Contemporary Role of Cardiac Magnetic Resonance in the Management of Patients with Suspected or Known Coronary Artery Disease
Abstract
:1. Introduction
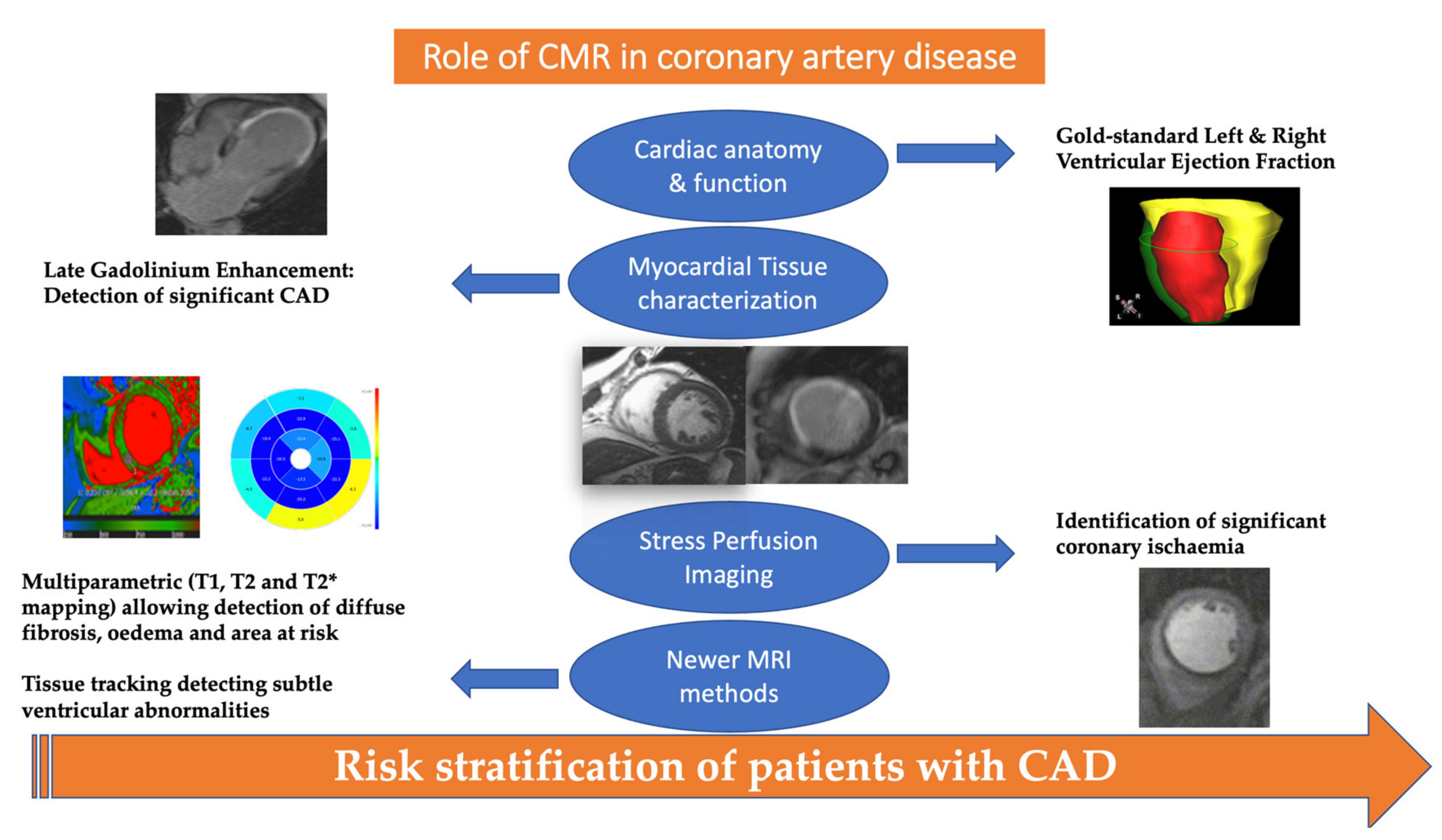
2. The Role of CMR in the Detection of Significant CAD
3. Role of CMR in the Risk Stratification of Patients with CAD
4. Limitations of CMR Imaging
5. Recent Advances in CMR Imaging
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the magnitude of coronary artery disease and acute coronary syndrome: A narrative review. J. Epidemiol. Glob. Health 2021. [Google Scholar] [CrossRef]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; Lopez-Sendon, J.; Alexander, K.P.; et al. Initial invasive or conservative strategy for stable coronary disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Lockie, T.; Nagel, E.; Redwood, S.; Plein, S. Use of cardiovascular magnetic resonance imaging in acute coronary syndromes. Circulation 2009, 119, 1671–1681. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Pandya, A.; Steel, K.; Bingham, S.; Jerosch-Herold, M.; Chen, Y.Y.; Mikolich, J.R.; Arai, A.E.; Bandettini, W.P.; Patel, A.R.; et al. Cost-effectiveness analysis of stress cardiovascular magnetic resonance imaging for stable chest pain syndromes. JACC Cardiovasc. Imaging 2020, 13, 1505–1517. [Google Scholar] [CrossRef]
- Greenwood, J.P.; Ripley, D.P.; Berry, C.; McCann, G.P.; Plein, S.; Bucciarelli-Ducci, C.; Dall’Armellina, E.; Prasad, A.; Bijsterveld, P.; Foley, J.R.; et al. Effect of care guided by cardiovascular magnetic resonance, myocardial perfusion scintigraphy, or NICE guidelines on subsequent unnecessary angiography rates: The CE-MARC 2 randomized clinical trial. JAMA 2016, 316, 1051–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulce, M.C.; Higgins, C.B. Evaluation of ventricular dimensions and function with magnetic resonance imaging. Am. J. Card. Imaging 1994, 8, 168–180. [Google Scholar] [PubMed]
- Beache, G.M.; Wedeen, V.J.; Dinsmore, R.E. Magnetic resonance imaging evaluation of left ventricular dimensions and function and pericardial and myocardial disease. Coron. Artery Dis. 1993, 4, 328–333. [Google Scholar] [CrossRef]
- Semelka, R.C.; Tomei, E.; Wagner, S.; Mayo, J.; Kondo, C.; Suzuki, J.; Caputo, G.R.; Higgins, C.B. Normal left ventricular dimensions and function: Interstudy reproducibility of measurements with cine MR imaging. Radiology 1990, 174, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Van Dijkman, P.R.; Hold, K.M.; van der Laarse, A.; Holman, E.R.; Ozdemir, H.I.; van der Nat, T.H.; de Roos, A.; van der Wall, E.E. Sequential analysis of infarcted and normal myocardium in piglets using in vivo gadolinium-enhanced MR images. Magn. Reson. Imaging 1993, 11, 207–218. [Google Scholar] [CrossRef]
- Kim, R.J.; Wu, E.; Rafael, A.; Chen, E.L.; Parker, M.A.; Simonetti, O.; Klocke, F.J.; Bonow, R.O.; Judd, R.M. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N. Engl. J. Med. 2000, 343, 1445–1453. [Google Scholar] [CrossRef]
