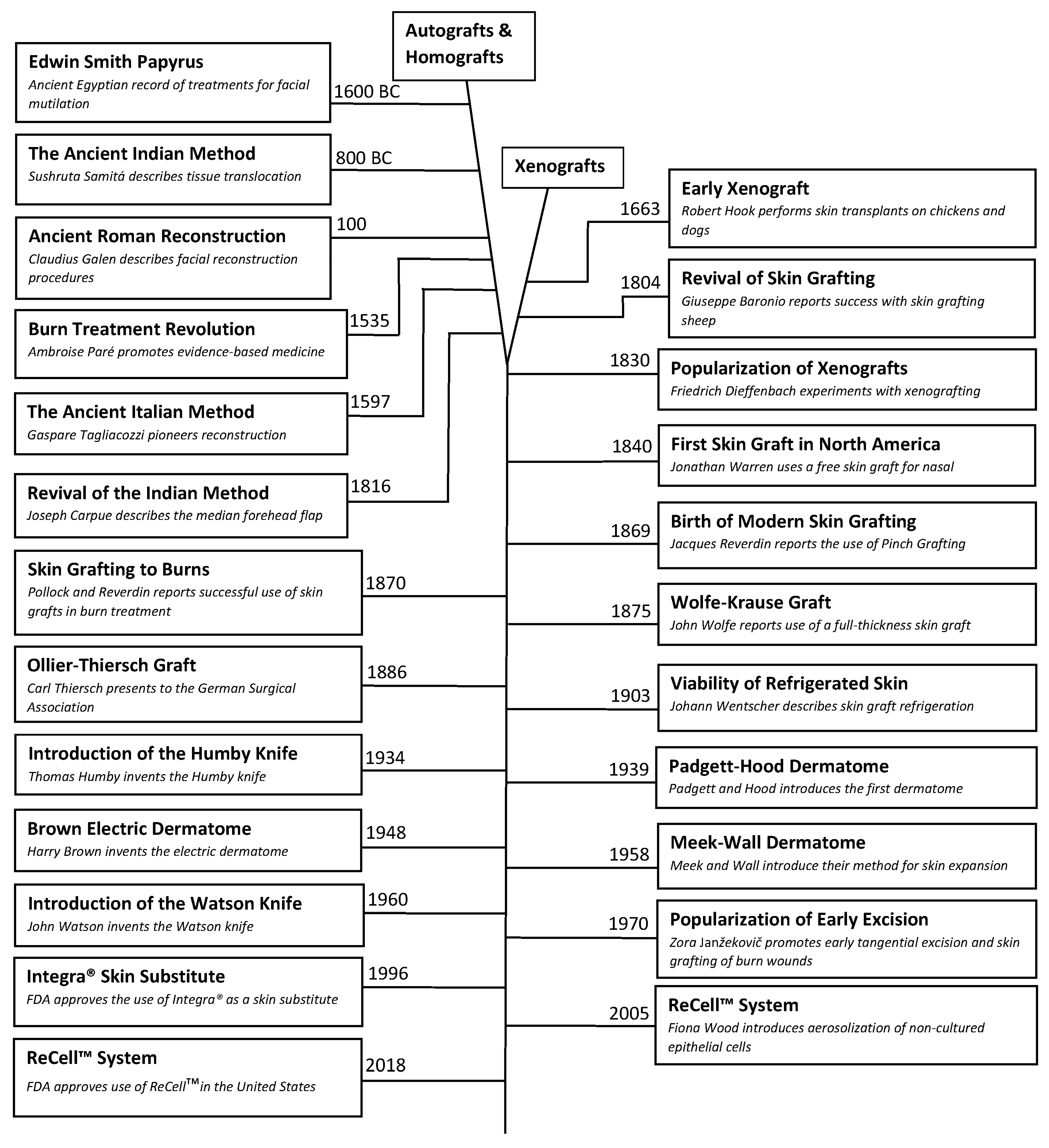
6.1. Burn Wound Management
The use of skin grafting in acute burn wound treatment did not occur until the utility of early wound excision in full thickness burn wounds was understood. Although the importance of early burn wound excision was first described by Paré in the sixteenth century. Wilhelm Fabricius Hildanus (1560–1634) is considered the “Father of German Surgery” and the first to write a book dedicated to the management of burn wounds, De Combustionibus (On Burns) in 1607. In his book, Hildanus advocated for the surgical removal of burn eschar to facilitate improved medication penetration. Although certainly ahead of his time in a surgical regard, Hildanus was ignorant of the teaching of Paré nearly 70 years prior, as many of the medications he utilized were of medieval origin [
32]. In the eighteenth century, Dieffenbach would describe the use of skin grafts to reconstruct wounds caused by burn injuries and in the late nineteenth century both Reverdin and Pollock would be credited with the successful use of skin grafts in chronic burn wounds. However, it would not be until the 1940 that skin grafting following tangential excision of acute burn wounds wound be connected in a therapeutic sequence.
