The Case of a 44-Year-Old Survivor of Unrepaired Tetralogy of Fallot, Right Aortic Arch and Abdominal Aortopulmonary Collateral Vessels
Abstract
:1. Introduction
2. Case Report
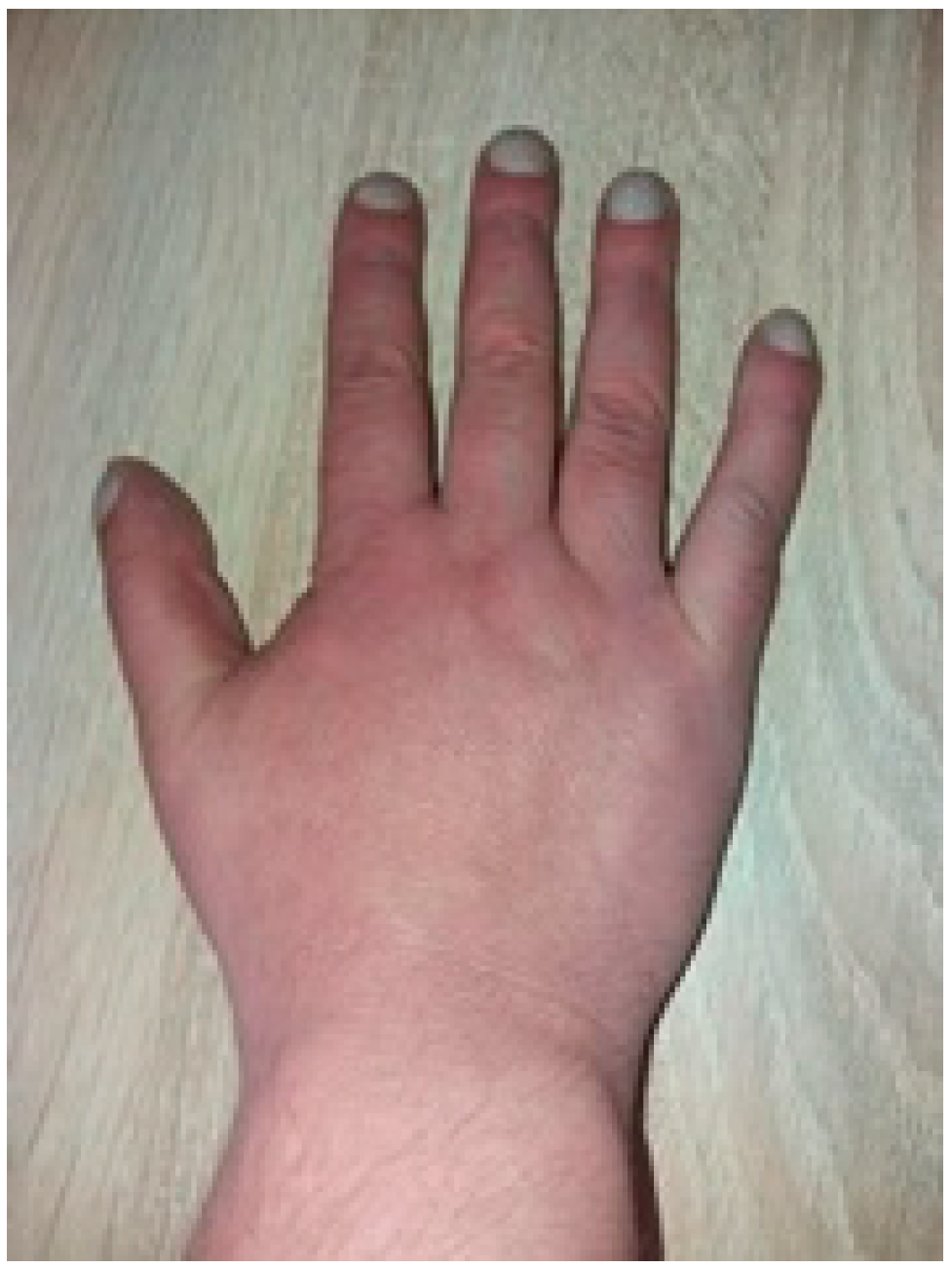
2.1. Initial Work Up
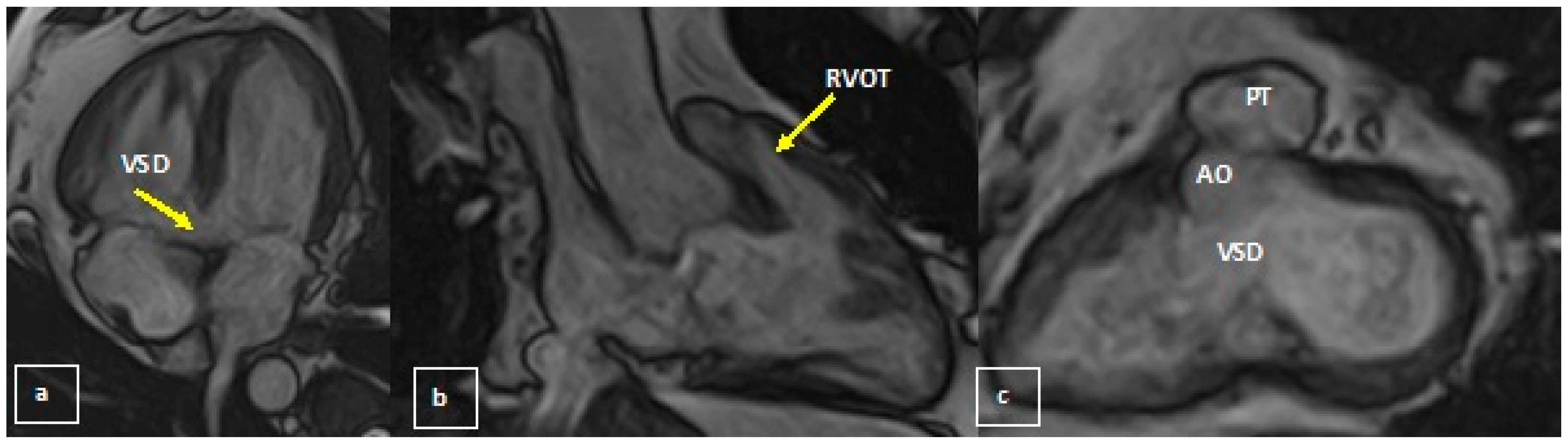
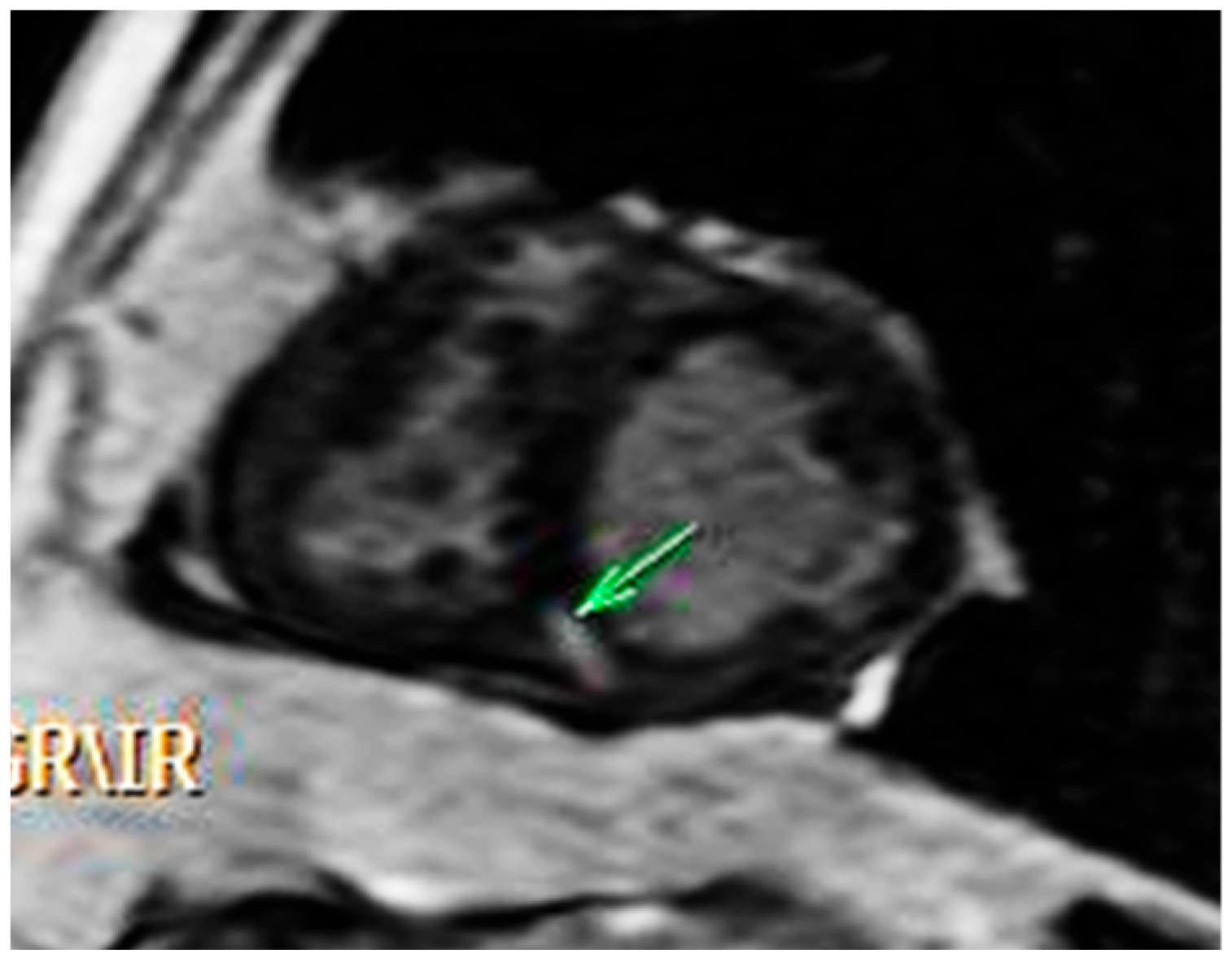
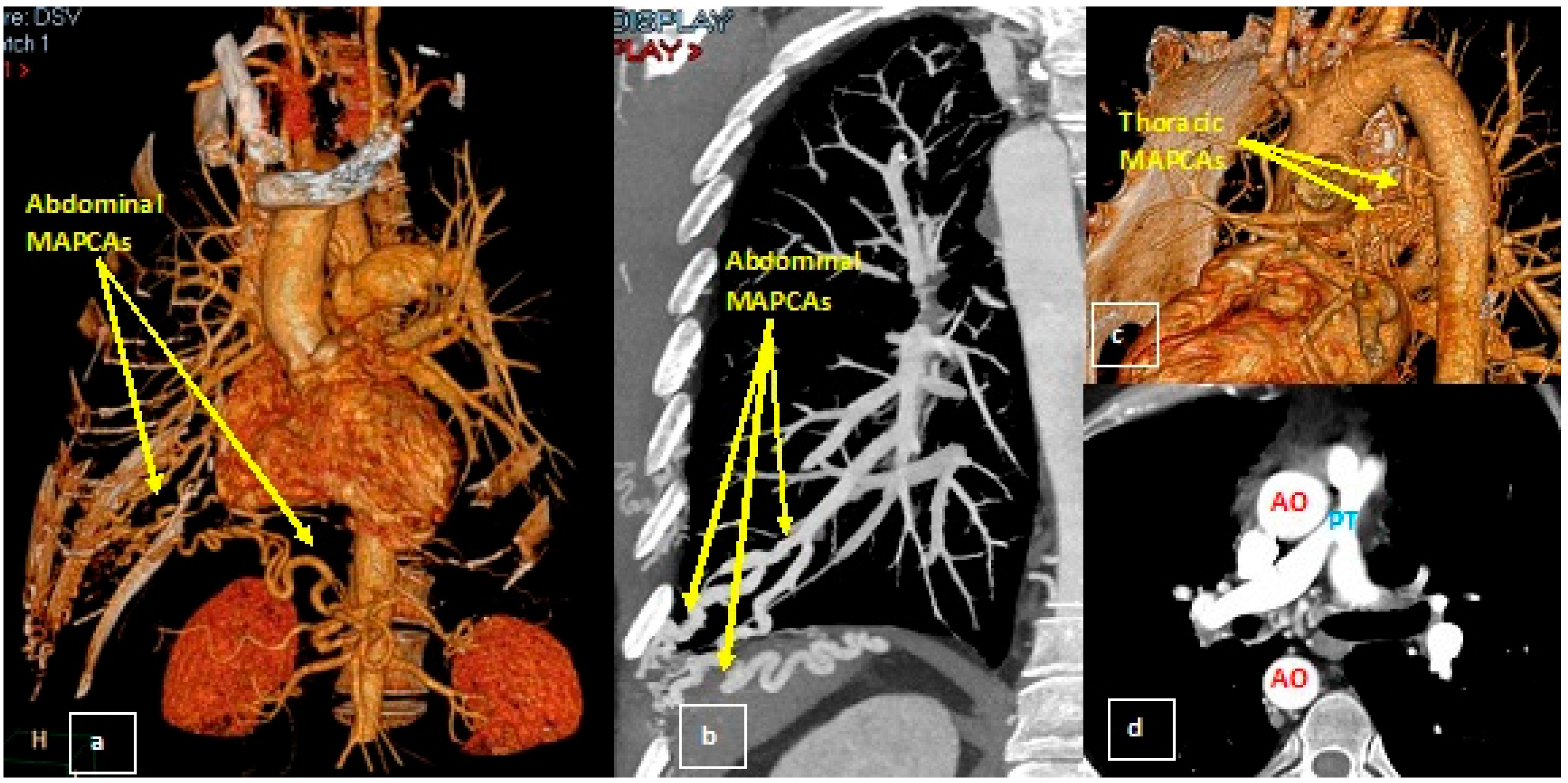
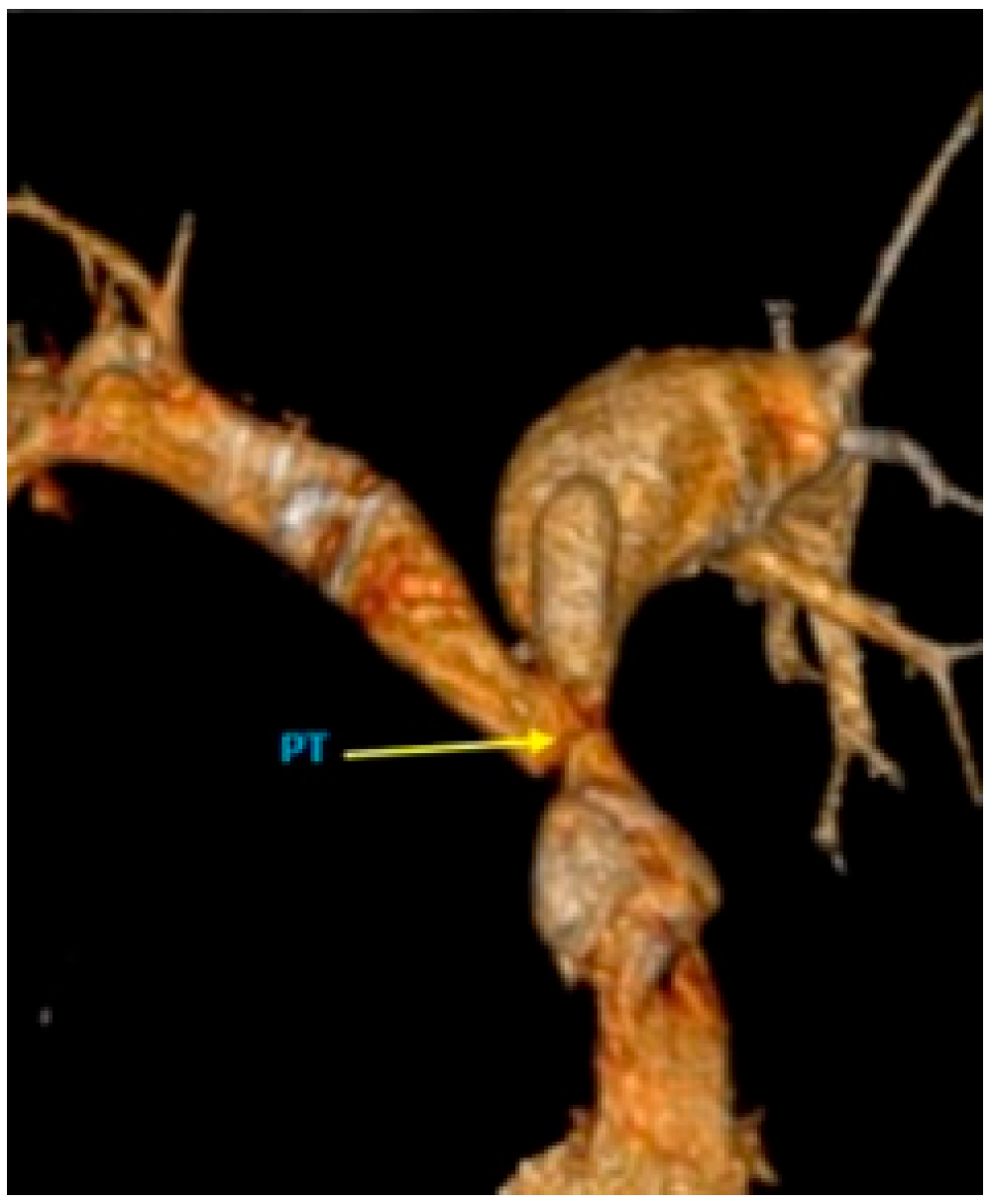
2.2. Diagnosis and Management
3. Discussion
Follow-Up
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prakash, S.S.; Sharma, R.; Agrawal, N.; Shankar, S. An extraordinary survival at the age of 67 years in an unoperated case tetralogy of Fallot presenting as systemic hypertension despite RV dysfunction. BMJ Case Rep. 2014, 2014, bcr2013202647. [Google Scholar] [CrossRef] [PubMed]
- Dobrocky, T.; Klink, T.; Weisstanner, C.; Heverhagen, J.; Christe, A. Imaging findings in uncorrected tetralogy of Fallot and pulmonary atresia with major aortopulmonary collateral arteries and septic embolism. Acta Radiol. Short Rep. 2014, 3, 2047981613515211. [Google Scholar] [CrossRef] [PubMed]