- Pennell, C.D.J.; Underwood, S.R.; Longmore, D.B. Detection of coronary artery disease using MR imaging with dipyridamole infusion. J. Comput. Assist. Tomogr. 1990, 14, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Taylor, A.; Yang, Y.; Kuruvilla, S.; Ragosta, M.; Meyer, C.H.; Kramer, C.M. Adenosine stress cardiovascular magnetic resonance with variable-density spiral pulse sequences accurately detects coronary artery disease: Initial clinical evaluation. Circ. Cardiovasc. Imaging 2014, 7, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.L.; Bandettini, W.P.; Shanbhag, S.; Leung, S.W.; Wilson, J.R.; Arai, A.E. Safety and tolerability of regadenoson CMR. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 753–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charoenpanichkit, C.; Hundley, W.G. The 20 year evolution of dobutamine stress cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2010, 12, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwood, J.P.; Maredia, N.; Younger, J.F.; Brown, J.M.; Nixon, J.; Everett, C.C.; Bijsterveld, P.; Ridgway, J.P.; Radjenovic, A.; Dickinson, C.J.; et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): A prospective trial. Lancet 2012, 379, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Nagel, E.; Greenwood, J.P.; McCann, G.P.; Bettencourt, N.; Shah, A.M.; Hussain, S.T.; Perera, D.; Plein, S.; Bucciarelli-Ducci, C.; Paul, M.; et al. Magnetic resonance perfusion or fractional flow reserve in coronary disease. N. Engl. J. Med. 2019, 380, 2418–2428. [Google Scholar] [CrossRef]
- Kozor, R.; Walker, S.; Parkinson, B.; Younger, J.; Hamilton-Craig, C.; Selvanayagam, J.B.; Greenwood, J.P.; Taylor, A.J. Cost-effectiveness of cardiovascular magnetic resonance in diagnosing coronary artery disease in the australian health care system. Heart Lung Circ. 2021, 30, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Pontone, G.; Guaricci, A.I.; Palmer, S.C.; Andreini, D.; Verdecchia, M.; Fusini, L.; Lorenzoni, V.; Guglielmo, M.; Muscogiuri, G.; Baggiano, A.; et al. Diagnostic performance of non-invasive imaging for stable coronary artery disease: A meta-analysis. Int. J. Cardiol. 2020, 300, 276–281. [Google Scholar] [CrossRef]
- De Knegt, M.C.; Rossi, A.; Petersen, S.E.; Wragg, A.; Khurram, R.; Westwood, M.; Saberwal, B.; Mathur, A.; Nieman, K.; Bamberg, F.; et al. Stress myocardial perfusion with qualitative magnetic resonance and quantitative dynamic computed tomography: Comparison of diagnostic performance and incremental value over coronary computed tomography angiography. Eur. Heart J. Cardiovasc. Imaging 2020. [Google Scholar] [CrossRef]
- Bunce, N.H.; Reyes, E.; Keegan, J.; Bunce, C.; Davies, S.W.; Lorenz, C.H.; Pennell, D.J. Combined coronary and perfusion cardiovascular magnetic resonance for the assessment of coronary artery stenosis. J. Cardiovasc. Magn. Reson. 2004, 6, 527–539. [Google Scholar] [CrossRef]
- Silva Vieira, M.; Henningsson, M.; Dedieu, N.; Vassiliou, V.S.; Bell, A.; Mathur, S.; Pushparajah, K.; Figueroa, C.A.; Hussain, T.; Botnar, R.; et al. Improved coronary magnetic resonance angiography using gadobenate dimeglumine in pediatric congenital heart disease. Magn. Reson. Imaging 2018, 49, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, T.T.; Ang, B.W.Y.; Bryant, J.A.; Chin, C.Y.; Yeo, K.K.; Wong, P.E.H.; Ho, K.W.; Tan, J.W.C.; Lee, P.T.; Chin, C.W.L.; et al. Multiparametric exercise stress cardiovascular magnetic resonance in the diagnosis of coronary artery disease: The EMPIRE trial. J. Cardiovasc. Magn. Reson. 2021, 23, 17. [Google Scholar] [CrossRef]
