Evaluation of the Age- and Sex-Related Changes of the Osteogenic Differentiation Potentials of Healthy Bone Marrow-Derived Mesenchymal Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Bone Marrow-Derived Mesenchymal Stem Cells
2.2. Cellular Morphology and Determination of Cell Viability
2.3. Immunofluorescence
2.4. Secretion of Human Vascular Endothelial Growth Factor from the BMSCs
2.5. Total RNA Extraction and Quantification Using a Real-Time Polymerase Chain Reaction
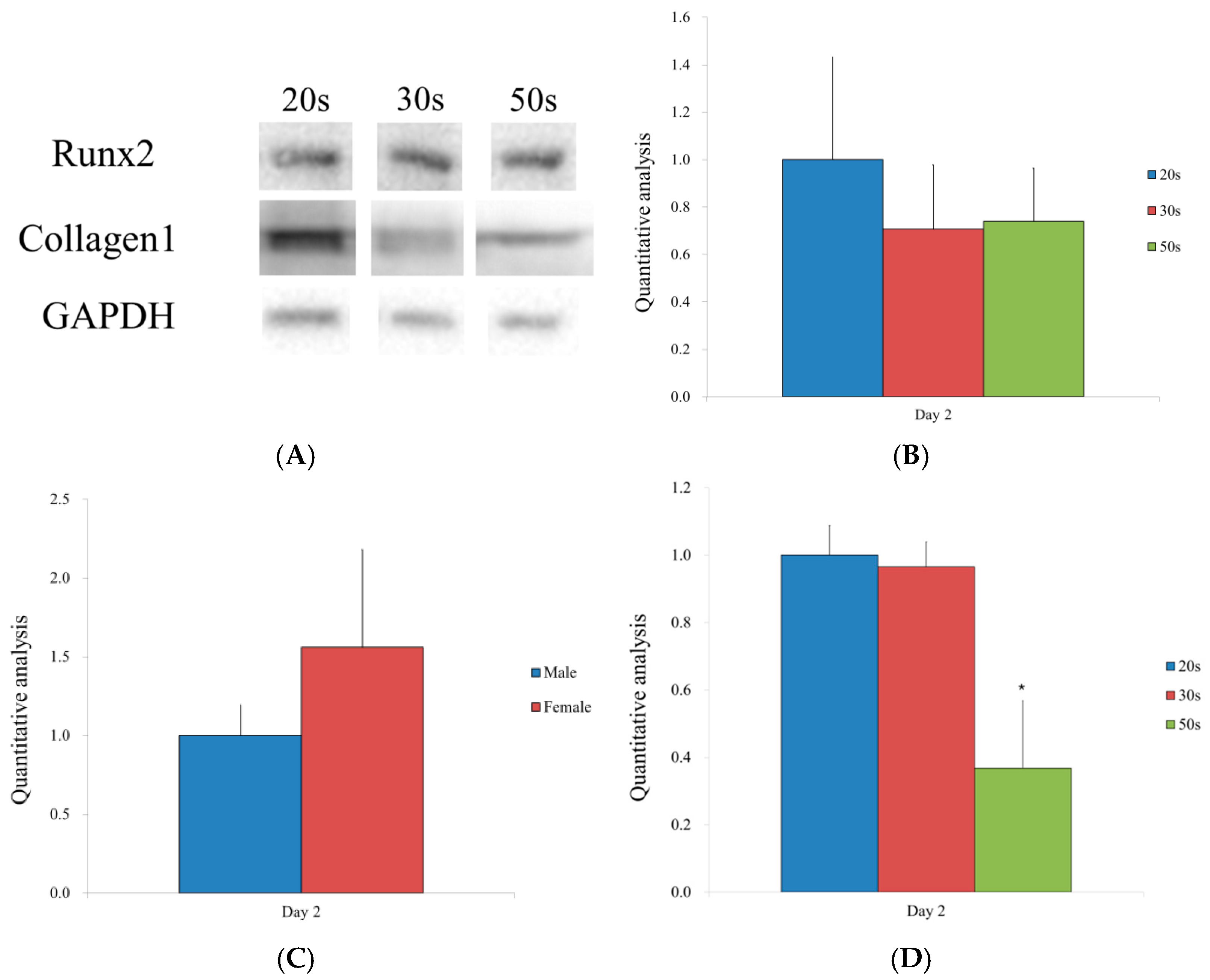
2.6. Western Blotting Analysis
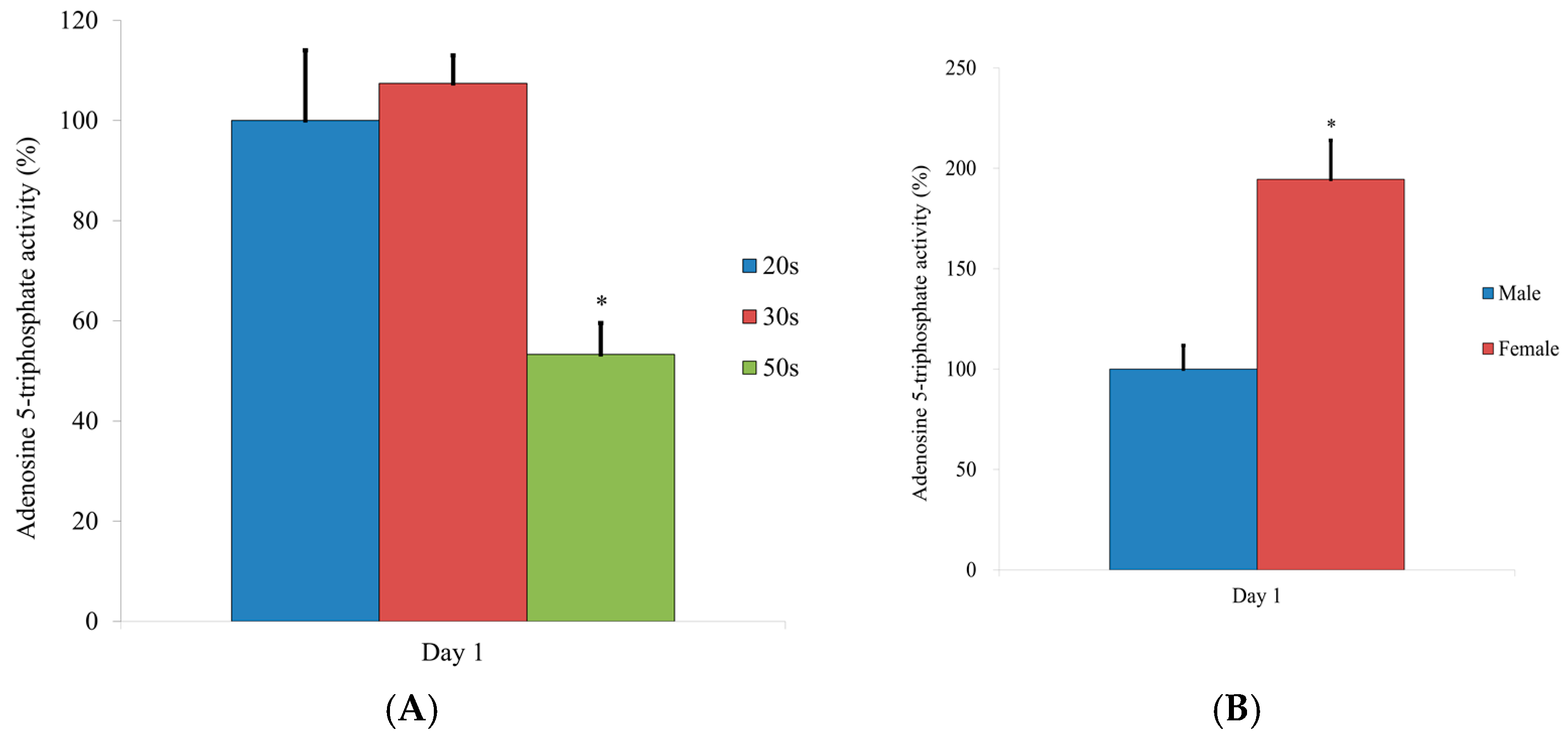
2.7. Evaluation of the Adenosine 5-Triphosphate Assay
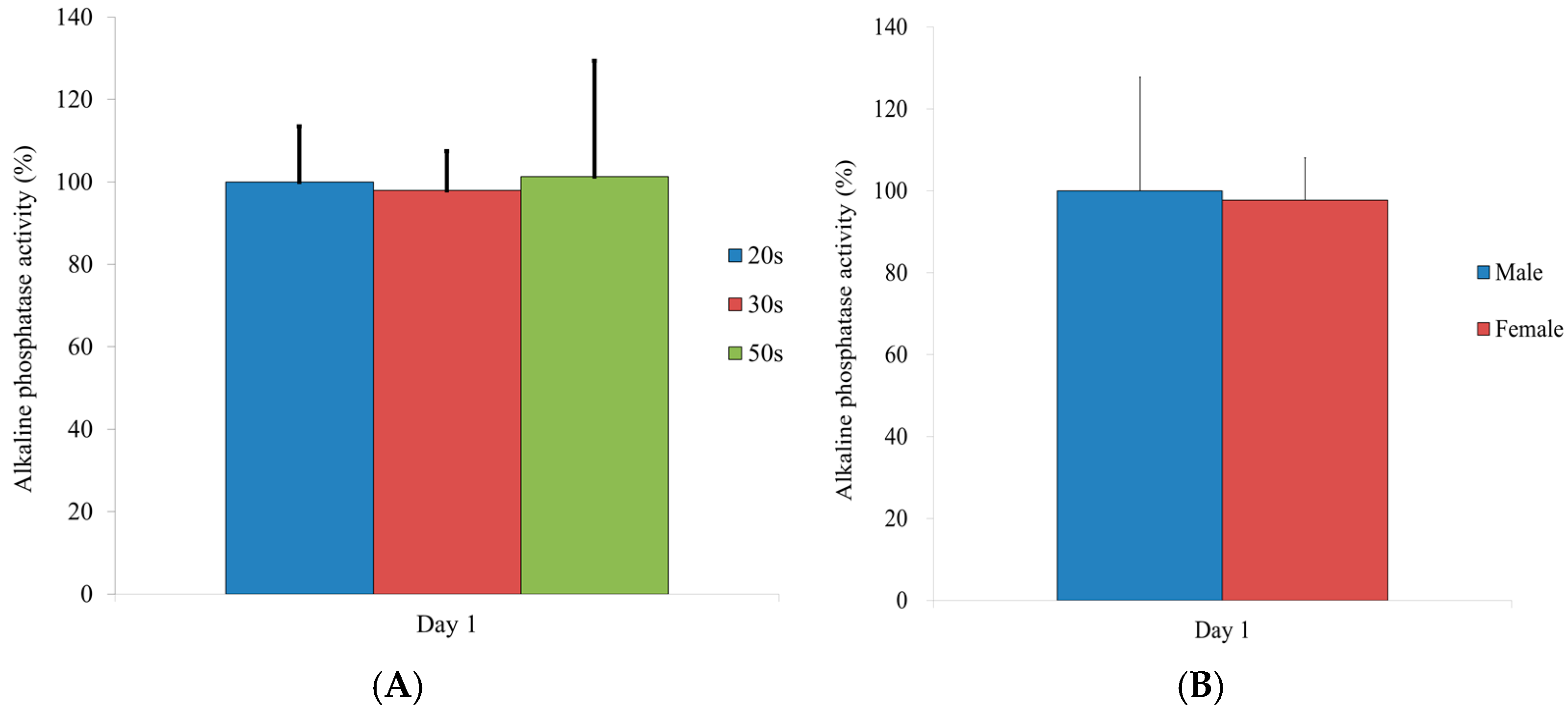
2.8. Alkaline Phosphatase Activity
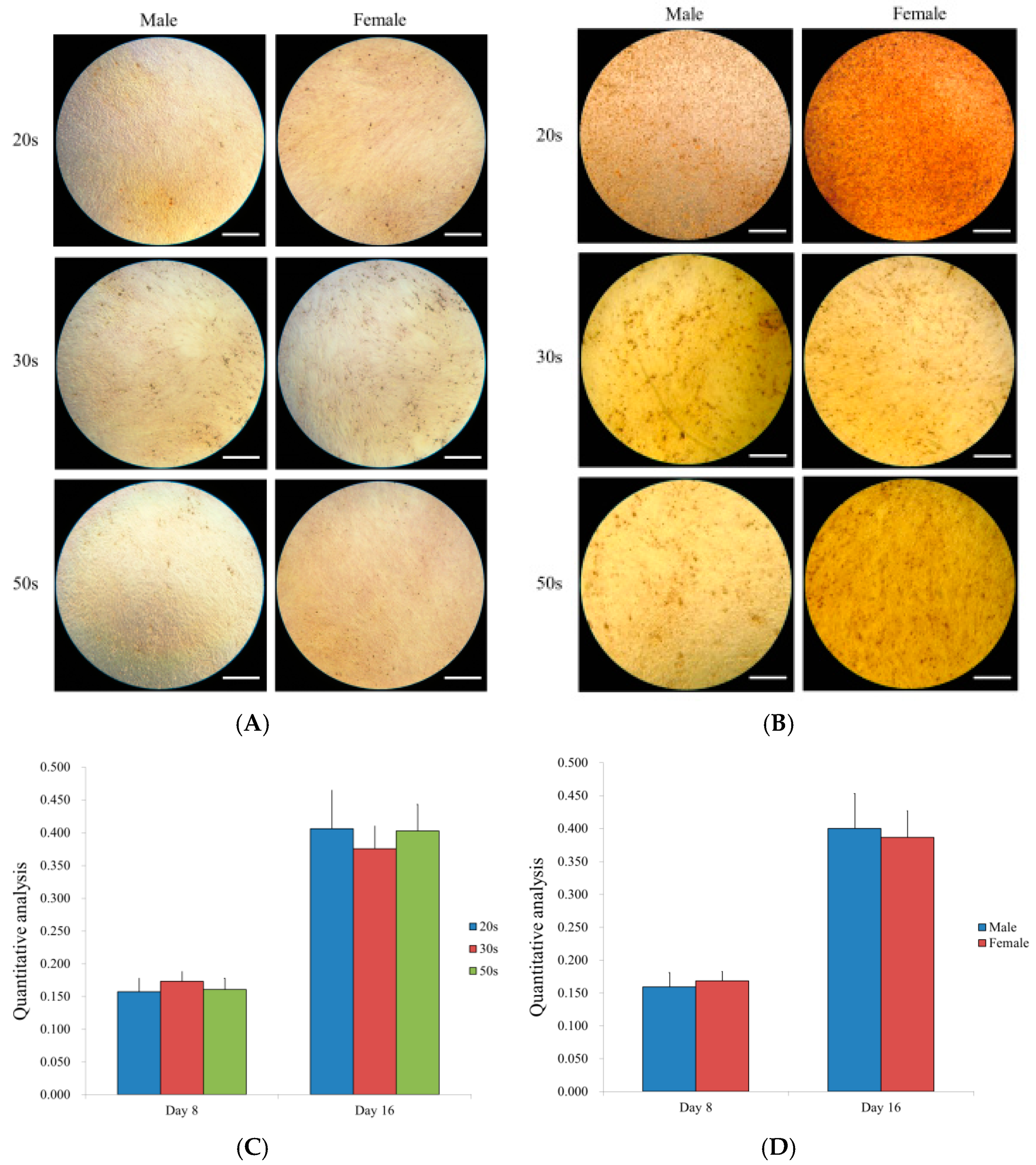
2.9. Alizarin Red S Staining
2.10. Statistical Analysis
3. Results
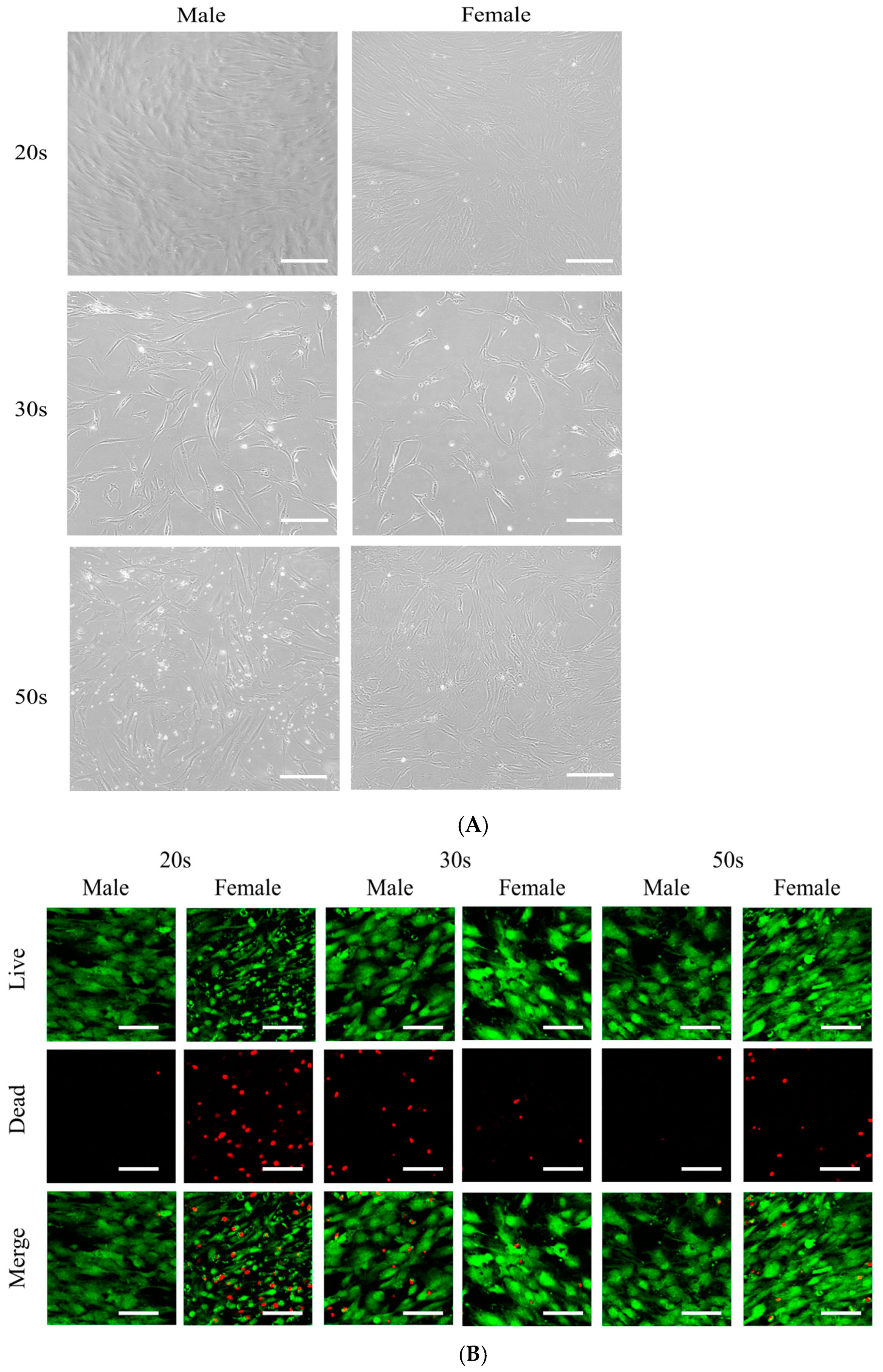
3.1. Cellular Morphology and Cell Viability
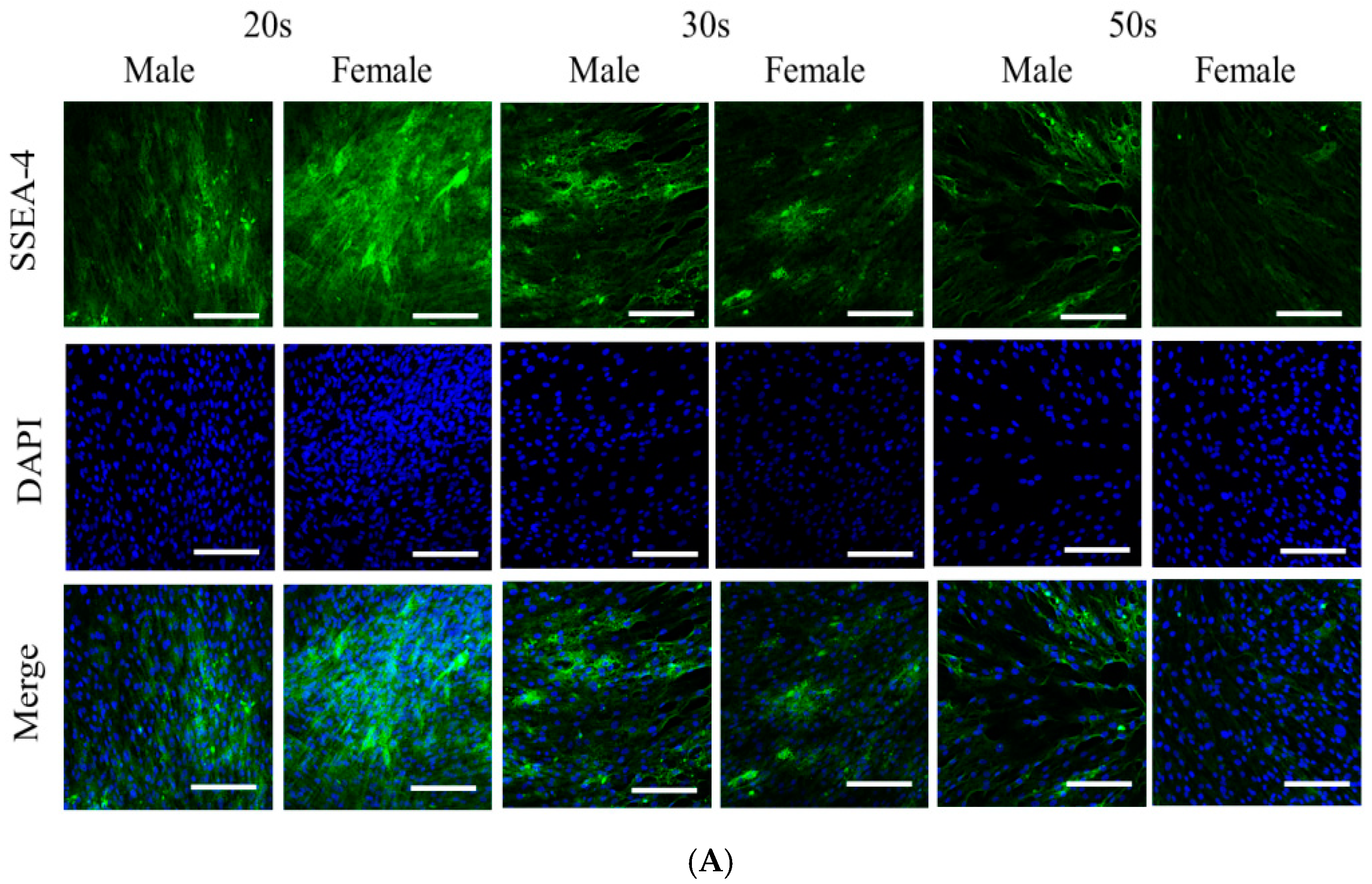
3.2. Immunofluorescence and Secretion of Human Vascular Endothelial Growth Factor from the BMSCs
3.3. Validation of mRNA Expression by qPCR
3.4. Western Blot
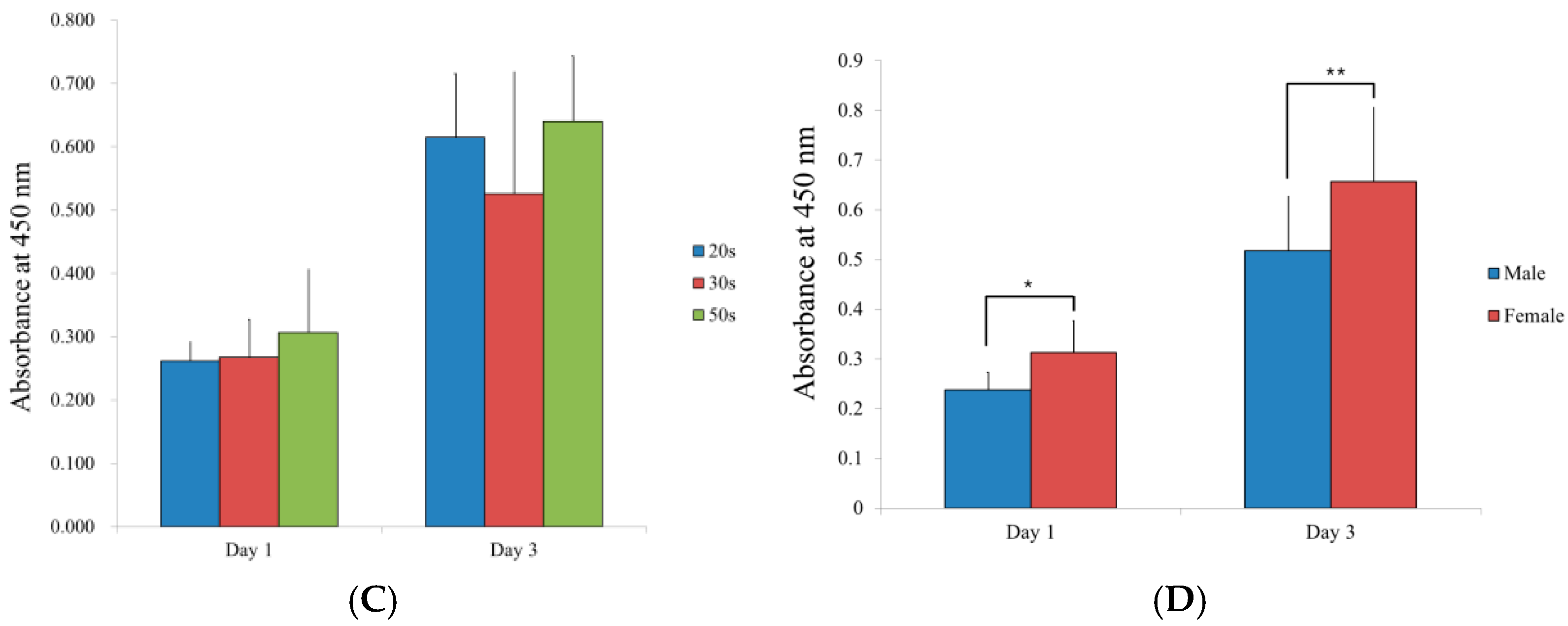
3.5. Evaluation of the Adenosine 5-Triphosphate Assay
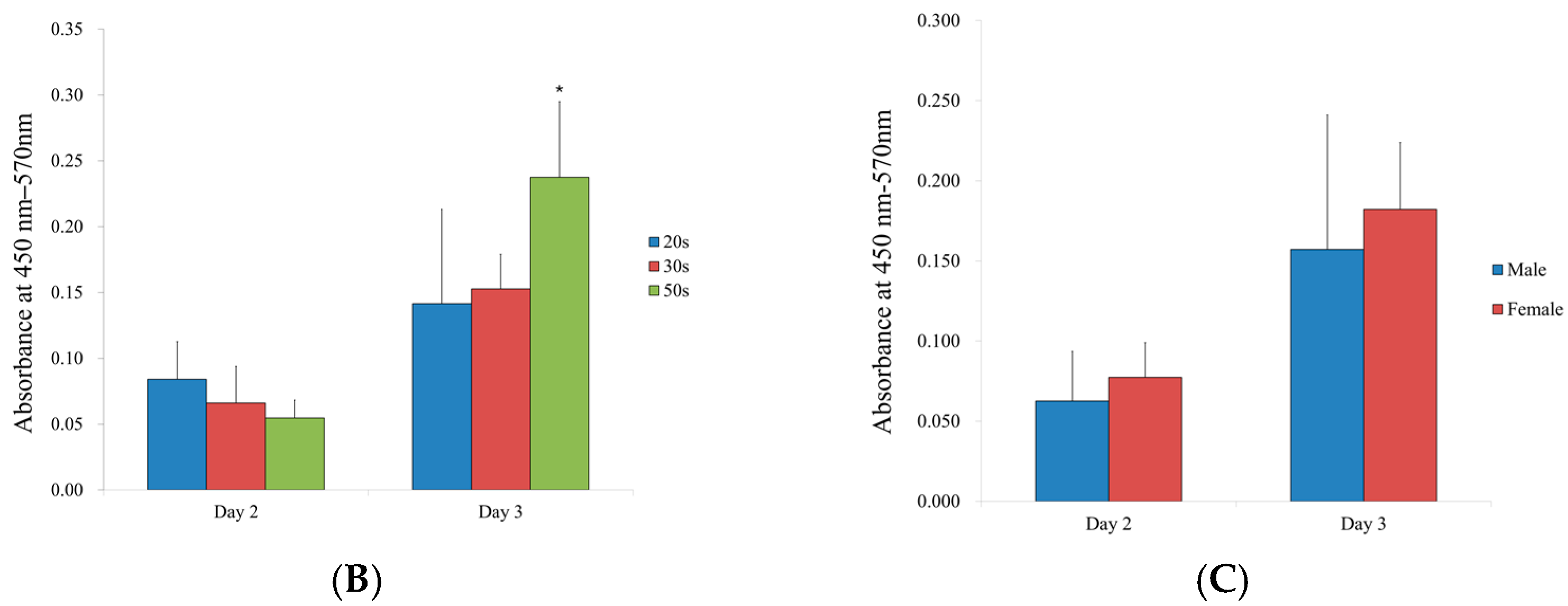
3.6. Alkaline Phosphatase Activity
3.7. Osteogenic Differentiation Potential by Alizarin Red S Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baker, N.; Boyette, L.B.; Tuan, R.S. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone 2015, 70, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Park, J.B.; Kim, I.; Lee, W.; Kim, H. Assessment of stem cell viability in the initial healing period in rabbits with a cranial bone defect according to the type and form of scaffold. J. Periodontal Implant Sci. 2019, 49, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Frenette, P.S.; Pinho, S.; Lucas, D.; Scheiermann, C. Mesenchymal stem cell: Keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu. Rev. Immunol. 2013, 31, 285–316. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J. Marrow stromal cells as stem cells for continual renewal of nonhematopoietic tissues and as potential vectors for gene therapy. J. Cell Biochem. 1998, 72, 284–285. [Google Scholar] [CrossRef]
- Karsenty, G.; Ferron, M. The contribution of bone to whole-organism physiology. Nature 2012, 481, 314–320. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, K.; Robinson, A.R.; Clauson, C.L.; Blair, H.C.; Robbins, P.D.; Niedernhofer, L.J.; Ouyang, H.J. DNA damage drives accelerated bone aging via an NF-kappa B-dependent mechanism. J. Bone Miner. Res. 2013, 28, 1214–1228. [Google Scholar] [CrossRef]
- Charif, N.; Li, Y.Y.; Targa, L.; Zhang, L.; Ye, J.S.; Li, Y.P.; Stoltz, J.F.; Han, H.Z.; de Isla, N. Aging of bone marrow mesenchymal stromal/stem cells: Implications on autologous regenerative medicine. Bio-Med. Mater. Eng. 2017, 28, S57–S63. [Google Scholar] [CrossRef]
- Wagner, W.; Bork, S.; Lepperdinger, G.; Joussen, S.; Ma, N.; Strunk, D.; Koch, C. How to track cellular aging of mesenchymal stromal cells? Aging 2010, 2, 224–230. [Google Scholar] [CrossRef]