- Boyer, R.; Kim, H.J.; Krishnan, R. Management of Unoperated Tetralogy of Fallot in a 59-Year-Old Patient. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620926908. [Google Scholar] [CrossRef] [PubMed]
- Spaziani, G.; Favilli, S.; Fonda, C.; Chiappa, E. Giant aorto-pulmonary collaterals in pulmonary atresia and ventricular septal defect: Long-term survival in unoperated adults. J. Cardiovasc. Med. 2013, 14, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Patrick, W.L.; Mainwaring, R.D.; Reinhartz, O.; Punn, R.; Tacy, T.; Hanley, F.L. Major Aortopulmonary Collateral Arteries With Anatomy Other Than Pulmonary Atresia/Ventricular Septal Defect. Ann. Thorac. Surg. 2017, 104, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Murthy, K.S.; Reddy, K.P.; Nagarajan, R.; Goutami, V.; Cherian, K.M. Management of ventricular septal defect with pulmonary atresia and major aorto pulmonary collateral arteries: Challenges and controversies. Ann. Pediatr. Cardiol. 2010, 3, 127–135. [Google Scholar] [CrossRef]
- D’Udekem, Y.; Alphonso, N.; Nørgaard, M.A.; Cochrane, A.D.; Grigg, L.E.; Wilkinson, J.L.; Brizard, C.P. Pulmonary atresia with ventricular septal defects and major aortopulmonary collateral arteries: Unifocalization brings no long-term benefits. J. Thorac. Cardiovasc. Surg. 2005, 130, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, S.A.E.F.; Hussein, M.M.; Sultan, A.A.; Bilal, M.M.Z.; El Gamal, M.A.F.; Sobh, D.M. Three steps approach for preoperative evaluation of tetralogy of Fallot patients: Role of 128 MDCT. Egypt J. Radiol. Nucl. Med. 2021, 52, 47. [Google Scholar] [CrossRef]
- Guta, A.C.; Nicula, A.I.; Radu, D.N.; Olaru-Lego, G.; Calin, A.; Predescu, L.P.; Radulescu, B.; Popescu, B.A.; Enache, R. P583A classical dilemma—To be or not to be constriction—Solved by CMR. Eur. Hear. J.-Cardiovasc. Imaging 2019, 20, jez108.019. [Google Scholar] [CrossRef]
