The p38/MK2 Axis in Monocytes of Fibromyalgia Syndrome Patients: An Explorative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Healthy Control (HC)
2.3. Assessment Tools
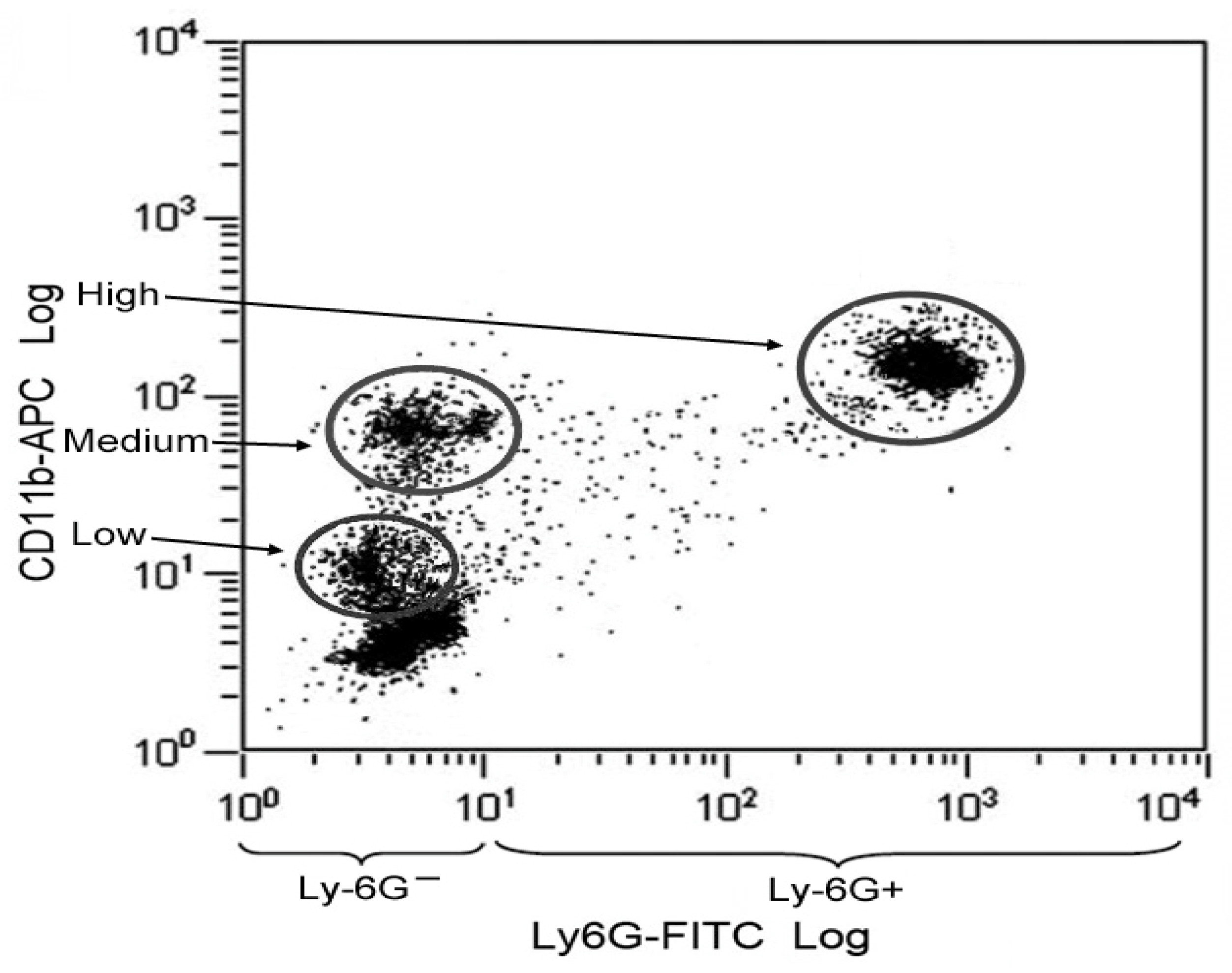
2.4. Identification of Monocytes and MK2 Analysis Methods
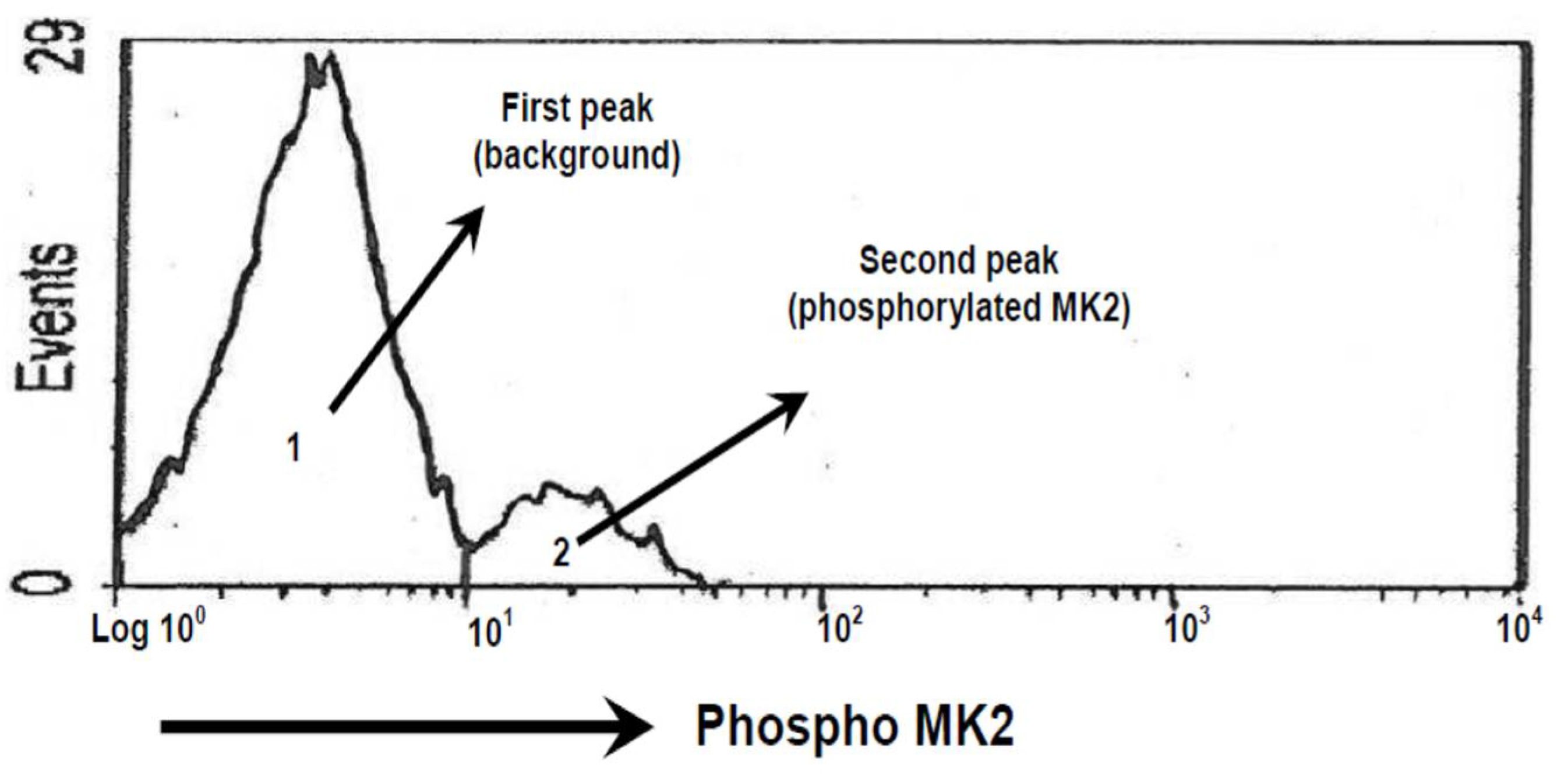
2.5. Calculation of the Area of Phosphorylation of MK2
2.6. Statistics Analysis
3. Results
3.1. Characteristics of FMS Patients and HCs
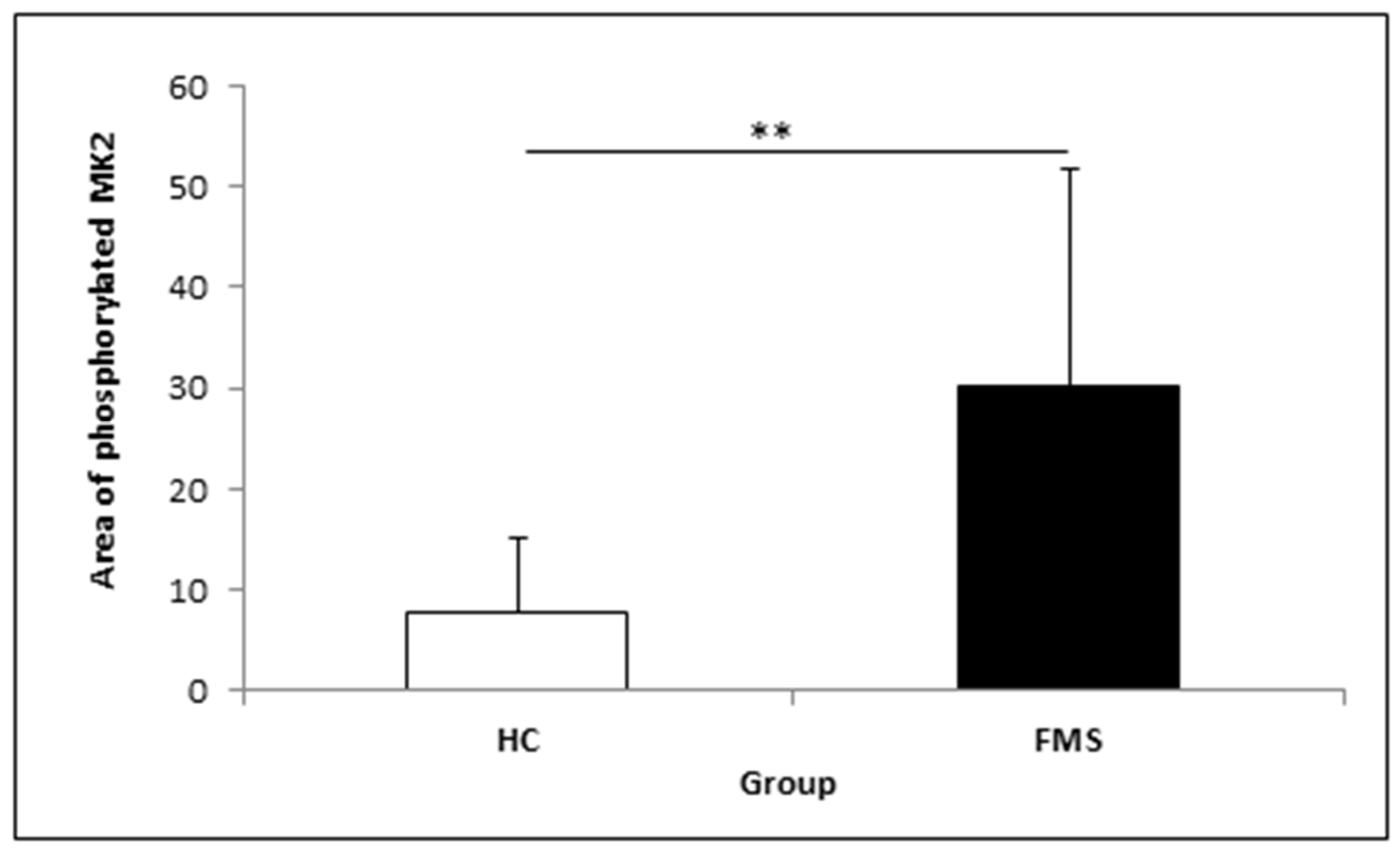
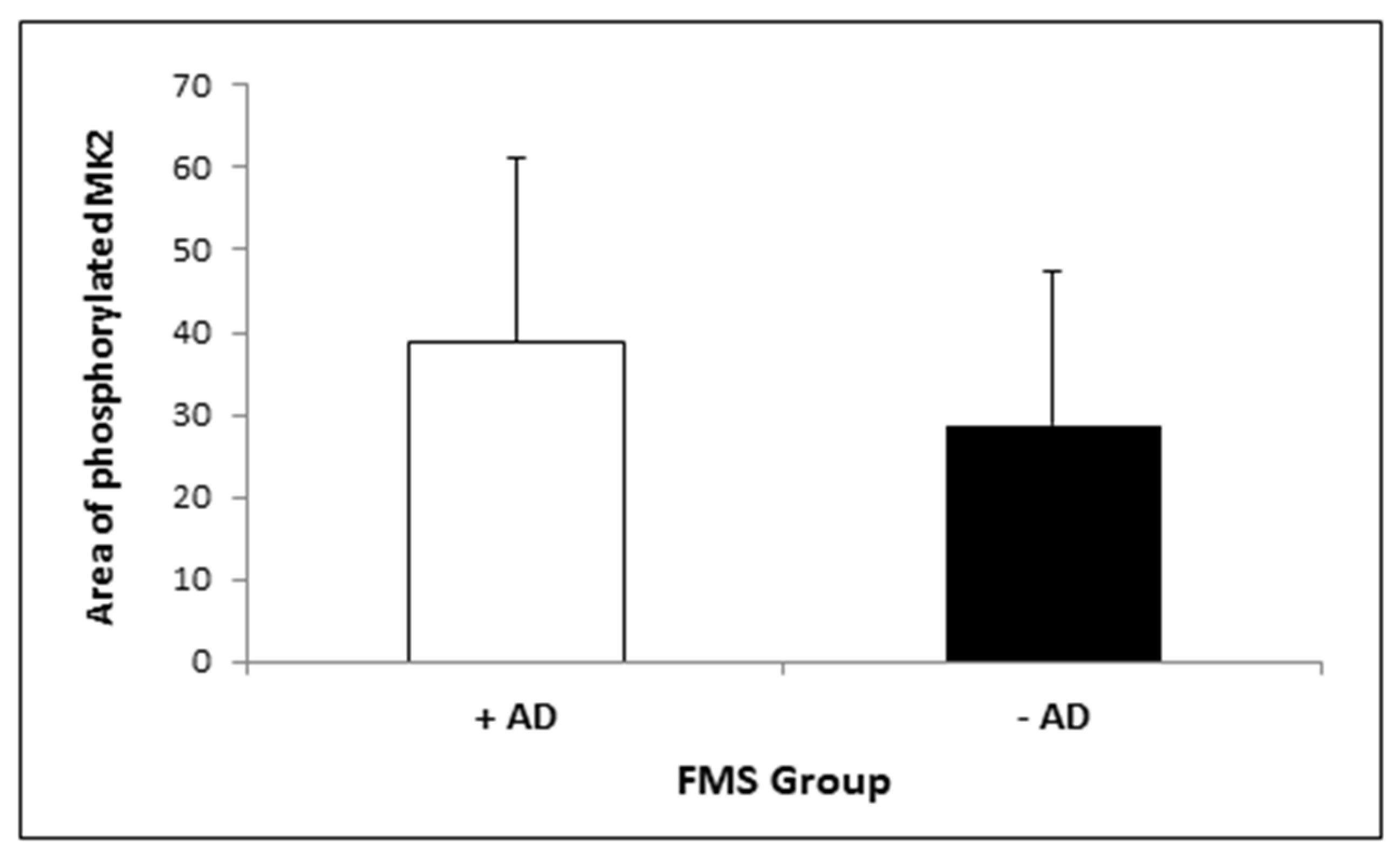
3.2. MK2 Measurement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sawaddiruk, P.; Paiboonworachat, S.; Chattipakorn, N.; Chattipakorn, S.C. Alterations of brain activity in fibromyalgia patients. J. Clin. Neurosci. 2017, 38, 13–22. [Google Scholar] [CrossRef]
- Staud, R. Evidence of involvement of central neural mechanisms in generating fibromyalgia pain. Curr. Rheumatol. Rep. 2002, 4, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Staud, R.; Smitherman, M.L. Peripheral and central sensitization in fibromyalgia: Pathogenetic role. Curr. Pain Headache Rep. 2002, 6, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Casale, R.; Sarzi-Puttini, P.; Botto, R.; Alciati, A.; Batticciotto, A.; Marotto, D.; Torta, R. Fibromyalgia and the concept of resilience. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 116), 105–113. [Google Scholar]
- Schoneveld, O.C.J.; Cidlowski, J.A. Glucocorticoids and immunity: Mechanisms of regulation. In Psychoneuroimmunology; Elsevier Academic Press: Burlington, MA, USA, 2007; Volume 4, pp. 45–61. [Google Scholar]
