Combined Systematic and MRI-US Fusion Prostate Biopsy Has the Highest Grading Accuracy When Compared to Final Pathology
Abstract
1. Introduction
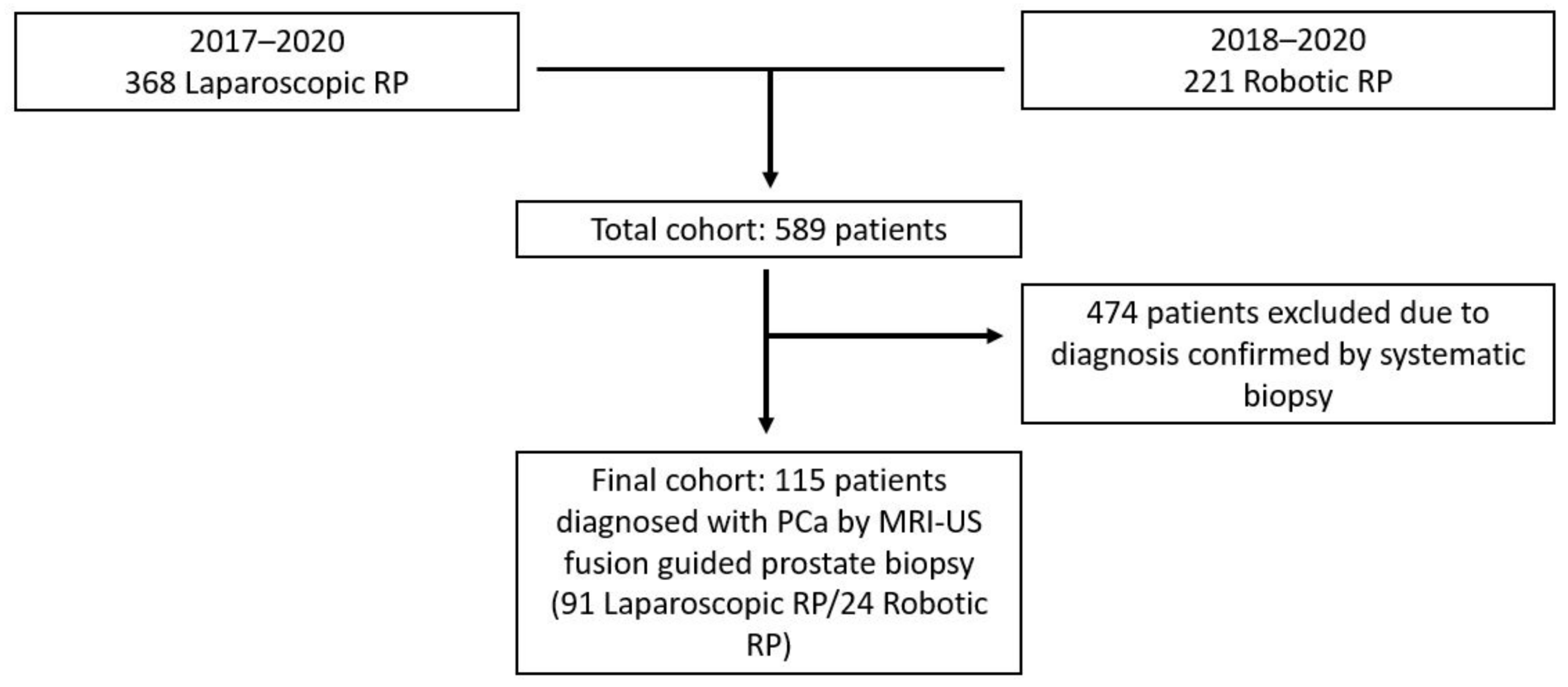
2. Materials and Methods
2.1. Study Population
2.2. MRI and MRI-US Fusion Prostate Biopsy Protocol
2.3. Surgical Approach
2.4. Pathology
2.5. Cohort and Definitions
2.6. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Biopsy GG vs. Radical Prostatectomy GG
3.3. Predictors of Upgrading/Downgrading
3.4. Predictors of Unfavorable RP Pathologic Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andras, I.; Crisan, N.; Vesa, S.; Rahota, R.; Romanciuc, F.; Lazar, A.; Socaciu, C.; Matei, D.V.; De Cobelli, O.; Bocsan, I.S.; et al. Serum Metabolomics Can Predict the Outcome of First Systematic Transrectal Prostate Biopsy in Patients with PSA <10 Ng/Ml. Future Oncol. 2017, 13, 1793–1800. [Google Scholar] [CrossRef]
- Ukimura, O.; Coleman, J.A.; De La Taille, A.; Emberton, M.; Epstein, J.I.; Freedland, S.J.; Giannarini, G.; Kibel, A.S.; Montironi, R.; Ploussard, G.; et al. Contemporary Role of Systematic Prostate Biopsies: Indications, Techniques, and Implications for Patient Care. Eur. Urol. 2013, 63, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Bullock, N.; Simpkin, A.; Fowler, S.; Varma, M.; Kynaston, H.; Narahari, K. Pathological Upgrading in Prostate Cancer Treated with Surgery in the United Kingdom: Trends and Risk Factors from the British Association of Urological Surgeons Radical Prostatectomy Registry. BMC Urol. 2019, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Cornford, P.; de Santis, M.; Fanti, S.; Gillessen, S.; Grummet, J.; Henry, A.M.; Lam, T.B.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. 2019. Available online: https://uroweb.org/guideline/prostate-cancer/ (accessed on 23 March 2021).