The 1940s was a period of significant advancement in the understanding of burn shock management, not in any small part due to the immense loss of life precipitated by multiple man-made disasters. In 1921, Frank Pell Underhill (1877–1932) a surgeon at Yale University, treated more than 20 victims of the infamous Rialto Theater fire in New Haven, Connecticut. Underhill noted similarities in the serous fluid within skin blisters and plasma, and went on to suggested that acute shock in burn victims was primarily a hypovolemic process due to fluid losses from injured skin [
36]. Then, 20 years later, Oliver Cope (1902–1994) and Francis D. Moore (1913–2001) from Massachusetts General Hospital would treat nearly 40 victims of the Coconut Grove Nightclub in Boston, Massachusetts. Their work would connect the amount of body surface injured to the volume of resuscitation fluid needed to stave off the precipitating shock [
37]. In 1942, Forrest Young of the University of Rochester correlated that victims of burn injuries suffered from sepsis and shock as a result of fluid losses and bacterial colonization of their wounds. He also surmised that full-thickness burn injuries would only heal by secondary intention. Therefore, he advocated for early excision and skin grafting to improve mortality by removing the source of sepsis [
38]. During this era, however, many believed it inopportune to operate during the acute period of shock, so the term “early surgical intervention” referred to a period of 10–21 days after the injury [
39,
40].
In 1960, Douglas MacGilchrist Jackson (1916–2002) and colleagues from Birmingham, England described in detail a series of cases where early excision down to fascia was performed on the day of injury for full thickness burn wounds and hemodynamic management with aggressive hemoglobin monitoring and transfusions as needed. They determined that 20–30% total body surface area (TBSA) burns could be operated on the day of injury without any increased risk of death, while achieving much earlier rates of graft take and wound closure [
41]. Other surgeons would advocate for similar time frames for early excision of burn wounds, but there was little momentum in the surgical community because like Jackson, they could not demonstrate a mortality improvement over the method that delayed excision by 2–3 weeks. This would change in the early 1970s when an unknown Eastern European surgeon, Zora Janžekovič (1918–2015) from the Slovenian city of Maribor, published her findings after performing tangential excisions on deep-second and third-degree burn wounds on 2615 patients [
42,
43]. Janžekovič described the austere post-war conditions in which she found herself practicing in isolation. Later in her life, Janžekovič wrote:
“The daily changing of dressings of the burn patient, piles of dressings full of pus, the terrible stench, but above all the horrible suffering of the patients—mostly children who were scared to death and emaciated, was a cry for help and a challenge for our personal engagement. Their suffering became our suffering. The feeling of our own helplessness and the incompetence of the then medical science were destroying us… Confronted with this terrible situation, I was forced to search for any kind of solution.”
She went on to abandon delaying wound excision until the wound had fully demarcated through the sequela of infection out of necessity. She believed that she could circumvent pathologic process by shaving the wound down to healthy tissue before infection had set in. Janžekovič found that tangential excision needed to be performed down to bleeding tissue, otherwise any applied graft would desiccate and fail. Beginning with small wounds and gaining confidence with larger injuries, Janžekovič is credited with formalizing the technique of tangential excision with immediate skin graft placement within 5 days of injury. Her results showed that patients with up to a 20% TBSA could be healed within 10 days, which was unheard-of at other more prestigious burn centers [
44]. Janžekovič would go on to be the first woman to receive the Evens Medal from the American Burn Association and the Zora Janžekovič (Golden Razor) Award from the European Club for Paediatric Burns. It should be noted that Janžekovič technique was primarily for deep second-degree burns that were small enough to be addressed with a single operative intervention. In the decades that followed her publication, more than 200 surgeons from around the world would come to learn her technique first-hand and go on to further build upon her accomplishments. John Francis Burke (1922–2011) and his colleagues from Harvard University showed that the combination of tangential excision for smaller burns and full-fascial excision for larger burns followed by immediate autograft placement markedly reduced mortality and allowed the successful treatment of children up to 80% TBSA [
45]. In the decades since, numerous surgeons continue to validate and build upon Janžekovič’s technique. Today tangential excision of all non-viable tissues within 72 h of injury followed by immediate skin graft placement for full-thickness wounds is considered the standard of care.