- Macedo, J.A.; Hesse, J.; Turner, J.D.; Ammerlaan, W.; Gierens, A.; Hellhammer, D.H.; Muller, C.P. Adhesion molecules and cytokine expression in fibromyalgia patients: Increased L-selectin on monocytes and neutrophils. J. Neuroimmunol. 2007, 188, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, S.; Zimmermann, C.; Kalideris, G.; Lesuis, S.L.; Arloth, J.; Uribe, A.; Dournes, C.; Balsevich, G.; Hartmann, J.; Masana, M.; et al. An adverse early life environment can enhance stress resilience in adulthood. Psychoneuroendocrinology 2017, 78, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Tsigos, C.; Kyrou, I.; Kassi, E.; Chrousos, G.P. Stress: Endocrine Physiology and Pathophysiology. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., Kaltsas, G., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Miller, A.H.; Haroon, E.; Raison, C.L.; Felger, J.C. Cytokine targets in the brain: Impact on neurotransmitters and neurocircuits. Depress. Anxiety 2013, 30, 297–306. [Google Scholar] [CrossRef]
- Altier, N.; Stewart, J. Dopamine receptor antagonists in the nucleus accumbens attenuate analgesia induced by ventral tegmental area substance P or morphine and by nucleus accumbens amphetamine. J. Pharm. Exp. Ther. 1998, 285, 208–215. [Google Scholar]
- Coulthard, L.R.; White, D.E.; Jones, D.L.; McDermott, M.F.; Burchill, S.A. p38(MAPK): Stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 2009, 15, 369–379. [Google Scholar] [CrossRef]
- Gaestel, M. MAPKAP kinases—MKs—Two’s company, three’s a crowd. Nat. Rev. Mol. Cell Biol. 2006, 7, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Suter, M.R. p38 MAPK, microglial signaling, and neuropathic pain. Mol. Pain 2007, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Chang, C.C.; Yang, Y.; Yuan, L.; Xu, L.; Ho, C.T.; Li, S. Resveratrol Alleviates Rheumatoid Arthritis via Reducing ROS and Inflammation, Inhibiting MAPK Signaling Pathways, and Suppressing Angiogenesis. J. Agric. Food Chem. 2018, 66, 12953–12960. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, N.; Zhang, R.; Zhao, W.; Chen, Y.; Wang, Z.; Xu, B.; Zhang, M.; Shi, X.; Zhang, Q.; et al. Preemptive intrathecal administration of endomorphins relieves inflammatory pain in male mice via inhibition of p38 MAPK signaling and regulation of inflammatory cytokines. J. Neuroinflamm. 2018, 15, 320. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Shenoy, R.; Palmer, J.E.; Baines, A.J.; Lai, R.Y.; Robertson, J.; Bird, N.; Ostenfeld, T.; Chizh, B.A. Clinical trial of the p38 MAP kinase inhibitor dilmapimod in neuropathic pain following nerve injury. Eur. J. Pain 2011, 15, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Auffray, C.; Sieweke, M.H.; Geissmann, F. Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009, 27, 669–692. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Pereira, M.; Petretto, E.; Gordon, S.; Bassett, J.H.D.; Williams, G.R.; Behmoaras, J. Common signalling pathways in macrophage and osteoclast multinucleation. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef]
- Grage-Griebenow, E.; Flad, H.D.; Ernst, M. Heterogeneity of human peripheral blood monocyte subsets. J. Leukoc Biol. 2001, 69, 11–20. [Google Scholar]
- Tilton, J.C.; Johnson, A.J.; Luskin, M.R.; Manion, M.M.; Yang, J.; Adelsberger, J.W.; Lempicki, R.A.; Hallahan, C.W.; McLaughlin, M.; Mican, J.M.; et al. Diminished production of monocyte proinflammatory cytokines during human immunodeficiency virus viremia is mediated by type I interferons. J. Virol. 2006, 80, 11486–11497. [Google Scholar] [CrossRef]
- Han, J.; Lee, J.D.; Tobias, P.S.; Ulevitch, R.J. Endotoxin induces rapid protein tyrosine phosphorylation in 70Z/3 cells expressing CD14. J. Biol. Chem. 1993, 268, 25009–25014. [Google Scholar] [CrossRef]