- Wegelin, O.; van Melick, H.H.E.; Hooft, L.; Bosch, J.L.H.R.; Reitsma, H.B.; Barentsz, J.O.; Somford, D.M. Comparing Three Different Techniques for Magnetic Resonance Imaging-Targeted Prostate Biopsies: A Systematic Review of in-Bore versus Magnetic Resonance Imaging-Transrectal Ultrasound Fusion versus Cognitive Registration. Is There a Preferred Technique? Eur. Urol. 2017, 71, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Laitinen, S.; Khan, S.; Vihinen, M.; Kowalski, J.; Yu, G.; Chen, L.; Ewing, C.M.; Eisenberger, M.A.; Carducci, M.A.; et al. Copy Number Analysis Indicates Monoclonal Origin of Lethal Metastatic Prostate Cancer. Nat. Med. 2009, 15, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Ploussard, G.; Valerio, M.; Marra, G.; Moschini, M.; Martini, A.; Roumiguié, M.; Fossati, N.; Stabile, A.; Beauval, J.B.; et al. Prognostic Implications of Multiparametric Magnetic Resonance Imaging and Concomitant Systematic Biopsy in Predicting Biochemical Recurrence After Radical Prostatectomy in Prostate Cancer Patients Diagnosed with Magnetic Resonance Imaging-Targeted Biopsy. Eur. Urol. Oncol. 2020, 3, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef]
- Pierorazio, P.M.; Walsh, P.C.; Partin, A.W.; Epstein, J.I. Prognostic Gleason grade grouping: Data based on the modified Gleason scoring system. BJU Int. 2013, 111, 753–760. [Google Scholar] [CrossRef]
- Diamand, R.; Oderda, M.; Al Hajj Obeid, W.; Albisinni, S.; Van Velthoven, R.; Fasolis, G.; Simone, G.; Ferriero, M.; Roche, J.B.; Piechaud, T.; et al. A Multicentric Study on Accurate Grading of Prostate Cancer with Systematic and MRI/US Fusion Targeted Biopsies: Comparison with Final Histopathology after Radical Prostatectomy. World J. Urol. 2019, 37, 2109–2117. [Google Scholar] [CrossRef]
- Le, J.D.; Stephenson, S.; Brugger, M.; Lu, D.Y.; Lieu, P.; Sonn, G.A.; Natarajan, S.; Dorey, F.J.; Huang, J.; Margolis, D.J.A.; et al. Magnetic Resonance Imaging-Ultrasound Fusion Biopsy for Prediction of Final Prostate Pathology. J. Urol. 2014, 192, 1367–1373. [Google Scholar] [CrossRef]
- Radtke, J.P.; Schwab, C.; Wolf, M.B.; Freitag, M.T.; Alt, C.D.; Kesch, C.; Popeneciu, I.V.; Huettenbrink, C.; Gasch, C.; Klein, T.; et al. Multiparametric Magnetic Resonance Imaging (MRI) and MRI–Transrectal Ultrasound Fusion Biopsy for Index Tumor Detection: Correlation with Radical Prostatectomy Specimen. Eur. Urol. 2016. [Google Scholar] [CrossRef]
- Kayano, P.P.; Carneiro, A.; Castilho, T.M.L.; Sivaraman, A.; Claros, O.R.; Baroni, R.H.; Garcia, R.G.; Mariotti, G.C.; Smaletz, O.; Filippi, R.Z.; et al. Comparison of Gleason Upgrading Rates in Transrectal Ultrasound Systematic Random Biopsies versus US-MRI Fusion Biopsies for Prostate Cancer. Int. Braz. J. Urol. 2018, 44, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Porpiglia, F.; Manfredi, M.; Mele, F.; Cossu, M.; Bollito, E.; Veltri, A.; Cirillo, S.; Regge, D.; Faletti, R.; Passera, R.; et al. Diagnostic Pathway with Multiparametric Magnetic Resonance Imaging Versus Standard Pathway: Results from a Randomized