6.2. Operative Equipment
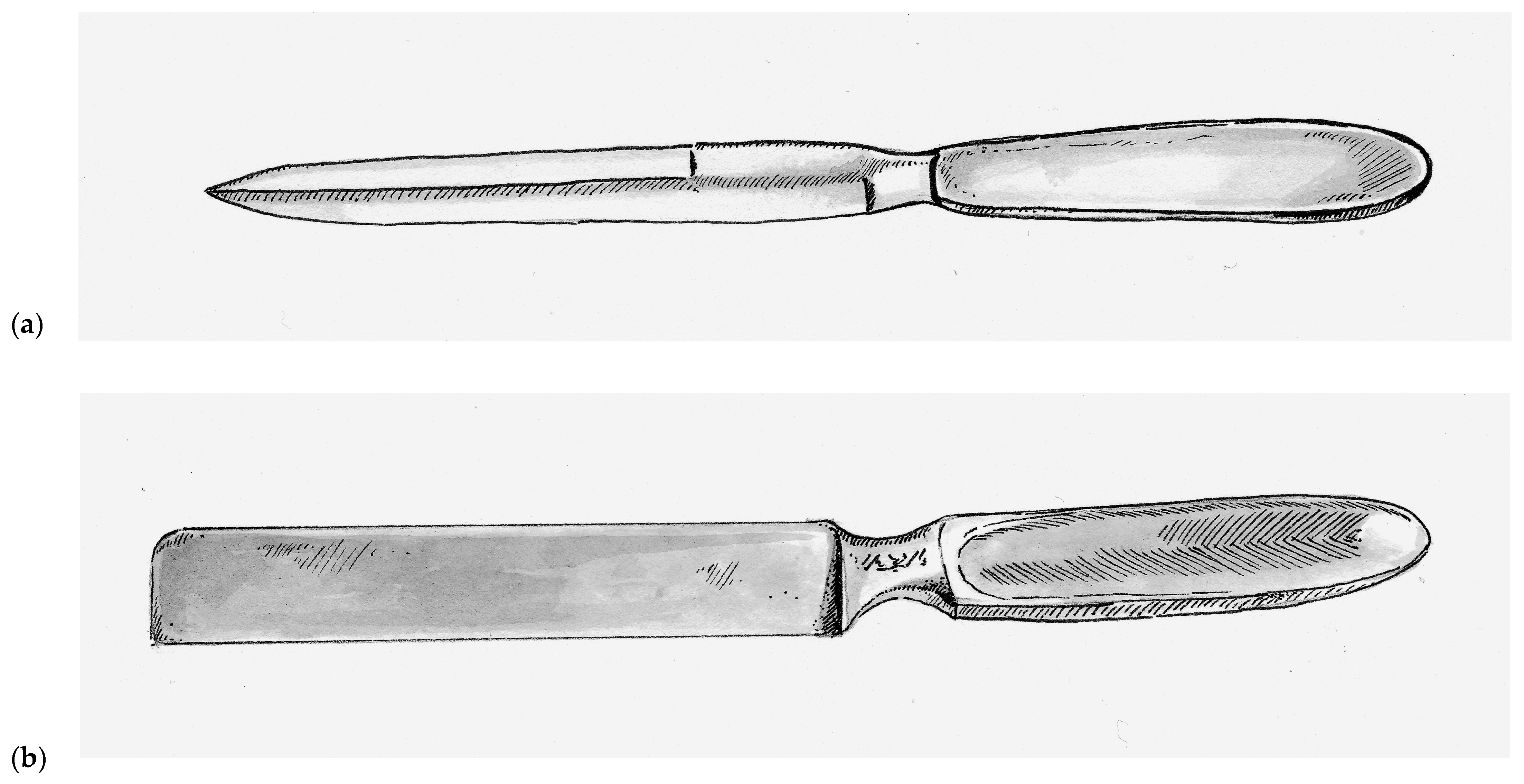
Prior to the nineteenth century, skin grafts were collected using scalpels and knives adapted for surgical procedures, such as the Catlin knife (also called the Catling knife, Amputation knife, and Interosseous knife), which was a double-bladed instrument that was typically 17 cm long and 1.5 cm wide with a simple handle at one end, as seen in
Figure 1a [
12]. As a basic single-piece instrument, it lacked any mechanism by which to control depth of excision. It was also used surgically for a number of different tasks [
12]. Although commonly used as far back as the seventeenth century, the Catling knife is still used by many surgeons today when performing extremity amputations. As one might imagine, the fine task of harvesting skin grafts with such a blunt instrument was challenging even in the most experienced hands and generally resulted in inconsistent graft thickness.
In 1920, the Thiersch’s skin grafting knife was introduced and was a rectangular stainless steel single-bladed instruments weighted toward the handle. This rectangular design, as seen in
Figure 1b, has persisted with nearly all subsequent hand-held skin grafting instruments. Vilary Papin Blair (1871–1975) an American surgeon at Washington University in St. Louis, who is best remembered as a pioneer for helping to distinguish plastic surgery from general surgery. Much of his reconstructive experience came from his time as a surgeon in the United States Army during World War I. He introduced the Blair knife in 1930, which was used in conjunction with a suction apparatus that put the skin under tension during harvest. This markedly improved the consistence of the grafts and allowed surgeons to harvest skin free-hand [
12]. The Hofmann and Finochietto knives were introduced shortly thereafter, both of which were equipped with rudimentary guards that could be adjusted with lateral screws to allow the surgeon to calibrate the depth of dissection.
