Assessment of Fluid Status by Bioimpedance Analysis and Central Venous Pressure Measurement and Their Association with the Outcomes of Severe Acute Kidney Injury
Abstract
1. Introduction
2. Materials and Methods
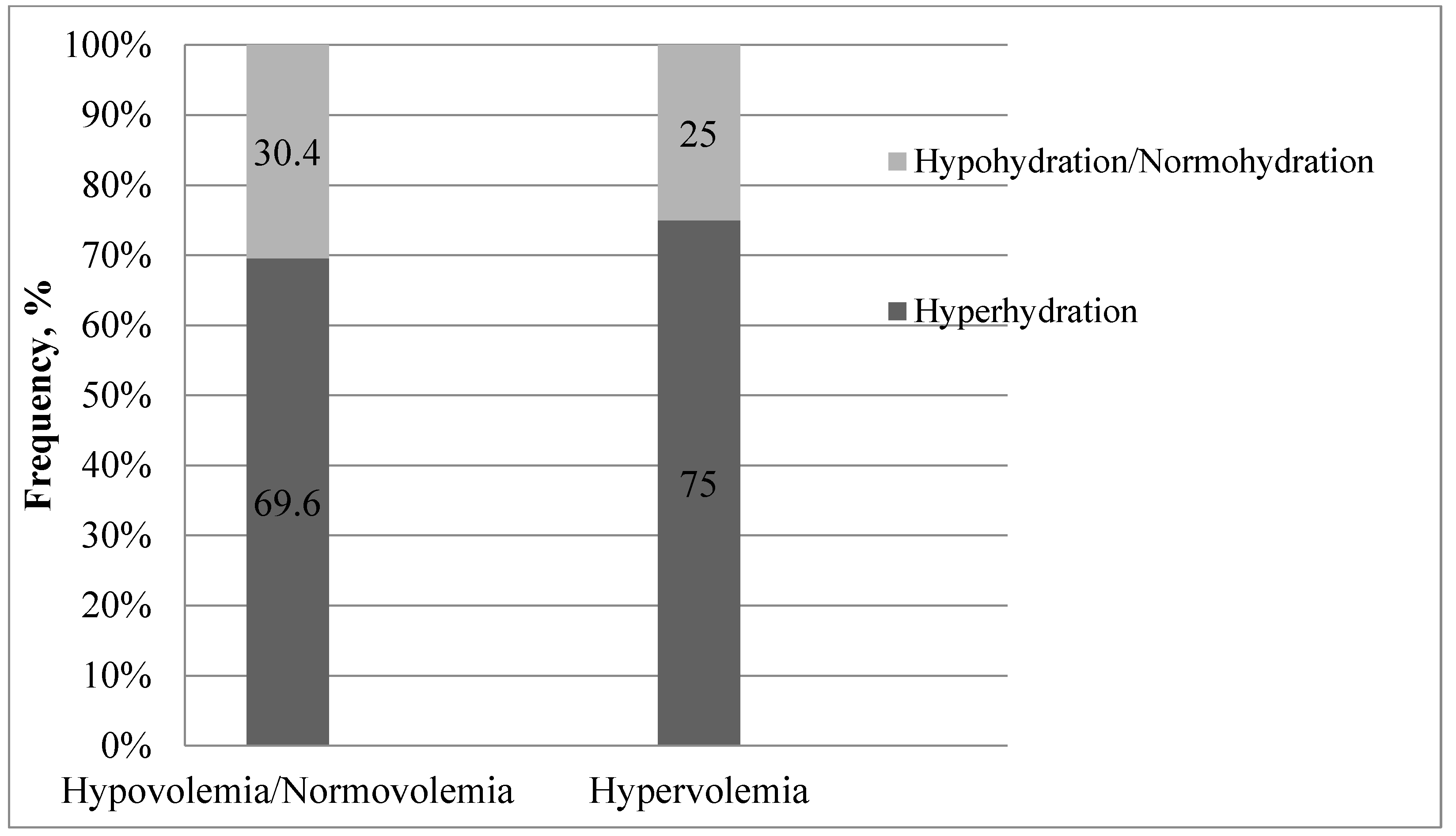
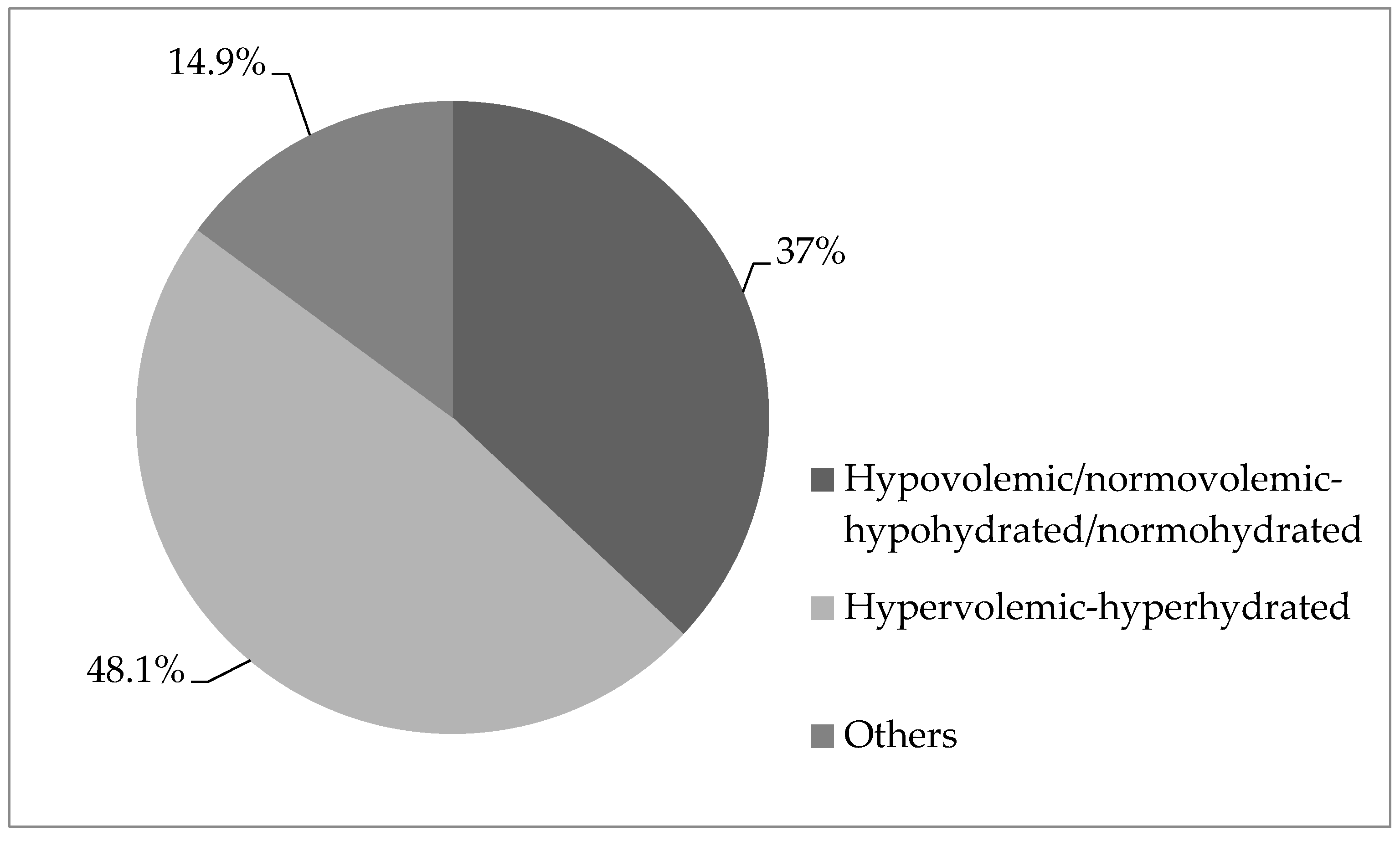
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, N.; Jiang, L.; Zhu, B.; Wen, Y.; Xi, X.-M.; The Beijing Acute Kidney Injury Trial (BAKIT) Workgroup. Fluid Balance and Mortality in Critically Ill Patients with Acute Kidney Injury: A Multicenter Prospective Epidemiological Study. Crit. Care 2015, 19, 371. [Google Scholar] [CrossRef]
- Brotfain, E.; Koyfman, L.; Toledano, R.; Borer, A.; Fucs, L.; Galante, O.; Frenkel, A.; Kutz, R.; Klein, M. Positive Fluid Balance as a Major Predictor of Clinical Outcome of Patients with Sepsis/Septic Shock after ICU Discharge. Am. J. Emerg. Med. 2016, 34, 2122–2126. [Google Scholar] [CrossRef]
- Granado, R.C.-D.; Mehta, R.L. Fluid Overload in the ICU: Evaluation and Management. BMC Nephrol. 2016, 17, 109. [Google Scholar] [CrossRef]
- Sosa, G.; Gandham, N.; Landeras, V.; Calimag, A.P.; Lerma, E. Abdominal Compartment Syndrome. Dis. Mon. 2019, 65, 5–19. [Google Scholar] [CrossRef]
- Salahuddin, N.; Sammani, M.; Hamdan, A.; Joseph, M.; Al-Nemary, Y.; AlQuaiz, R.; Dahli, R.; Maghrabi, K. Fluid Overload Is an Independent Risk Factor for Acute Kidney Injury in Critically Ill Patients: Results of a Cohort Study. BMC Nephrol. 2017, 18, 45. [Google Scholar] [CrossRef]
- De Oliveira, F.S.V.; Freitas, F.G.R.; Ferreira, E.M.; de Castro, I.; Bafi, A.T.; De Azevedo, L.C.P.; Machado, F.R. Positive Fluid Balance as a Prognostic Factor for Mortality and Acute Kidney Injury in Severe Sepsis and Septic Shock. J. Crit. Care 2015, 30, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Kim, J.H.; Lee, D.W.; Lee, S.B.; Rhee, H.; Seong, E.Y.; Kwak, I.S.; Song, S.H. Fluid Overload and Survival in Critically Ill Patients with Acute Kidney Injury Receiving Continuous Renal Replacement Therapy. PLoS ONE 2017, 12, e0172137. [Google Scholar] [CrossRef] [PubMed]