- Martin, C.; Olmos, E.; Collignon, M.L.; De Isla, N.; Blanchard, F.; Chevalot, I.; Marc, A.; Guedon, E. Revisiting MSC expansion from critical quality attributes to critical culture process parameters. Process. Biochem. 2017, 59, 231–243. [Google Scholar] [CrossRef]
- Infante, A.; Rodríguez, C.I. Osteogenesis and aging: Lessons from mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 244. [Google Scholar] [CrossRef]
- Fan, M.; Chen, W.; Liu, W.; Du, G.Q.; Jiang, S.L.; Tian, W.C.; Sun, L.; Li, R.K.; Tian, H. The effect of age on the efficacy of human mesenchymal stem cell transplantation after a myocardial infarction. Rejuvenation Res. 2010, 13, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Mareschi, K.; Ferrero, I.; Rustichelli, D.; Aschero, S.; Gammaitoni, L.; Aglietta, M.; Madon, E.; Fagioli, F. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J. Cell Biochem. 2006, 97, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef]
- Dexheimer, V.; Mueller, S.; Braatz, F.; Richter, W. Reduced reactivation from dormancy but maintained lineage choice of human mesenchymal stem cells with donor age. PloS ONE 2011, 6, e22980. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, S.; Kalajzic, I.; Aguila, H.L.; Canalis, E. Sex and genetic factors determine osteoblastic differentiation potential of murine bone marrow stromal cells. PloS ONE 2014, 9, e86757. [Google Scholar] [CrossRef]