- Neininger, A.; Kontoyiannis, D.; Kotlyarov, A.; Winzen, R.; Eckert, R.; Volk, H.D.; Holtmann, H.; Kollias, G.; Gaestel, M. MK2 targets AU-rich elements and regulates biosynthesis of tumor necrosis factor and interleukin-6 independently at different post-transcriptional levels. J. Biol. Chem. 2002, 277, 3065–3068. [Google Scholar] [CrossRef]
- Zhao, J.; Evans, G.; Li, W.; Green, L.; Chu, S.; Marder, P.; Na, S. Rapid and quantitative detection of p38 kinase pathway in mouse blood monocyte. Vitr. Cell Dev. Biol. Anim. 2008, 44, 145–153. [Google Scholar] [CrossRef]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatry Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Comalada, M.; Xaus, J.; Valledor, A.F.; Lopez-Lopez, C.; Pennington, D.J.; Celada, A. PKC epsilon is involved in JNK activation that mediates LPS-induced TNF-alpha, which induces apoptosis in macrophages. Am. J. Physiol. Cell Physiol. 2003, 285, C1235–C1245. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.; Sundler, R. Signalling to translational activation of tumour necrosis factor-alpha expression in human THP-1 cells. Cytokine 2000, 12, 1784–1787. [Google Scholar] [CrossRef]
- Tiedje, C.; Ronkina, N.; Tehrani, M.; Dhamija, S.; Laass, K.; Holtmann, H.; Kotlyarov, A.; Gaestel, M. The p38/MK2-driven exchange between tristetraprolin and HuR regulates AU-rich element-dependent translation. PLoS Genet. 2012, 8, e1002977. [Google Scholar] [CrossRef] [PubMed]
- Ortega, E.; Bote, M.E.; Giraldo, E.; Garcia, J.J. Aquatic exercise improves the monocyte pro- and anti-inflammatory cytokine production balance in fibromyalgia patients. Scand. J. Med. Sci. Sports 2012, 22, 104–112. [Google Scholar] [CrossRef]