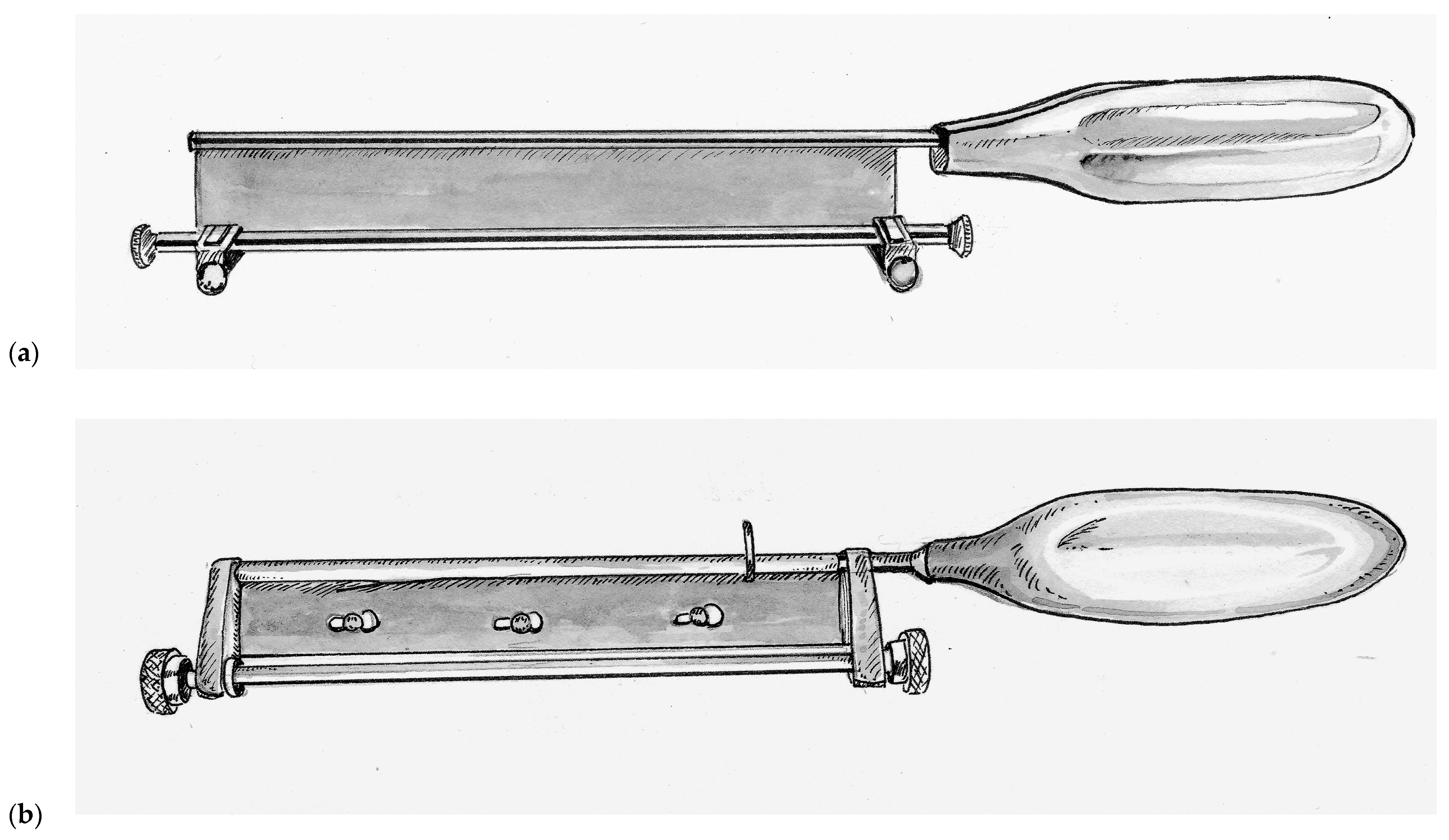
Improving upon this design, Thomas Graham Humby (1909–1970) a British plastic surgeon training at the Great Ormonde Street Hospital for Sick Children (London, UK) under the famous Sir Heneage Ogilvie introduced the Humby knife in 1934, as seen in
Figure 2a [
46]. Humby added a guard with a roller mechanism to his knife that allowed detailed calibration of the depth of tissue excised. In addition, the Humby knife had a rectangular metal frame equipped with 1/8th inch hooks at either end and a ratchet mechanism that enabled the surgeon to keep the donor tissue under tension while sliding the knife within the construct of the frame. Its use revolutionized skin grafting, enabling surgeons to single handedly and consistently excise rectangular strips of skin of consistent depth. The framework of the Humby knife was later abandoned as it could not reasonably be used for a number of potential donor sites. Subsequent variations of the Humby knife design include the Modified Humby (1936, fixed blade for rigidity), Bodenham (1949, partially supported replaceable blade that must be dismantled to change blades), Braithwaite (1955, leaf-type fully supportive replaceable blade that can be changed without disassembly) [
47,
48,
49]. All of these knives shared two design flaws. First, the mechanism for depth adjustment is dependent on two separate knurled collars mounted on either end of the back of the handle allowing room for asymmetry and user error. Second, the roller guard and the handle are fixed while the blade must have some slack in the end-bearings in order for it to slide freely from side-to-side during use. As a result, grafts would often curl around the guard, become irregular and at times became gradually thicker across a harvest.