- Carter-Arnold, J.L.; Neilsen, N.L.; Amelse, L.L.; Odoi, A.; Dhar, M.S. In vitro analysis of equine, bone marrow-derived mesenchymal stem cells demonstrates differences within age- and gender-matched horses. Equine Vet. J. 2014, 46, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.H.; Kim, S.M.; Lim, J.Y.; Ryu, C.H.; Jun, J.A.; Jeun, S.S. Mesenchymal stem cells expressing brain-derived neurotrophic factor enhance endogenous neurogenesis in an ischemic stroke model. Biomed. Res. Int. 2014, 2014, 129145. [Google Scholar] [CrossRef]
- Min, S.K.; Oh, J.; Park, J.B. The Effects of Morinda citrifolia (Noni) on the Cellular Viability and Osteogenesis of Stem Cell Spheroids. Medicina 2020, 56, 389. [Google Scholar] [CrossRef]
- Mendes, S.C.; Tibbe, J.M.; Veenhof, M.; Bakker, K.; Both, S.; Platenburg, P.P.; Oner, F.C.; De Bruijn, J.D.; Van Blitterswijk, C.A. Bone tissue-engineered implants using human bone marrow stromal cells: Effect of culture conditions and donor age. Tissue Eng. 2002, 8, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Greenberger, J.S.; Epperly, M.W.; Goff, J.P.; Adler, C.; LeBoff, M.S.; Glowacki, J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell 2008, 7, 335–343. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Schiller, P.C.; Ricordi, C.; Roos, B.A.; Howard, G.A. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J. Bone Miner. Res. 1999, 14, 1115–1122. [Google Scholar] [CrossRef]
- Muschler, G.F.; Nitto, H.; Boehm, C.A.; Easley, K.A. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J. Orthop. Res. 2001, 19, 117–125. [Google Scholar] [CrossRef]
- Colter, D.C.; Class, R.; DiGirolamo, C.M.; Prockop, D.J. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc. Natl. Acad. Sci. USA 2000, 97, 3213–3218. [Google Scholar] [CrossRef]