- Garzotto, F.; Ostermann, M.; Martín-Langerwerf, D.; Sánchez-Sánchez, M.; Teng, J.; Robert, R.; Marinho, A.; Herrera-Gutierrez, M.E.; Mao, H.J.; Benavente, D.; et al. The Dose Response Multicentre Investigation on Fluid Assessment (DoReMIFA) in Critically Ill Patients. Crit. Care 2016, 20, 196. [Google Scholar] [CrossRef]
- Magder, S. Understanding Central Venous Pressure: Not a Preload Index? Curr. Opin. Crit. Care 2015, 21, 369–375. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Vincent, J.-L. Should We Measure the Central Venous Pressure to Guide Fluid Management? Ten Answers to 10 Questions. Crit. Care 2018, 22, 43. [Google Scholar] [CrossRef]
- Park, K.H.; Shin, J.-H.; Hwang, J.H.; Kim, S.H. Utility of Volume Assessment Using Bioelectrical Impedance Analysis in Critically Ill Patients Receiving Continuous Renal Replacement Therapy: A Prospective Observational Study. Korean J. Crit. Care Med. 2017, 32, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Slobod, D.; Yao, H.; Mardini, J.; Natkaniec, J.; Correa, J.A.; Jayaraman, D.; Weber, C.L. Bioimpedance-Measured Volume Overload Predicts Longer Duration of Mechanical Ventilation in Intensive Care Unit Patients. Can. J. Anaesth. J. Can. D’anesthesie 2019, 66, 1458–1463. [Google Scholar] [CrossRef]
- Samoni, S.; Vigo, V.; Reséndiz, L.I.B.; Villa, G.; De Rosa, S.; Nalesso, F.; Ferrari, F.; Meola, M.; Brendolan, A.; Malacarne, P.; et al. Impact of Hyperhydration on the Mortality Risk in Critically Ill Patients Admitted in Intensive Care Units: Comparison between Bioelectrical Impedance Vector Analysis and Cumulative Fluid Balance Recording. Crit. Care 2016, 20, 95. [Google Scholar] [CrossRef]