John Watson (1914–2009), who served as a pilot in the Royal Air Force during World War II, went on to become a plastic surgeon at the Queen Victoria Hospital in East Grinstead, United Kingdom, after the war. There, he would operate on many former aircrew who had sustained disfiguring burn injuries during the war. Inspired by a potato peeler and wanting to create a user-friendly instrument that emulated the renowned dexterity of his mentor Sir Archibald McIndoe (1900–1960). So inspiring was McIndoe’s work that he was knighted for his contributions in reconstructing injured veterans of World War II. Watson introduced his knife in the year of McIndoe’s passing, 1960. The Watson knife, as seen in
Figure 2b, is unique in its simple design with a single more ridged knurled control knob for depth adjustment and precision-fit end-bearings that do not slide [
50,
51]. The result was a knife that is easier to operate and maintain, and for this reason the Watson knife is still used at many burn centers today.
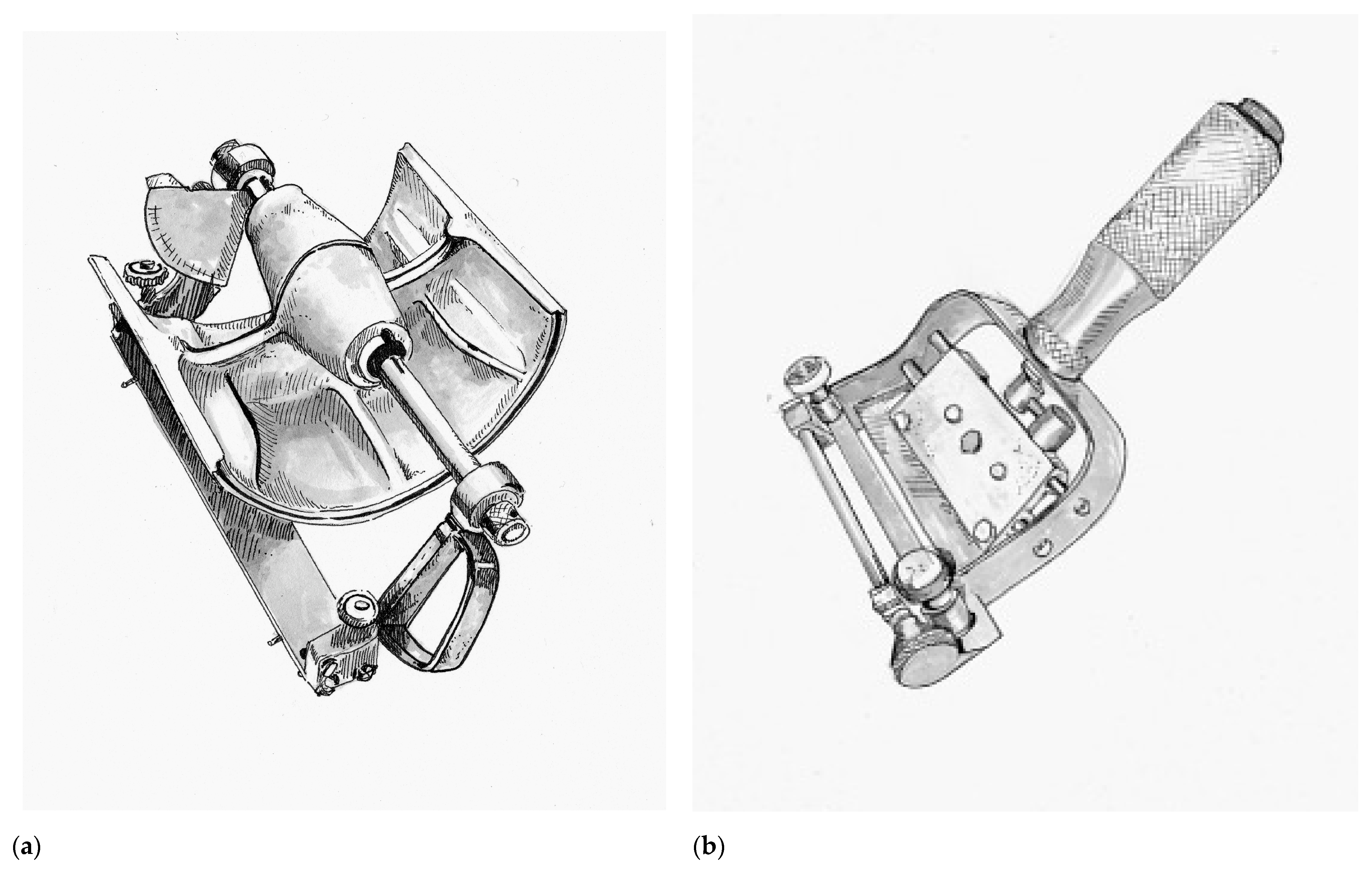
In 1937, a remarkable advancement in surgical technology occurred with the introduction of the Padgett–Hood dermatome. Earl Calvin Padgett (1893–1946), an American physician from Kansas City who trained under Blair during residency. Padgett in collaboration with his colleague George J. Hood from the Department of Engineering designed a simple to use dermatome that allowed calibrated collection of skin grafts and presented their invention at the Western Surgical Association in 1938. Unlike many of its predecessors, the Padgett–Hood dermatome earned instant recognition. One of the founding members of the American Board of Plastic Surgery, George Warren Pierce, called it “the greatest contribution in many decades to the technique of skin grafting” [
52].