Prospective Study in Biopsy-Naïve Patients with Suspected Prostate Cancer. Eur. Urol. 2017, 72, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Rührup, J.; Preisser, F.; Theißen, L.; Wenzel, M.; Roos, F.C.; Becker, A.; Kluth, L.A.; Bodelle, B.; Köllermann, J.; Chun, F.K.H.; et al. MRI-Fusion Targeted vs. Systematic Prostate Biopsy–How Does the Biopsy Technique Affect Gleason Grade Concordance and Upgrading After Radical Prostatectomy? Front. Surg. 2019, 6, 1–6. [Google Scholar] [CrossRef]
- Demirtaş, A.; Sönmez, G.; Tombul, Ş.T.; Demirtaş, T.; Akgün, H. Comparison of the Upgrading Rates of International Society of Urological Pathology Grades and Tumor Laterality in Patients Undergoing Standard 12-Core Prostate Biopsy versus Fusion Prostate Biopsy for Prostate Cancer. Urol. Int. 2019, 103, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Porpiglia, F.; De Luca, S.; Passera, R.; Manfredi, M.; Mele, F.; Bollito, E.; De Pascale, A.; Cossu, M.; Aimar, R.; Veltri, A. Multiparametric-Magnetic Resonance/Ultrasound Fusion Targeted Prostate Biopsy Improves Agreement Between Biopsy and Radical Prostatectomy Gleason Score. Anticancer Res. 2016, 36, 4833–4840. [Google Scholar] [CrossRef]
- Ploussard, G.; Manceau, C.; Beauval, J.B.; Lesourd, M.; Almeras, C.; Gautier, J.R.; Loison, G.; Salin, A.; Soulié, M.; Tollon, C.; et al. Decreased Accuracy of the Prostate Cancer EAU Risk Group Classification in the Era of Imaging-Guided Diagnostic Pathway: Proposal for a New Classification Based on MRI-Targeted Biopsies and Early Oncologic Outcomes after Surgery. World J. Urol. 2020, 38, 2493–2500. [Google Scholar] [CrossRef]
- Borkowetz, A.; Platzek, I.; Toma, M.; Renner, T.; Herout, R.; Baunacke, M.; Laniado, M.; Baretton, G.; Froehner, M.; Zastrow, S.; et al. Direct Comparison of Multiparametric Magnetic Resonance Imaging (MRI) Results with Final Histopathology in Patients with Proven Prostate Cancer in MRI/Ultrasonography-Fusion Biopsy. BJU Int. 2016, 118, 213–220. [Google Scholar] [CrossRef]
- Calio, B.P.; Sidana, A.; Sugano, D.; Gaur, S.; Maruf, M.; Jain, A.L.; Merino, M.J.; Choyke, P.L.; Wood, B.J.; Pinto, P.A.; et al. Risk of Upgrading from Prostate Biopsy to Radical Prostatectomy Pathology—Does Saturation Biopsy of Index Lesion during Multiparametric Magnetic Resonance Imaging-Transrectal Ultrasound Fusion Biopsy Help? J. Urol. 2018, 199, 976–982. [Google Scholar] [CrossRef]
- Ploussard, G.; Beauval, J.-B.; Renard-Penna, R.; Lesourd, M.; Manceau, C.; Almeras, C.; Gautier, J.-R.; Loison, G.; Portalez, D.; Salin, A.; et al. Assessment of the Minimal Targeted Biopsy Core Number per MRI Lesion for Improving Prostate Cancer Grading Prediction. J. Clin. Med. 2020, 9, 225. [Google Scholar] [CrossRef]
- Cata, E.; Andras, I.; Ferro, M.; Kadula, P.; Leucuta, D.; Musi, G.; Matei, D.V.; de Cobelli, O.; Tamas-Szora, A.; Caraiani, C.; et al. Systematic Sampling during MRI-US Fusion Prostate Biopsy Can Overcome Errors of Targeting—Prospective Single Center Experience after 300 Cases in First Biopsy Setting. Transl. Androl. Urol. 2020, 9, 2510–2518. [Google Scholar] [CrossRef]