- Zhang, L.; Song, X.; Dong, L.; Li, J.; Dou, R.; Fan, Z.; An, J.; Li, D. Additive value of 3T cardiovascular magnetic resonance coronary angiography for detecting coronary artery disease. J. Cardiovasc. Magn. Reson. 2018, 20, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, M.; Hundemer, A.; Zwicker, C.; Altiok, E.; Krohn, T.; Mottaghy, F.M.; Lente, C.; Kelm, M.; Marx, N.; Hoffmann, R. Detection of coronary artery disease in postmenopausal women: The significance of integrated stress imaging tests in a 4-year prognostic study. Clin. Res. Cardiol. 2015, 104, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Motwani, M.; Maredia, N.; Fairbairn, T.A.; Kozerke, S.; Radjenovic, A.; Greenwood, J.P.; Plein, S. High-resolution versus standard-resolution cardiovascular MR myocardial perfusion imaging for the detection of coronary artery disease. Circ. Cardiovasc. Imaging 2012, 5, 306–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, J.Y.; Ko, S.M.; Song, I.Y.; Yi, J.G.; Hwang, H.K.; Shin, J.K. Comparison of the diagnostic accuracies of 1.5T and 3T stress myocardial perfusion cardiovascular magnetic resonance for detecting significant coronary artery disease. Korean J. Radiol. 2018, 19, 1007–1020. [Google Scholar] [CrossRef]
- Desroche, L.M.; Milleron, O.; Safar, B.; Ou, P.; Garbarz, E.; Lavie-Badie, Y.; Abtan, J.; Millischer, D.; Pathak, A.; Durand-Zaleski, I.; et al. Cardiovascular magnetic resonance may avoid unnecessary coronary angiography in patients with unexplained left ventricular systolic dysfunction: A retrospective diagnostic pilot study. J. Card. Fail. 2020, 26, 1067–1074. [Google Scholar] [CrossRef]
- Romero, J.; Xue, X.; Gonzalez, W.; Garcia, M.J. CMR imaging assessing viability in patients with chronic ventricular dysfunction due to coronary artery disease: A meta-analysis of prospective trials. JACC Cardiovasc. Imaging 2012, 5, 494–508. [Google Scholar] [CrossRef] [Green Version]
- Mavrogeni, S.I.; Markousis-Mavrogenis, G.; Karapanagiotou, O.; Toutouzas, K.; Argyriou, P.; Velitsista, S.; Kanoupakis, G.; Apostolou, D.; Hautemann, D.; Sfikakis, P.P.; et al. Silent myocardial perfusion abnormalities detected by stress cardiovascular magnetic resonance in antiphospholipid syndrome: A case-control study. J. Clin. Med. 2019, 8, 1084. [Google Scholar] [CrossRef] [Green Version]
- Doan, T.T.; Wilkinson, J.C.; Loar, R.W.; Pednekar, A.S.; Masand, P.M.; Noel, C.V. Regadenoson stress perfusion cardiac magnetic resonance imaging in children with kawasaki disease and coronary artery disease. Am. J. Cardiol. 