Female patients with major depressive disorder from the outpatient clinic-No history of suicide attempt/ideation |
| Maes | 1989 | Belgium | Cross-sectional | 17 | 100 | 48.5 (10.5) | Female patients with major depressive disorder and suicidal ideation | Suicidal ideation was determined when the Hamilton Rating Scale for Depression score on item 3 (suicide) was three, and when the item on suicide of the Structured Clinical Interview for DSM-III (depression section) was definitely positive | 17 | 100 | 50.4 (10.0) | Female patients with major depressive disorder with no suicide ideation |
| Ozcan | 2016 | Turkey | Case/Control | 115 | 67.8 | 25.9 (11.6) | Suicide attempters who were admitted consecutively to the Emergency Room | Evaluation of DSM IV-TR criteria and details of previous and current psychiatric history within the last six months were recorded by means of a semi-structured interview by a senior and experienced psychiatry resident | 243 | 68.4a | 26.4 (6.6)a | Subjects who were admitted consecutively to the hospital with no suicide attempt |
| Peng | 2018 | China | Cross-sectional | 69 | 59.9 | 36.4 (15.5) | Suicidal attempters incorporated after a suicide attempt at a university hospital | The Hamilton Depression Rating Scale and the Beck Scale for Suicidal Ideation | 202 | 61.4 | 36.4 (15.7) | Subjects with a major depressive episode without suicidal behavior |
| Pompili | 2012 | Italy | Cross-sectional | 79 | 63.3 | 40.8 (13.9) | Patients suffering from mood disorders and psychosis consecutively admitted to the emergency department-Suicide attempt | An interview a psychiatrist performing mental examinations relied on the MINI and one section of this instrument dedicated to the assessment of suicidal risk, with questions about past and current suicidality | 360 | 50.6 | 41.8 (13.3) | Patients suffering from mood disorders and psychosis consecutively admitted to the emergency department-No suicide attempt |
| Sensitivity Analyses | MD [95% CI] | I2 (%) | References Included | |
|---|---|---|---|---|
| Recent suicide attempt | ||||
| TSH (mIU/L) | −0.03 [−0.28 to 0.22] | 72 | [19,20,21,23,25,27,28,29] | |
| FT4 (ng/dL) | −0.08 [−0.26 to 0.11] | 98 | [19,20,21,27,28,29] | |
| TT4 (µg/dL) | −0.35 [−0.11 to 0.82] | 0 | [17,25] | |
| FT3 (pg/mL) | −0.19 [−0.39 to 0.01] | 91 | [19,20,21,27,28,29] | |
| TT3 (ng/dL) | −1.41 [−2.73 to 5.54] | 0 | [17,25] | |
| Recent suicidal ideation | ||||
| TSH (mIU/L) | −0.30 [−0.51 to −0.09] | 0 | [22,25,26] | |
| FT4 (ng/dL) | −0.07 [−0.35 to 0.49] | 84 | [22,26] | |
| History of suicide attempt | ||||
| TSH (mIU/L) | 0.18 [0.01 to 0.35] | 0 | [18,20,21,24] | |
| FT4 (ng/dL) | −0.06 [−0.10 to −0.01] | 0 | [18,20,21,24] | |
| FT3 (pg/mL) | −0.27 [−0.41 to −0.12] | 22 | [20,21,24] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toloza, F.J.K.; Mao, Y.; Menon, L.; George, G.; Borikar, M.; Thumma, S.; Motahari, H.; Erwin, P.; Owen, R.; Maraka, S. Association of Thyroid Function with Suicidal Behavior: A Systematic Review and Meta-Analysis. Medicina 2021, 57, 714. https://doi.org/10.3390/medicina57070714
Toloza FJK, Mao Y, Menon L, George G, Borikar M, Thumma S, Motahari H, Erwin P, Owen R, Maraka S. Association of Thyroid Function with Suicidal Behavior: A Systematic Review and Meta-Analysis. Medicina. 2021; 57(7):714. https://doi.org/10.3390/medicina57070714
Chicago/Turabian StyleToloza, Freddy J. K., Yuanjie Mao, Lakshmi Menon, Gemy George, Madhura Borikar, Soumya Thumma, Hooman Motahari, Patricia Erwin, Richard Owen, and Spyridoula Maraka. 2021. "Association of Thyroid Function with Suicidal Behavior: A Systematic Review and Meta-Analysis" Medicina 57, no. 7: 714. https://doi.org/10.3390/medicina57070714
APA StyleToloza, F. J. K., Mao, Y., Menon, L., George, G., Borikar, M., Thumma, S., Motahari, H., Erwin, P., Owen, R., & Maraka, S. (2021). Association of Thyroid Function with Suicidal Behavior: A Systematic Review and Meta-Analysis. Medicina, 57(7), 714. https://doi.org/10.3390/medicina57070714