- Shoag, J.E.; Cai, P.Y.; Gross, M.D.; Gaffney, C.; Li, D.; Mao, J.; Nowels, M.; Scherr, D.S.; Sedrakyan, A.; Hu, J.C. Impact of Prebiopsy Magnetic Resonance Imaging on Biopsy and Radical Prostatectomy Grade Concordance. Cancer 2020, 126, 2986–2990. [Google Scholar] [CrossRef] [PubMed]
- Beksac, A.T.; Sobotka, S.; Xu, P.; Gupta, A.; Treacy, P.J.; Weil, R.; Mahajan, K.; Prasad, S.; Cumarasamy, S.; Martini, A.; et al. Downgrading of Grade Group After Radical Prostatectomy: Comparison of Multiparametric Magnetic Resonance Imaging Guided Fusion Biopsy and Standard 12-Core Biopsy. Urology 2019, 127, 80–85. [Google Scholar] [CrossRef]
- Arsov, C.; Becker, N.; Rabenalt, R.; Hiester, A.; Quentin, M.; Dietzel, F.; Antoch, G.; Gabbert, H.E.; Albers, P.; Schimmöller, L. The Use of Targeted MR-Guided Prostate Biopsy Reduces the Risk of Gleason Upgrading on Radical Prostatectomy. J. Cancer Res. Clin. Oncol. 2015, 141, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Robertson, N.L.; Hu, Y.; Ahmed, H.U.; Freeman, A.; Barratt, D.; Emberton, M. Prostate Cancer Risk Inflation as a Consequence of Image-Targeted Biopsy of the Prostate: A Computer Simulation Study. Eur. Urol. 2014, 65, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Manceau, C.; Fromont-Hankard, G.; Beauval, J.B.; Lesourd, M.; Almeras, C.; Bajeot, A.S.; Gautier, J.R.; Soulié, M.; Loison, G.; Salin, A.; et al. The Prognostic Value of High-Grade Prostate Cancer Pattern on MRI-Targeted Biopsies: Predictors for Downgrading and Importance of Concomitant Systematic Biopsies. World J. Urol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Stackhouse, D.A.; Sun, L.; Schroeck, F.R.; Jayachandran, J.; Caire, A.A.; Acholo, C.O.; Robertson, C.N.; Albala, D.M.; Polascik, T.J.; Donatucci, C.F.; et al. Factors predicting prostatic biopsy Gleason sum under grading. J. Urol. 2009, 182, 118–122. [Google Scholar] [CrossRef]
- Epstein, J.I.; Feng, Z.; Trock, B.J.; Pierorazio, P.M. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: Incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur. Urol. 2012, 61, 1019–1024. [Google Scholar] [CrossRef]
- Hambrock, T.; Hoeks, C.; Hulsbergen-van de Kaa, C.; Scheenen, T.; Fütterer, J.; Bouwense, S.; van Oort, I.; Schröder, F.; Huisman, H.; Barentsz, J. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur. Urol. 2012, 61, 177–184. [Google Scholar] [CrossRef]
- Leyh-Bannurah, S.R.; Kachanov, M.; Karakiewicz, P.I.; Beyersdorff, D.; Pompe, R.S.; Oh-Hohenhorst, S.J.; Fisch, M.; Maurer, T.; Graefen, M.; Budäus, L. Combined Systematic versus Stand-Alone Multiparametric MRI-Guided Targeted Fusion Biopsy: Nomogram Prediction of Non-Organ-Confined Prostate Cancer. World J. Urol. 2021, 39, 81–88. [Google Scholar] [CrossRef]