2019, 124, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Nanni, S.; Lovato, L.; Ghetti, G.; Vagnarelli, F.; Mineo, G.; Fattori, R.; Saia, F.; Marzocchi, A.; Marrozzini, C.; Zompatori, M.; et al. Utility of stress perfusion-cardiac magnetic resonance in follow-up of patients undergoing percutaneous coronary interventions of the left main coronary artery. Int. J. Cardiovasc. Imaging 2017, 33, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli-Ducci, C.; Auger, D.; Di Mario, C.; Locca, D.; Petryka, J.; O’Hanlon, R.; Grasso, A.; Wright, C.; Symmonds, K.; Wage, R.; et al. CMR guidance for recanalization of coronary chronic total occlusion. JACC Cardiovasc. Imaging 2016, 9, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Pica, S.; Di Giovine, G.; Bollati, M.; Testa, L.; Bedogni, F.; Camporeale, A.; Pontone, G.; Andreini, D.; Monti, L.; Gasparini, G.; et al. Cardiac magnetic resonance for ischaemia and viability detection. Guiding patient selection to revascularization in coronary chronic total occlusions: The CARISMA_CTO study design. Int. J. Cardiol. 2018, 272, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Van Kranenburg, M.; Magro, M.; Thiele, H.; de Waha, S.; Eitel, I.; Cochet, A.; Cottin, Y.; Atar, D.; Buser, P.; Wu, E.; et al. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc. Imaging 2014, 7, 930–939. [Google Scholar] [CrossRef]
- Tamarappoo, B.; Samuel, T.J.; Elboudwarej, O.; Thomson, L.E.J.; Aldiwani, H.; Wei, J.; Mehta, P.; Cheng, S.; Sharif, B.; AlBadri, A.; et al. Left ventricular circumferential strain and coronary microvascular dysfunction: A report from the Women’s Ischemia Syndrome Evaluation Coronary Vascular Dysfunction (WISE-CVD) project. Int. J. Cardiol. 2021, 327, 25–30. [Google Scholar] [CrossRef]
- Zorach, B.; Shaw, P.W.; Bourque, J.; Kuruvilla, S.; Balfour, P.C., Jr.; Yang, Y.; Mathew, R.; Pan, J.; Gonzalez, J.A.; Taylor, A.M.; et al. Quantitative cardiovascular magnetic resonance perfusion imaging identifies reduced flow reserve in microvascular coronary artery disease. J. Cardiovasc. Magn. Reson. 2018, 20, 14. [Google Scholar] [CrossRef] [Green Version]
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur. Heart J. 2017, 38, 143–153. [Google Scholar] [CrossRef]
- Leurent, G.; Langella, B.; Fougerou, C.; Lentz, P.A.; Larralde, A.; Bedossa, M.; Boulmier, D.; Le Breton, H. Diagnostic contributions of cardiac magnetic resonance imaging in patients presenting with elevated troponin, acute chest pain syndrome and unobstructed coronary arteries. Arch. Cardiovasc. Dis. 2011, 104, 161–170. [Google Scholar] [CrossRef]
- Pasupathy, S.; Air, T.; Dreyer, R.P.; Tavella, R.; Beltrame, J.F. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 2015, 131, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Catalano, O.; Moro, G.; Mori, A.; Perotti, M.; Gualco, A.; Frascaroli, M.; Pesarin, C.; Napolitano, C.; Ntusi, N.A.B.; Priori, S.G. Cardiac magnetic resonance in stable coronary artery disease: Added prognostic value to conventional risk profiling. Biomed. Res. Int. 2018, 2018, 2806148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteban-Fernandez, A.; Bastarrika, G.; Castanon, E.; Coma-Canella, I.; Barba-Cosials, J.; Jimenez-Martin, M.; Alpendurada, F.; Gavira, J.J.; Azcarate-Aguero, P.M. Prognostic role of stress cardiac magnetic resonance in the elderly. Rev. Esp. Cardiol. (Engl. Ed.) 2020, 73, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Filho, O.R.; Seabra, L.F.; Mongeon, F.P.; Abdullah, S.M.; Francis, S.A.; Blankstein, R.; Di Carli, M.F.; Jerosch-Herold, M.; Kwong, R.Y. Stress myocardial perfusion imaging by CMR provides strong prognostic value to cardiac events regardless of patient’s sex. JACC Cardiovasc. Imaging 2011, 4, 850–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puntmann, V.O.; Carr-White, G.; Jabbour, A.; Yu, C.Y.; Gebker, R.; Kelle, S.; Rolf, A.; Zitzmann, S.; Peker, E.; D’Angelo, T.; et al. Native T1 and ECV of noninfarcted myocardium and outcome in patients with coronary artery disease. J. Am. Coll. Cardiol. 