- Uceyler, N.; Hauser, W.; Sommer, C. Systematic review with meta-analysis: Cytokines in fibromyalgia syndrome. BMC Musculoskelet. Disord. 2011, 12, 245. [Google Scholar] [CrossRef]
- Nugraha, B.; Korallus, C.; Gutenbrunner, C. Serum level of brain-derived neurotrophic factor in fibromyalgia syndrome correlates with depression but not anxiety. Neurochem. Int. 2013, 62, 281–286. [Google Scholar] [CrossRef]
- Yasuda, S.; Sugiura, H.; Tanaka, H.; Takigami, S.; Yamagata, K. p38 MAP kinase inhibitors as potential therapeutic drugs for neural diseases. Cent. Nerv. Syst. Agents Med. Chem. 2011, 11, 45–59. [Google Scholar] [CrossRef]
- Humo, M.; Ayazgok, B.; Becker, L.J.; Waltisperger, E.; Rantamaki, T.; Yalcin, I. Ketamine induces rapid and sustained antidepressant-like effects in chronic pain induced depression: Role of MAPK signaling pathway. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 100, 109898. [Google Scholar] [CrossRef]
- Budziszewska, B.; Szymanska, M.; Leskiewicz, M.; Basta-Kaim, A.; Jaworska-Feil, L.; Kubera, M.; Jantas, D.; Lason, W. The decrease in JNK- and p38-MAP kinase activity is accompanied by the enhancement of PP2A phosphate level in the brain of prenatally stressed rats. J. Physiol. Pharm. 2010, 61, 207–215. [Google Scholar]
- Bennett, R. Fibromyalgia: Present to future. Curr. Rheumatol. Rep. 2005, 7, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Buskila, D. Developments in the scientific and clinical understanding of fibromyalgia. Arthritis Res. Ther 2009, 11, 242. [Google Scholar] [CrossRef]
- Staud, R. Heart rate variability as a biomarker of fibromyalgia syndrome. Future Rheumatol. 2008, 3, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.R.; Brouillard, D.; Simpson, C.S.; Hopman, W.M.; Abdollah, H. Dysautonomia among patients with fibromyalgia: A noninvasive assessment. J. Rheumatol. 2000, 27, 2660–2665. [Google Scholar]