- Antonetti, I.; Lorch, D.; Coe, B.; Maxey, T.S.; Nallamshetty, L.; Dadlani, G.H.; Berlowitz, M.S.; Cohen, A.J.; Guglin, M.E. Unrepaired Tetralogy of Fallot with Major Aortopulmonary Collateral Arteries in an Adult Patient. Congenit. Heart Dis. 2013, 8, E24–E30. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulou, S.C.; Manginas, A.; Kelekis, N.L.; Noutsias, M. Cardiovascular imaging approach in pre and postoperative tetralogy of Fallot 11 Medical and Health Sciences 1103 Clinical Sciences. BMC Cardiovasc. Disord. 2019, 19, 7. [Google Scholar]
- Geva, T. Repaired tetralogy of Fallot: The roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J. Cardiovasc. Magn. Reson. 2011, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, C.; Dubois, J.; Rypens, F.; Raboisson, M.J.; Déry, J. Tetralogy of Fallot: Preoperative assessment with MR and CT imaging. Diagn. Interv. Imaging 2016, 97, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Ross, O.; Griksaitis, M.J. Tetralogy of Fallot. BJA Educ. 2019, 19, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, E.; Splendiani, A.; Barile, A.; Squillaci, E.; Di Cesare, A.; Brunese, L.; Masciocchi, C. CT and MR imaging of the thoracic aorta. Open Med. 2016, 11, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Baicus, C.; Balanescu, P.; Zeh, S.; Oprisan, E.; Lapadatu, R.; Gurghean, A.; Padureanu, V.; Rezus, C.; Mitu, F.; Jurcut, R.; et al. Characteristics of shared decision making in Romania from the patient perspective: A cross-sectional multicentric study. J. Eval. Clin. Pract. 2019, 25, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciltea, R.; Nicula, A.I.; Bajdechi, M.; Scafa-Udriste, A.; Rimbas, R.; Iana, G.; Vinereanu, D. The Case of a 44-Year-Old Survivor of Unrepaired Tetralogy of Fallot, Right Aortic Arch and Abdominal Aortopulmonary Collateral Vessels. Medicina 2022, 58, 1011. https://doi.org/10.3390/medicina58081011