- Patel, U.; Dasgupta, P.; Challacombe, B.; Cahill, D.; Brown, C.; Patel, R.; Kirby, R. Pre-Biopsy 3-Tesla MRI and Targeted Biopsy of the Index Prostate Cancer: Correlation with Robot-Assisted Radical Prostatectomy. BJU Int. 2017, 119, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Rud, E.; Baco, E.; Klotz, D.; Rennesund, K.; Svindland, A.; Berge, V.; Lundeby, E.; Wessel, N.; Hoff, J.-R.; Berg, R.E.; et al. Does Preoperative Magnetic Resonance Imaging Reduce the Rate of Positive Surgical Margins at Radical Prostatectomy in a Randomised Clinical Trial? Eur. Urol. 2015, 68, 487–496. [Google Scholar] [CrossRef]
- Pooli, A.; Johnson, D.C.; Shirk, J.; Markovic, D.; Sadun, T.Y.; Sisk, A.E.J.; Mohammadian Bajgiran, A.; Afshari Mirak, S.; Felker, E.R.; Hughes, A.K.; et al. Predicting Pathological Tumor Size in Prostate Cancer Based on Multiparametric Prostate Magnetic Resonance Imaging and Preoperative Findings. J. Urol. 2021, 205, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Loghin, A.; Predal, O.; Bacârea, V.; Moldovan, C.; Porav-Hodade, D.; Dema, A.; Berger, N.; Borda, A. Predictive Preoperatory Variables of the Prostate Tumor Volume. Rom. J. Morphol. Embryol. 2011, 52 (Suppl. 1), 363–368. [Google Scholar] [PubMed]
- Poulos, C.K.; Daggy, J.K.; Cheng, L. Prostate Needle Biopsies: Multiple Variables Are Predictive of Final Tumor Volume in Radical Prostatectomy Specimens. Cancer 2004, 101, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, D.N.; Sisk, A.E., Jr.; Priester, A.; Felker, E.R.; Kwan, L.; Delfin, M.K.; Reiter, R.E.; Marks, L.S. Cancer Core Length from Targeted Biopsy: An Index of Prostate Cancer Volume and Pathological Stage. BJU Int. 2019, 124, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A.; Veeratterapillay, R.; Voysey, A.; Kelly, K.; Johnson, M.I.; Aning, J.; Soomro, N.A. Positive Surgical Margins and Biochemical Recurrence Following Minimally-Invasive Radical Prostatectomy—An Analysis of Outcomes from a UK Tertiary Referral Centre. BMC Urol. 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Msezane, L.P.; Gofrit, O.N.; Lin, S.; Shalhav, A.L.; Zagaja, G.P.; Zorn, K.C. Prostate Weight: An Independent Predictor for Positive Surgical Margins during Robotic-Assisted Laparoscopic Radical Prostatectomy. Can. J. Urol. 2007, 14, 3697–3701. [Google Scholar]
| Variables | Value (IQR) | ||
|---|---|---|---|
| Age, years Median (IQR) | 64 (60–67) | ||
| PSA, ng/mL Median (IQR) | 8 (5–10.1) | ||
| Prostate volume, g Median (IQR) | 41 (33–52) | ||
| PSA density, ng/mL/g Median (IQR) | 0.17 (0.12–0.28) | ||
| Lesion dimension on MRI, mm Median (IQR) | 15 (10–21) | ||
| PIRADS score * | 3—16.5% 4—44.3% 5—39.2% | ||
| Number of targeted biopsy cores Median (IQR) | 3 (3–4) | ||
| Biopsy GG | Combined biopsy GG 1—25.5% 2—50.4% 3—14.8% 4—4.3% 5—5.2% | MRI-US fusion GG 1—33.72% 2—41.86% 3—10.46% 4—9.3% 5—4.65% | Systematic biopsy GG 1—28.3% 2—48.11% 3—12.26% 4—6.89% 5—3.77% |
| Maximum cancer core length, mm Median (IQR) | Combined biopsy 6 (4–11) | MRI-US fusion biopsy 6 (3–9) | Systematic biopsy 5 (3–9) |
| Radical prostatectomy GG | 1—16.5% 2—62.6% 3—15.7% 4—0 5—5.2% | ||
| pT | pT2—86 (74.78%) pT3—29 (25.21%) | ||
| Surgical margins | Overall R0—79.14% R1—20.86% | pT2 R0—83.73% R1—16.27% | pT3 R0—65.52% R1—34.48% |
| MRI-US Fusion Biopsy | Systematic Biopsy | Combined Biopsy | p | |