- Qiao, Z.G.; Vaeroy, H.; Morkrid, L. Electrodermal and microcirculatory activity in patients with fibromyalgia during baseline, acoustic stimulation and cold pressor tests. J. Rheumatol. 1991, 18, 1383–1389. [Google Scholar] [PubMed]
- Nugraha, B.; Korallus, C.; Kielstein, H.; Gutenbrunner, C. CD3+CD56+natural killer T cells in fibromyalgia syndrome patients: Association with the intensity of depression. Clin. Exp. Rheumatol. 2013, 31, S9–S15. [Google Scholar]
- Dimmek, J.D.; Korallus, C.; Buyny, S.; Gutenbrunner, C.; Lichtinghagen, R.; Jacobs, R.; Nugraha, B. Brain-Derived Neurotrophic Factor and Immune Cells in Osteoarthritis, Chronic Low Back Pain, and Chronic Widespread Pain Patients: Association with Anxiety and Depression. Medicina 2021, 57, 327. [Google Scholar] [CrossRef]
- Kenney, M.J.; Ganta, C.K. Autonomic nervous system and immune system interactions. Compr. Physiol. 2014, 4, 1177–1200. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, P.J.; Rice, F.L. Fibromyalgia syndrome pathology and environmental influences on afflictions with medically unexplained symptoms. Rev. Environ. Health 2016, 31, 281–294. [Google Scholar] [CrossRef]
- Mai, L.; Zhu, X.; Huang, F.; He, H.; Fan, W. p38 mitogen-activated protein kinase and pain. Life Sci. 2020, 256, 117885. [Google Scholar] [CrossRef] [PubMed]
- Hauser, W.; Ablin, J.; Perrot, S.; Fitzcharles, M.A. Management of fibromyalgia: Practical guides from recent evidence-based guidelines. Pol. Arch. Intern. Med. 2017, 127, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Hauser, W.; Fluss, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef]