- Bergman, R.J.; Gazit, D.; Kahn, A.J.; Gruber, H.; McDougall, S.; Hahn, T.J. Age-related changes in osteogenic stem cells in mice. J. Bone Miner. Res. 1996, 11, 568–577. [Google Scholar] [CrossRef]
- Bertram, H.; Mayer, H.; Schliephake, H. Effect of donor characteristics, technique of harvesting and in vitro processing on culturing of human marrow stroma cells for tissue engineered growth of bone. Clin. Oral Implant. Res. 2005, 16, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schafer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11, 146. [Google Scholar] [CrossRef]
- Wagner, W.; Bork, S.; Horn, P.; Krunic, D.; Walenda, T.; Diehlmann, A.; Benes, V.; Blake, J.; Huber, F.X.; Eckstein, V.; et al. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- McCann, R.M.; Marsh, D.R.; Horner, A.; Clarke, S.A. Body mass index is more predictive of progenitor number in bone marrow stromal cell population than age in men: Expanding the predictors of the progenitor compartment. Tissue Eng. Part. A 2010, 16, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Battula, V.L.; Bareiss, P.M.; Treml, S.; Conrad, S.; Albert, I.; Hojak, S.; Abele, H.; Schewe, B.; Just, L.; Skutella, T.; et al. Human placenta and bone marrow derived MSC cultured in serum-free, b-FGF-containing medium express cell surface frizzled-9 and SSEA-4 and give rise to multilineage differentiation. Differentiation 2007, 75, 279–291. [Google Scholar] [CrossRef]