The Padgett–Hood dermatome consisted of an aluminum drum that utilizes a traction-adhesion principle to feed donor tissue into a rotating blade, as seen in
Figure 3a. The distance between the blade and the rotary drum can be calibrated down to a thousandth of an inch. The Padgett–Hood dermatome had several advantages compared to previous free hand tools. It vastly improved the quality and consistency of harvested graft, even when utilized on uneven donor surfaces. This not only increased the body surface that could be considered for donor harvest, but also increased the pool of surgeons that could perform skin grafting. No longer were skin grafts an art restricted to the most dexterous and experienced plastic surgeons, rather, now they could be performed by nearly any surgeon in training. Padgett’s fortunate timing just prior to the onset of World War II cannot be understated [
29]. Blair Rogers claims the Padgett–Hood dermatome “probably did more than any other single achievement in our specialty to bring the advantages of rapid, free skin transplantation to the innumerable casualties of that conflict” [
31].
Following the war, John Davies Reese (1893–1958) would improve upon the Padgett–Hood dermatome in 1946. In contrast to the cast-aluminum of its predecessor, the Reese dermatome was a heavily machined, more precise and more reliable instrument. Notable disadvantages of the Reese dermatome were its considerable weight and inability for depth to be adjusted during a graft harvest [
53]. The Reese dermatome would be overshadowed by the introduction of the first electric dermatome by the American surgeon Harry M. Brown (1914–1948) in 1948, as seen in
Figure 3b [
12]. Brown’s dermatome was hand-held and relatively easy to operate, which allowed the harvest of large amounts of skin graft rather quickly with minimal effort. Brown actually thought of this new instrument while being held prisoner by the Japanese during World War II, but unfortunately was killed in a tragic accident shortly after his invention was introduced. A number of contemporary electric dermatomes including the Stryker, Padgett, and the Zimmer are based directly on the design of the Brown electric dermatome [
12].
6.3. Skin Graft Expansion
Split thickness skin grafts first became popular in the 1930s after Blair and James Barrett Brown (1899–1971) first articulated the differences between full-thickness, intermediate-thickness and epidermal skin grafts. Their work showed that donor sites for STSG healed through epithelialization from local hair follicles and the underlying basal layer. The preservation of donor tissue so that it could potentially be re-harvested made STSG an attractive option over full thickness grafts, particularly in the treatment of large surface area wounds. The invention of the dermatome made the collection of STSG common practice to any interested surgeon. Building upon these advancements, Cicero Parker Meek (1914–1979) from South Carolina introduced a novel micrografting technique in 1958 that enabled a graft to be utilized over a wound ranging from six to nine-fold greater in size [
54]. Meek built upon the early wisdom of Reverdin’s pinch grafts and Lawson’s Fourpenny grafts by recognizing that epithelialization occurred from the graft edge. He hypothesized that by maximizing the epithelialization boarder he could expedite wound healing. Therefore, Meek would cut each square inch of harvested graft in a 16 by 16 grid pattern (generating 256 micrografts each 1/16
th of a square inch) using the Meek–Wall microdermatome. The result increased the epithelialization boarder 16-fold from 4 inches to 64 inches. The micrografts would then be placed onto bandages and applied to the wound [
55]. In addition to the expansion of applicable surface area for harvested donor tissue, the Meek’s technique allowed serosanguineous fluids to drain freely around the micrografts. Although the Meek–Wall microdermatome is still utilized at many specialized burn centers today, its routine use did not gain momentum as it was expensive to acquire and cumbersome to operate.