- Hise, A.C.d.R.; Gonzalez, M.C. Assessment of Hydration Status Using Bioelectrical Impedance Vector Analysis in Critical Patients with Acute Kidney Injury. Clin. Nutr. 2018, 37, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, S.; Lalle, I.; Magrini, L.; Russo, V.; Navarin, S.; Castello, L.; Avanzi, G.C.; Di Stasio, E.; Maisel, A. Additive Diagnostic and Prognostic Value of Bioelectrical Impedance Vector Analysis (BIVA) to Brain Natriuretic Peptide ‘grey-Zone’ in Patients with Acute Heart Failure in the Emergency Department. Eur. Heart J. Acute Cardiovasc. Care 2014, 3, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-F.; Tseng, C.-M.; Liu, I.-F.; Tsai, S.-H.; Kuo, W.-S.; Tsao, T.-P. Clinical Significance of Bioimpedance Spectroscopy in Critically Ill Patients. J. Intensive Care Med. 2019, 34, 495–502. [Google Scholar] [CrossRef]
- Núñez, J.; Mascarell, B.; Stubbe, H.; Ventura, S.; Bonanad, C.; Bodí, V.; Nunez, E.; Minana, G.; Fácila, L.; Bayes-Genis, A.; et al. Bioelectrical Impedance Vector Analysis and Clinical Outcomes in Patients with Acute Heart Failure. J. Cardiovasc. Med. 2016, 17, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhu, B.; Jiang, L.; Wen, Y.; Du, B.; Li, W.; Liu, G.; Li, W.; Wen, J.; He, Y.; et al. Dose–Response Association between Fluid Overload and in-Hospital Mortality in Critically Ill Patients: A Multicentre, Prospective, Observational Cohort Study. BMJ Open 2020, 10. [Google Scholar] [CrossRef]
- Woodward, C.W.; Lambert, J.; Ortiz-Soriano, V.; Li, Y.; Ruiz-Conejo, M.; Bissell, B.D.; Kelly, A.; Adams, P.; Yessayan, L.; Morris, P.E.; et al. Fluid Overload Associates with Major Adverse Kidney Events in Critically Ill Patients with Acute Kidney Injury Requiring Continuous Renal Replacement Therapy. Crit. Care Med. 2019, 17, e753–e760. [Google Scholar] [CrossRef]
- Hatton, G.E.; Du, R.E.; Wei, S.; Harvin, J.A.; Finkel, K.W.; Wade, C.E.; Kao, L.S. Positive Fluid Balance and Association with Post-Traumatic Acute Kidney Injury. J. Am. Coll. Surg. 2020, 230, 190–199.e1. [Google Scholar] [CrossRef]