|---|---|---|---|---|
| Concordance, n (%) | 39 (45.3%) | 64 (60.4%) | 71 (61.7%) | <0.0001 |
| Upgrading, n (%) | 30 (34.9%) | 26 (24.5%) | 24 (20.9%) | |
| Downgrading, n (%) | 17 (19.8%) | 16 (15.1%) | 20 (17.4%) |
| Variable | Upgraded | Downgraded | Concordant | p |
|---|---|---|---|---|
| PSA, ng/mL Median (IQR) | 9 (5.7–12.8) | 8.8 (5.3–10.2) | 7.5 (4.8–10) | 0.39 |
| Prostate volume, g Median (IQR) | 52 (37.5–50.2) | 42.1 (37.5–50.2) | 39.9 (28.8–46.7) | 0.03 |
| PSA density, ng/mL/g Median (IQR) | 0.17 (0.14–0.23) | 0.2 (0.09–0.26) | 0.17 (0.12–0.32) | 0.9 |
| Previous negative biopsy | Yes 75% No 25% | Yes 65% No 35% | Yes 74.6% No 25.4% | 0.67 |
| Lesion location on MRI | Anterior 13.04% Transitional 30.43% Peripheral 56.52% | Anterior 5.26% Transitional 26.31% Peripheral 68.42% | Anterior 7.35% Transitional 25% Peripheral 67.64% | 0.83 |
| Lesion dimension on MRI, mm Median (IQR) | 19 (12.1–24.2) | 12 (9–20.2) | 15 (12–19) | 0.28 |
| PIRADS score * | 3—9.52% 4—38.09% 5—52.38% | 3—16.66% 4—58.33% 5—25% | 3—19.56% 4—43.47% 5—36.95% | 0.52 |
| Number of targeted biopsy cores Median (IQR) | 3 (3–4) | 3 (3–3) | 3 (3–4) | 0.89 |
| %PBC/total Median (IQR) | 29.91 (13.3–43.3) | 27.62 (16.6–40) | 33.33 (21.4–53.3) | 0.24 |
| %PBC/targeted Median (IQR) | 41.66 (0–100) | 66.67 (0–100) | 50 (25–100) | 0.8 |
| %PBC/systematic Median (IQR) | 20.83 (8.3–41.6) | 25 (16.6–33.3) | 33.33 (16.6–50) | 0.21 |
| MCCL on combined biopsy, mm Median (IQR) | 5 (2.5–11.5) | 6.5 (4.5–8) | 6 (4–11) | 0.48 |
| MCCL on targeted biopsy, mm Median (IQR) | 5 (2.6–9.5) | 6 (5–7) | 6 (3–9) | 0.96 |
| MCCL on systematic biopsy, mm Median (IQR) | 5 (3–9) | 4 (2.2–6.5) | 6 (4–11) | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andras, I.; Cata, E.D.; Serban, A.; Kadula, P.; Telecan, T.; Buzoianu, M.; Bungardean, M.; Stanca, D.V.; Coman, I.; Crisan, N. Combined Systematic and MRI-US Fusion Prostate Biopsy Has the Highest Grading Accuracy When Compared to Final Pathology. Medicina 2021, 57, 519. https://doi.org/10.3390/medicina57060519
Andras I, Cata ED, Serban A, Kadula P, Telecan T, Buzoianu M, Bungardean M, Stanca DV, Coman I, Crisan N. Combined Systematic and MRI-US Fusion Prostate Biopsy Has the Highest Grading Accuracy When Compared to Final Pathology. Medicina. 2021; 57(6):519. https://doi.org/10.3390/medicina57060519
Chicago/Turabian StyleAndras, Iulia, Emanuel Darius Cata, Andreea Serban, Pierre Kadula, Teodora Telecan, Maximilian Buzoianu, Maria Bungardean, Dan Vasile Stanca, Ioan Coman, and Nicolae Crisan. 2021. "Combined Systematic and MRI-US Fusion Prostate Biopsy Has the Highest Grading Accuracy When Compared to Final Pathology" Medicina 57, no. 6: 519. https://doi.org/10.3390/medicina57060519
APA StyleAndras, I., Cata, E. D., Serban, A., Kadula, P., Telecan, T., Buzoianu, M., Bungardean, M., Stanca, D. V., Coman, I., & Crisan, N. (2021). Combined Systematic and MRI-US Fusion Prostate Biopsy Has the Highest Grading Accuracy When Compared to Final Pathology. Medicina, 57(6), 519. https://doi.org/10.3390/medicina57060519