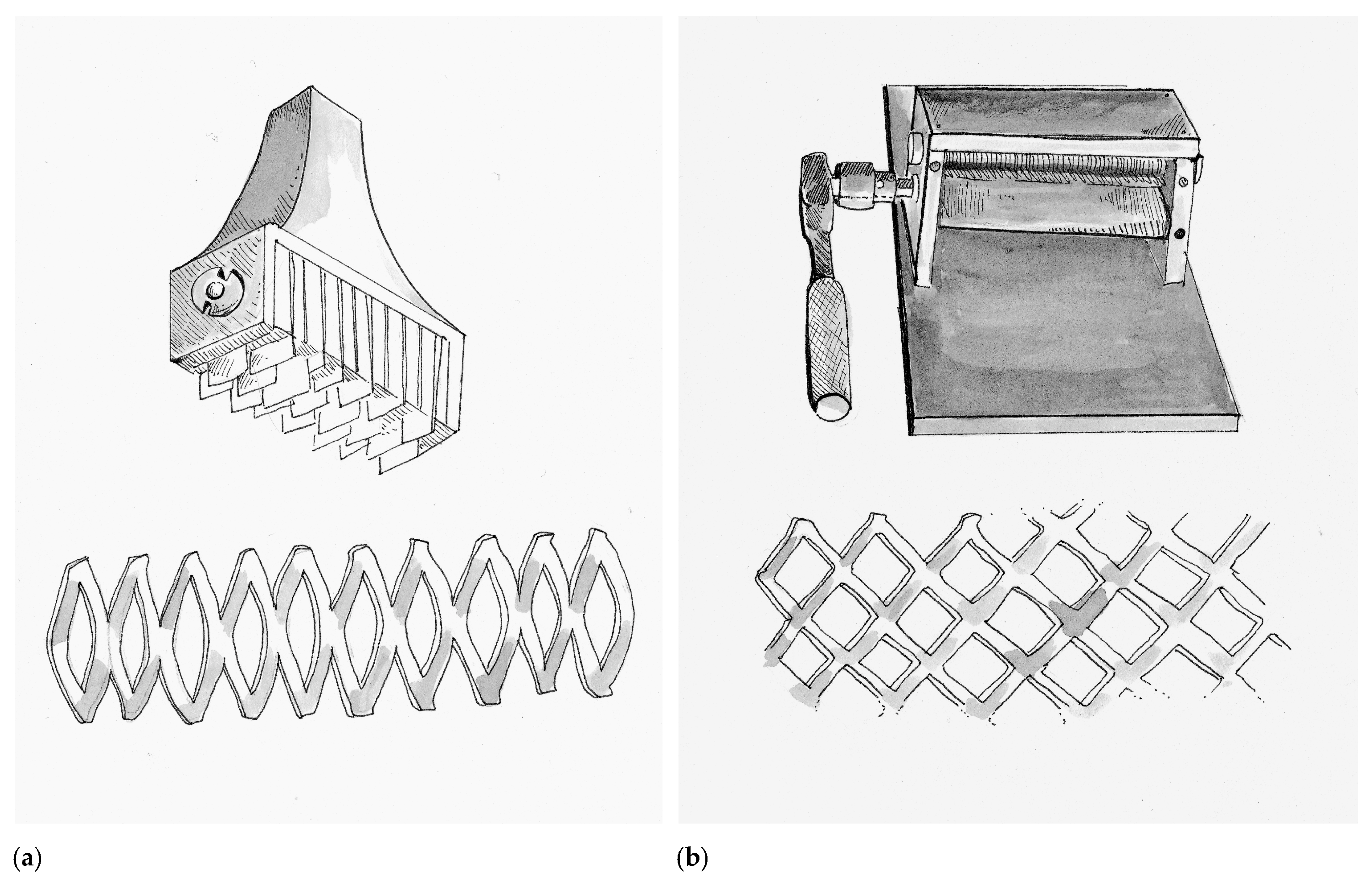
Although the concept of micrografting would not be forgotten, at this time in history the Meek’s technique served as the bridge to skin meshing, which also allowed for wound fluid drainage and expansion of donor tissue over a larger wound surface area. Credit for the first prototype skin mesher belongs to the Swiss surgeon Otto Lanz (1865–1935) in Amsterdam (Netherlands), who trained under the Emil Theodor Kocher (1841–1917) the first surgeon to be awarded the Nobel Prize. Lanz invented a tool in 1907 he called the Hautschlitzapparat, which made 19 parallel cuts into a harvested skin graft, allowing it to expand in a manner that he described as a skin-net [
56]. The Hautschlitzapparat and the accompanying tissue pattern are depicted in
Figure 4a. Lanz described that the meshed skin graft would allow it to cover twice the surface area as the original donor tissue, a principle called the concertina effect. Lanz would apply one-half of the graft over the wound of interest and the other half would be used to re-cover the donor wound. Lanz’s idea was based on the children’s activity where a strip of paper is cut in alternating intervals to make an accordion-toy. He did this because it bothered him that when using Thiersch grafts often the wound would heal prior to the donor site. In 1930 Beverly Douglas (1891–1975) described the “sieve graft”, which was a skin graft where he punched out holes. The removed “holes” were then re-applied on the donor site, while the graft was applied to the wound. The intention of the sieve graft was to facilitate drainage of wound fluids, which if undrained can dissociate the graft from the underlying wound bed and compromise viability. In 1937, multiple surgeons like Lester Reynolds Dragstedt (1893–1975) and František (Francis) Burian (1881–1965) continued to adapt and modify the sieve graft by manually making staggered incisions in the graft instead of hole-punches. As a result, fluid drainage was still achieved while the graft could now expand to cover larger surface areas. Although at that time, this technique was used primarily on full-thickness skin grafts, today it has been adapted for STSG and is commonly referred to as “pie-crusting”.