- Ge, Q.; Zhang, H.; Hou, J.; Wan, L.; Cheng, W.; Wang, X.; Dong, D.; Chen, C.; Xia, J.; Guo, J.; et al. VEGF secreted by mesenchymal stem cells mediates the differentiation of endothelial progenitor cells into endothelial cells via paracrine mechanisms. Mol. Med. Rep. 2018, 17, 1667–1675. [Google Scholar] [CrossRef]
- Lee, H.; Min, S.K.; Park, J.B. Effects of demographic factors on adipogenic and chondrogenic differentiation in bone marrow-derived stem cells. Exp. Ther. Med. 2019, 17, 3548–3554. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.M. Monitoring gene expression: Quantitative real-time rt-PCR. Methods Mol. Biol. 2013, 1027, 19–45. [Google Scholar] [CrossRef]
- Taylor, S.C.; Posch, A. The design of a quantitative western blot experiment. Biomed. Res. Int. 2014, 2014, 361590. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Park, J.; Kwon, I.K.; Lee, S.W.; Park, S.J.; Ahn, S.J. Static magnetic fields promote osteoblastic/cementoblastic differentiation in osteoblasts, cementoblasts, and periodontal ligament cells. J. Periodontal Implant Sci. 2017, 47, 273–291. [Google Scholar] [CrossRef]
- Gilbert, L.; He, X.; Farmer, P.; Rubin, J.; Drissi, H.; van Wijnen, A.J.; Lian, J.B.; Stein, G.S.; Nanes, M.S. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J. Biol. Chem. 2002, 277, 2695–2701. [Google Scholar] [CrossRef]
- Thiagarajan, L.; Abu-Awwad, H.A.M.; Dixon, J.E. Osteogenic programming of human mesenchymal stem cells with highly efficient intracellular delivery of RUNX2. STEM Cells Transl. Med. 2017, 6, 2146–2159. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.J.; Qiu, Z.Y.; Wu, J.J.; Kong, X.D.; Weng, X.S.; Cui, F.Z.; Wang, X.M. Osteogenic differentiation gene expression profiling of hMSCs on hydroxyapatite and mineralized collagen. Tissue Eng. Part. A 2016, 22, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Noh, W.C.; Park, J.W.; Lee, J.M.; Suh, J.Y. Gene expression pattern during osteogenic differentiation of human periodontal ligament cells in vitro. J. Periodontal Implant Sci. 2011, 41, 167–175. [Google Scholar] [CrossRef]
- Lee, J.W.; Fang, X.; Krasnodembskaya, A.; Howard, J.P.; Matthay, M.A. Concise review: Mesenchymal stem cells for acute lung injury: Role of paracrine soluble factors. STEM Cells 2011, 29, 913–919. [Google Scholar] [CrossRef]
- van Poll, D.; Parekkadan, B.; Cho, C.H.; Berthiaume, F.; Nahmias, Y.; Tilles, A.W.; Yarmush, M.L. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 2008, 47, 1634–1643. [Google Scholar] [CrossRef]
- Crisostomo, P.R.; Wang, M.J.; Herring, C.M.; Markel, T.A.; Meldrum, K.K.; Lillemoe, K.D.; Meldrum, D.R. Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: Role of the 55 kDa TNF receptor (TNFR1). J. Mol. Cell Cardiol. 2007, 42, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Crisostomo, P.R.; Wang, M.J.; Herring, C.M.; Morrell, E.D.; Seshadri, P.; Meldrum, K.K.; Meldrum, D.R. Sex dimorphisms in activated mesenchymal stem cell function. Shock 2006, 26, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, J.; Li, C.; Yu, Y. Role of bone morphogenetic protein-2 in osteogenic differentiation of mesenchymal stem cells. Mol. Med. Rep. 2015, 12, 4230–4237. [Google Scholar] [CrossRef] [PubMed]