Ciltea R, Nicula AI, Bajdechi M, Scafa-Udriste A, Rimbas R, Iana G, Vinereanu D. The Case of a 44-Year-Old Survivor of Unrepaired Tetralogy of Fallot, Right Aortic Arch and Abdominal Aortopulmonary Collateral Vessels. Medicina. 2022; 58(8):1011. https://doi.org/10.3390/medicina58081011
Chicago/Turabian StyleCiltea, Roxana, Alina Ioana Nicula, Mircea Bajdechi, Alexandru Scafa-Udriste, Roxana Rimbas, Gheorghe Iana, and Dragos Vinereanu. 2022. "The Case of a 44-Year-Old Survivor of Unrepaired Tetralogy of Fallot, Right Aortic Arch and Abdominal Aortopulmonary Collateral Vessels" Medicina 58, no. 8: 1011. https://doi.org/10.3390/medicina58081011
APA StyleCiltea, R., Nicula, A. I., Bajdechi, M., Scafa-Udriste, A., Rimbas, R., Iana, G., & Vinereanu, D. (2022). The Case of a 44-Year-Old Survivor of Unrepaired Tetralogy of Fallot, Right Aortic Arch and Abdominal Aortopulmonary Collateral Vessels. Medicina, 58(8), 1011. https://doi.org/10.3390/medicina58081011