2018, 71, 766–778. [Google Scholar] [CrossRef]
- Indorkar, R.; Kwong, R.Y.; Romano, S.; White, B.E.; Chia, R.C.; Trybula, M.; Evans, K.; Shenoy, C.; Farzaneh-Far, A. Global coronary flow reserve measured during stress cardiac magnetic resonance imaging is an independent predictor of adverse cardiovascular events. JACC Cardiovasc. Imaging 2019, 12, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Saito, N.; Nakachi, T.; Fukui, K.; Iwasawa, T.; Taguri, M.; Kosuge, M.; Kimura, K. Stress perfusion coronary flow reserve versus cardiac magnetic resonance for known or suspected CAD. J. Am. Coll. Cardiol. 2017, 70, 869–879. [Google Scholar] [CrossRef]
- Kaolawanich, Y.; Boonyasirinant, T. Incremental prognostic value of aortic stiffness in addition to myocardial ischemia by cardiac magnetic resonance imaging. BMC Cardiovasc. Disord. 2020, 20, 287. [Google Scholar] [CrossRef]
- Marcos-Garces, V.; Gavara, J.; Monmeneu, J.V.; Lopez-Lereu, M.P.; Perez, N.; Rios-Navarro, C.; De Dios, E.; Moratal, D.; Minana, G.; Nunez, J.; et al. A novel clinical and stress cardiac magnetic resonance (C-CMR-10) score to predict long-term all-cause mortality in patients with known or suspected chronic coronary syndrome. J. Clin. Med. 2020, 9, 1957. [Google Scholar] [CrossRef]
- Freed, B.H.; Narang, A.; Bhave, N.M.; Czobor, P.; Mor-Avi, V.; Zaran, E.R.; Turner, K.M.; Cavanaugh, K.P.; Chandra, S.; Tanaka, S.M.; et al. Prognostic value of normal regadenoson stress perfusion cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2013, 15, 108. [Google Scholar] [CrossRef] [Green Version]
- Kelle, S.; Chiribiri, A.; Vierecke, J.; Egnell, C.; Hamdan, A.; Jahnke, C.; Paetsch, I.; Wellnhofer, E.; Fleck, E.; Klein, C.; et al. Long-term prognostic value of dobutamine stress CMR. JACC Cardiovasc. Imaging 2011, 4, 161–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.W.; Sultan, F.A.T.; Awan, S.; Ahmed, I. Prognostic significance of CMR findings in patients with known coronary artery disease—Experience from a South Asian country. J. Clin. Imaging Sci. 2020, 10, 75. [Google Scholar] [CrossRef]
- Zhou, W.; Lee, J.C.Y.; Leung, S.T.; Lai, A.; Lee, T.F.; Chiang, J.B.; Cheng, Y.W.; Chan, H.L.; Yiu, K.H.; Goh, V.K.; et al. Long-term prognosis of patients with coronary microvascular disease using stress perfusion cardiac magnetic resonance. JACC Cardiovasc. Imaging 2021, 14, 602–611. [Google Scholar] [CrossRef]
- Pezel, T.; Sanguineti, F.; Kinnel, M.; Landon, V.; Bonnet, G.; Garot, P.; Hovasse, T.; Unterseeh, T.; Champagne, S.; Louvard, Y.; et al. Safety and prognostic value of vasodilator stress cardiovascular magnetic resonance in patients with heart failure and reduced ejection fraction. Circ. Cardiovasc. Imaging 2020, 13, e010599. [Google Scholar] [CrossRef] [PubMed]