- Widegren, U.; Ryder, J.W.; Zierath, J.R. Mitogen-activated protein kinase signal transduction in skeletal muscle: Effects of exercise and muscle contraction. Acta Physiol. Scand. 2001, 172, 227–238. [Google Scholar] [CrossRef]
- Handschin, C.; Spiegelman, B.M. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 2008, 454, 463–469. [Google Scholar] [CrossRef]
| HC (n = 13) | FMS (n = 32) | p | |
|---|---|---|---|
| Age (years) | 52.77 ± 6.50 | 54.59 ± 9.10 | 0.456 |
| Pain Intensity (VAS) | 0.70 ± 0.52 | 5.75 ± 1.95 | <0.001 |
| Fatigue (VAS) | 1.58 ± 2.14 | 5.83 ± 2.46 | <0.001 |
| Anxiety (HADS-A Score) | 4.69 ± 2.92 | 9.08 ± 4.28 | 0.002 |
| Depression (HADS-D Score) | 2.54 ± 2.54 | 7.48 ± 4.28 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugraha, B.; Scheibe, R.; Korallus, C.; Gaestel, M.; Gutenbrunner, C. The p38/MK2 Axis in Monocytes of Fibromyalgia Syndrome Patients: An Explorative Study. Medicina 2021, 57, 396. https://doi.org/10.3390/medicina57040396
Nugraha B, Scheibe R, Korallus C, Gaestel M, Gutenbrunner C. The p38/MK2 Axis in Monocytes of Fibromyalgia Syndrome Patients: An Explorative Study. Medicina. 2021; 57(4):396. https://doi.org/10.3390/medicina57040396
Chicago/Turabian StyleNugraha, Boya, Renate Scheibe, Christoph Korallus, Matthias Gaestel, and Christoph Gutenbrunner. 2021. "The p38/MK2 Axis in Monocytes of Fibromyalgia Syndrome Patients: An Explorative Study" Medicina 57, no. 4: 396. https://doi.org/10.3390/medicina57040396
APA StyleNugraha, B., Scheibe, R., Korallus, C., Gaestel, M., & Gutenbrunner, C. (2021). The p38/MK2 Axis in Monocytes of Fibromyalgia Syndrome Patients: An Explorative Study. Medicina, 57(4), 396. https://doi.org/10.3390/medicina57040396