- Reible, B.; Schmidmaier, G.; Moghaddam, A.; Westhauser, F. Insulin-like growth factor-1 as a possible alternative to bone morphogenetic protein-7 to induce osteogenic differentiation of human mesenchymal stem cells in vitro. Int. J. Mol. Sci. 2018, 19, 1674. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-J.; Lee, H.; Na, C.-B.; Song, I.-S.; Ryu, J.-J.; Park, J.-B. Evaluation of the Age- and Sex-Related Changes of the Osteogenic Differentiation Potentials of Healthy Bone Marrow-Derived Mesenchymal Stem Cells. Medicina 2021, 57, 520. https://doi.org/10.3390/medicina57060520
Lee H-J, Lee H, Na C-B, Song I-S, Ryu J-J, Park J-B. Evaluation of the Age- and Sex-Related Changes of the Osteogenic Differentiation Potentials of Healthy Bone Marrow-Derived Mesenchymal Stem Cells. Medicina. 2021; 57(6):520. https://doi.org/10.3390/medicina57060520
Chicago/Turabian StyleLee, Hyun-Jin, Hyuna Lee, Chae-Bin Na, In-Seok Song, Jae-Jun Ryu, and Jun-Beom Park. 2021. "Evaluation of the Age- and Sex-Related Changes of the Osteogenic Differentiation Potentials of Healthy Bone Marrow-Derived Mesenchymal Stem Cells" Medicina 57, no. 6: 520. https://doi.org/10.3390/medicina57060520
APA StyleLee, H.-J., Lee, H., Na, C.-B., Song, I.-S., Ryu, J.-J., & Park, J.-B. (2021). Evaluation of the Age- and Sex-Related Changes of the Osteogenic Differentiation Potentials of Healthy Bone Marrow-Derived Mesenchymal Stem Cells. Medicina, 57(6), 520. https://doi.org/10.3390/medicina57060520