- Pezel, T.; Sanguineti, F.; Kinnel, M.; Hovasse, T.; Garot, P.; Unterseeh, T.; Champagne, S.; Louvard, Y.; Morice, M.C.; Garot, J. Prognostic value of dipyridamole stress perfusion cardiovascular magnetic resonance in elderly patients >75 years with suspected coronary artery disease. Eur. Heart J. Cardiovasc. Imaging 2020. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, M.J.; McVey, C.M.; Berger, J.S.; Kramer, C.M.; Salerno, M. Prognostic value of stress cardiac magnetic resonance imaging in patients with known or suspected coronary artery disease: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2013, 62, 826–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gargiulo, P.; Dellegrottaglie, S.; Bruzzese, D.; Savarese, G.; Scala, O.; Ruggiero, D.; D’Amore, C.; Paolillo, S.; Agostoni, P.; Bossone, E.; et al. The prognostic value of normal stress cardiac magnetic resonance in patients with known or suspected coronary artery disease: A meta-analysis. Circ. Cardiovasc. Imaging 2013, 6, 574–582. [Google Scholar] [CrossRef] [Green Version]
- Kwong, R.Y.; Ge, Y.; Steel, K.; Bingham, S.; Abdullah, S.; Fujikura, K.; Wang, W.; Pandya, A.; Chen, Y.Y.; Mikolich, J.R.; et al. Cardiac magnetic resonance stress perfusion imaging for evaluation of patients with chest pain. J. Am. Coll. Cardiol. 2019, 74, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Zemrak, F.; Petersen, S.E. Late gadolinium enhancement CMR predicts adverse cardiovascular outcomes and mortality in patients with coronary artery disease: Systematic review and meta-analysis. Prog. Cardiovasc. Dis. 2011, 54, 215–229. [Google Scholar] [CrossRef]
- Gerber, B.L.; Rousseau, M.F.; Ahn, S.A.; le Polain de Waroux, J.B.; Pouleur, A.C.; Phlips, T.; Vancraeynest, D.; Pasquet, A.; Vanoverschelde, J.L. Prognostic value of myocardial viability by delayed-enhanced magnetic resonance in patients with coronary artery disease and low ejection fraction: Impact of revascularization therapy. J. Am. Coll. Cardiol. 2012, 59, 825–835. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Lu, M.; Ouyang, W.; Li, B.; Yang, Y.; Zhao, S.; Sun, H. Prognostic value of myocardial scar by magnetic resonance imaging in patients undergoing coronary artery bypass graft. Int. J. Cardiol. 2021, 326, 49–54. [Google Scholar] [CrossRef]
- Alexandre, J.; Saloux, E.; Dugue, A.E.; Lebon, A.; Lemaitre, A.; Roule, V.; Labombarda, F.; Provost, N.; Gomes, S.; Scanu, P.; et al. Scar extent evaluated by late gadolinium enhancement CMR: A powerful predictor of long term appropriate ICD therapy in patients with coronary artery disease. J. Cardiovasc. Magn. Reson. 2013, 15, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, P.A.; Morgan, J.M.; Carroll, N.; Murday, D.C.; Roberts, P.R.; Peebles, C.R.; Harden, S.P.; Curzen, N.P. The extent of left ventricular scar quantified by late gadolinium enhancement MRI is associated with spontaneous ventricular arrhythmias in patients with coronary artery disease and implantable cardioverter-defibrillators. Circ. Arrhythm. Electrophysiol. 2011, 4, 324–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghbayan, H.; Lougheed, N.; Deva, D.P.; Chan, K.K.W.; Lima, J.A.C.; Yan, A.T. Peri-infarct quantification by cardiac magnetic resonance to predict outcomes in ischemic cardiomyopathy: Prognostic systematic review and meta-analysis. Circ. Cardiovasc. Imaging 2019, 12, e009156. [Google Scholar] [CrossRef]