- Li, D.-K.; Wang, X.-T.; Liu, D.-W. Association between Elevated Central Venous Pressure and Outcomes in Critically Ill Patients. Ann. Intensive Care 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Uthoff, H.; Breidthardt, T.; Klima, T.; Aschwanden, M.; Arenja, N.; Socrates, T.; Heinisch, C.; Noveanu, M.; Frischknecht, B.; Baumann, U.; et al. Central Venous Pressure and Impaired Renal Function in Patients with Acute Heart Failure. Eur. J. Heart Fail. 2011, 13, 432–439. [Google Scholar] [CrossRef] [PubMed]
| Characteristic of Patients | Value |
|---|---|
| Gender: Male/female, n (%) | 24 (51.1)/23 (48.9) |
| Age (mean ± SD), years | 70.13 ± 13.7 |
| Length of stay (mean ± SD) in ICU, days | 26.04 ± 28.3 |
| Length of stay till getting into ICU (mean ± SD), days | 4.45 ± 12.9 |
| Length of stay in ICU till the beginning of RRT (mean ± SD), days | 1.32 ± 2.0 |
| Chronic kidney disease (the 1st CKD stage), n (%) | 2 (4.3) |
| Chronic kidney disease (the 2nd–4th CKD stage), n (%) | 15 (31.9) |
| Use of vasopressors, n (%) | 25 (53.2) |
| Application of APV, n (%) | 34 (72.3) |
| Heart failure, n (%) | 26 (55.3) |
| Liver damage, n (%) | 11 (23.4) |
| Sepsis without a septic shock, n (%) | 7 (14.9) |
| Sepsis with a septic shock, n (%) | 21 (44.7) |
| Before the first RRT procedure: | |
| Pulmonary oedema n (%) | 8 (17) |
| Oliguria, n (%) | 39 (83) |
| Serum potassium (mean ± SD), mmol/L | 5.20 ± 1.1 |
| Serum sodium (mean ± SD), mmol/L | 139.32 ± 8.9 |
| Serum urea (mean ± SD), mmol/L | 32.64 ± 11.8 |
| Serum creatinine (mean ± SD), µmol/L | 511.83 ± 169.3 |
| pH of blood (mean ± SD) | 7.28 ± 0.1 |
| Serum bicarbonate (HCO3) (mean ± SD), mmol/L | 16.3 ± 3.9 |
| BIA Parameters | CVP ≤ 12 cm H2O (n = 23) | CVP > 12 cm H2O (n = 24) | p-Value |
|---|---|---|---|
| ICW/body weight | 0.36 ± 0.07 | 0.31 ± 0.06 | 0.013 |
| ECW/body weight | 0.26 ± 0.06 | 0.23 ± 0.04 | 0.042 |
| TBW/body weight | 0.63 ± 0.13 | 0.55 ± 0.10 | 0.02 |
| ECW/TBW | 0.41 ± 0.11 | 0.42 ± 0.04 | 0.317 |
| Outcomes | CVP before the First RRT Procedure | ||
|---|---|---|---|
| ≤12 cm H2O (n = 23) | >12 cm H2O (n = 24) | p-Value | |
| Survivors, n (%) | 9 (39.1) | 11 (45.8) | 0.642 |
| Nonsurvivors, n (%) | 14 (60.9) | 13 (54.2) | 0.642 |
| Outcomes | ECW/TBW before the First RRT Procedure | ||
|---|---|---|---|
| ≤0.39 (n = 13) | >0.39 (n = 34) | p-Value | |
| Survivors, n (%) | 8 (61.5) | 12 (35.3) | 0.104 |
| Nonsurvivors, n (%) | 5 (38.5) | 22 (64.7) | 0.104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpavičiūtė, J.; Skarupskienė, I.; Balčiuvienė, V.; Vaičiūnienė, R.; Žiginskienė, E.; Bumblytė, I.A. Assessment of Fluid Status by Bioimpedance Analysis and Central Venous Pressure Measurement and Their Association with the Outcomes of Severe Acute Kidney Injury. Medicina 2021, 57, 518. https://doi.org/10.3390/medicina57060518
Karpavičiūtė J, Skarupskienė I, Balčiuvienė V, Vaičiūnienė R, Žiginskienė E, Bumblytė IA. Assessment of Fluid Status by Bioimpedance Analysis and Central Venous Pressure Measurement and Their Association with the Outcomes of Severe Acute Kidney Injury. Medicina. 2021; 57(6):518. https://doi.org/10.3390/medicina57060518
Chicago/Turabian StyleKarpavičiūtė, Justina, Inga Skarupskienė, Vilma Balčiuvienė, Rūta Vaičiūnienė, Edita Žiginskienė, and Inga Arūnė Bumblytė. 2021. "Assessment of Fluid Status by Bioimpedance Analysis and Central Venous Pressure Measurement and Their Association with the Outcomes of Severe Acute Kidney Injury" Medicina 57, no. 6: 518. https://doi.org/10.3390/medicina57060518
APA StyleKarpavičiūtė, J., Skarupskienė, I., Balčiuvienė, V., Vaičiūnienė, R., Žiginskienė, E., & Bumblytė, I. A. (2021). Assessment of Fluid Status by Bioimpedance Analysis and Central Venous Pressure Measurement and Their Association with the Outcomes of Severe Acute Kidney Injury. Medicina, 57(6), 518. https://doi.org/10.3390/medicina57060518