- Kwon, D.H.; Hachamovitch, R.; Adeniyi, A.; Nutter, B.; Popovic, Z.B.; Wilkoff, B.L.; Desai, M.Y.; Flamm, S.D.; Marwick, T. Myocardial scar burden predicts survival benefit with implantable cardioverter defibrillator implantation in patients with severe ischaemic cardiomyopathy: Influence of gender. Heart 2014, 100, 206–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghadimi, M.; Sapra, A. Magnetic Resonance Imaging Contraindications; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Scatteia, A.; Baritussio, A.; Bucciarelli-Ducci, C. Strain imaging using cardiac magnetic resonance. Heart Fail. Rev. 2017, 22, 465–476. [Google Scholar] [CrossRef]
- Seetharam, K.; Lerakis, S. Cardiac magnetic resonance imaging: The future is bright. F1000Research 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Knott, K.D.; Camaioni, C.; Ramasamy, A.; Augusto, J.A.; Bhuva, A.N.; Xue, H.; Manisty, C.; Hughes, R.K.; Brown, L.A.E.; Amersey, R.; et al. Quantitative myocardial perfusion in coronary artery disease: A perfusion mapping study. J. Magn. Reson. Imaging 2019, 50, 756–762. [Google Scholar] [CrossRef] [Green Version]
- Knott, K.D.; Seraphim, A.; Augusto, J.B.; Xue, H.; Chacko, L.; Aung, N.; Petersen, S.E.; Cooper, J.A.; Manisty, C.; Bhuva, A.N.; et al. The prognostic significance of quantitative myocardial perfusion: An artificial intelligence-based approach using perfusion mapping. Circulation 2020, 141, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
| Advantages | Disadvantages | |
|---|---|---|
| Stress echocardiography |
|
|
| SPECT |
|
|
| CMR |
|
|
| CCTA |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazoukis, G.; Papadatos, S.S.; Michelongona, A.; Lampropoulos, K.; Farmakis, D.; Vassiliou, V. Contemporary Role of Cardiac Magnetic Resonance in the Management of Patients with Suspected or Known Coronary Artery Disease. Medicina 2021, 57, 649. https://doi.org/10.3390/medicina57070649
Bazoukis G, Papadatos SS, Michelongona A, Lampropoulos K, Farmakis D, Vassiliou V. Contemporary Role of Cardiac Magnetic Resonance in the Management of Patients with Suspected or Known Coronary Artery Disease. Medicina. 2021; 57(7):649. https://doi.org/10.3390/medicina57070649
Chicago/Turabian StyleBazoukis, George, Stamatis S. Papadatos, Archontoula Michelongona, Konstantinos Lampropoulos, Dimitrios Farmakis, and Vassilis Vassiliou. 2021. "Contemporary Role of Cardiac Magnetic Resonance in the Management of Patients with Suspected or Known Coronary Artery Disease" Medicina 57, no. 7: 649. https://doi.org/10.3390/medicina57070649
APA StyleBazoukis, G., Papadatos, S. S., Michelongona, A., Lampropoulos, K., Farmakis, D., & Vassiliou, V. (2021). Contemporary Role of Cardiac Magnetic Resonance in the Management of Patients with Suspected or Known Coronary Artery Disease. Medicina, 57(7), 649. https://doi.org/10.3390/medicina57070649