In 1964 James Carlton Tanner Jr. (1921–1996) and his chief resident Jacques J. Vandeput from Grady Memorial Hospital in Atlanta, Georgia created a simple device called the Tanner–Vandeput mesh-dermatome that could expand STSG by a ratio of 1:3. The Tanner–Vandeput mesh-dermatome and its accompanying tissue pattern are depicted in
Figure 4b. In their landmark paper titled “The Mesh Skin Graft” they introduced the term “meshed graft” [
57]. The Tanner–Vandeput mesher consisted of two four-inch rollers, one knurled to grip the skin and the other with multiple parallel staggered cutting blades to cause the meshing pattern. Harvested skin grafts were placed between the two rollers and then rotated to incise a meshed pattern into the graft [
58]. As mentioned, however, micrografting would not be forgotten. In 1966, Vandeput combined the concepts of the Meek–Wall dermatome and the Tanner–Vandeput mesher to create “ultra-postage skin grafts” that were 1/20th of an inch squared [
59].
The Tanner–Vandeput mesher was originally sold under the commercial name Mesh-Dermatome I by the Zimmer Company (Dover, OH, USA) in 1964 and utilized a plastic feeding tray called a dermacarrier. A number of iterations have since been made over the years to improve upon the design. The Mesh-Dermatome II (Zimmer Company) introduced in 1970 changed the blade angles relative to the dermacarrier and the meshing pattern (hexagon) to allow variability in the meshing ratio from 1:1 up to 9:1. In 1991, the Zimmer Skingraft Mesher (Zimmer Company) utilized a ratchet and cog-wheel mechanism to pull the dermacarrier through the cutting mechanism. This model also allowed interchangeable bladed rollers to allow for rapid variation in ratio (ranging from 1:1 to 4:1). The two companies did away with the dermacarrier and introduced a double-cutting-roller design—Collin (Arcueil, France) in 1986 and Brennen Med (St. Paul, MN, USA) in 1988. The primary benefit of the double-roller model is that it did not require sharpening as it did not relay on blades piercing the skin graft in order to form interstices. Rather, the two rollers, one of which is notched, performed a scissor-like pinching action. The Brennen Skingraft Mesher (Brennen Med) which was modified in 1993 from the original design to be more user-friendly offers a number of meshing patterns, but, unlike its Zimmer counterpart, a different instrument is needed for each meshing ratio (ranging from 1:1 to 8:1) [
60]. Meshed grafts are the mainstay of modern burn wound care and use nearly universally by all burn centers. They have several key advantages over unmeshed (sheet) grafts beyond surface area expansion, fluid drainage, and expedited wound closure. Meshed grafts are more versatile as the interstices allow the shape of the donor tissue to be adapted to asymmetric wounds and across irregular body contours [
58]. Unmeshed skin grafts in contrast develop more robust vascularization, reduced scar contracture and are more aesthetically pleasing due to the lack of interstices.
Severely injured burn victims and limited donor site availability have continued to challenge burn surgeons to push the limits of skin graft expansion. In 2012, Florian Hackl and colleagues from Brigham and Women’s Hospital in Boston, Massachusetts described the Xpansion
TM technique, which afforded an expansion ratio of 100:1 by mincing skin grafts into 0.8 mm × 0.8 mm micrografts and then spreading them onto the wound bed [
61]. Studies have found that mincing grafts leads to over expression of growth factors like tumor necrosis factor alpha, platelet-derived growth factor, and basic fibroblast growth factor, which are thought to promote wound healing [
62]. Further, developing Hackl’s technique, Denesh Kadam an Indian surgeon from Karnataka, India developed a technique called “Pixel Grafting”. Similar to the Xpansion
TM technique, skin grafts are minced into digital pixel sized grafts (0.3 mm × 0.3 mm) and are able to achieve expiation ratio of up to 700:1 [
63].
6.4. Homografts and Immunologic Discoveries in Skin Grafting
When Reverdin first introduced the concept of skin grafting it gained popularity rapidly due to the remarkable nature of the short-term results. By the 1870s, however, Reverdin’s techniques were losing popularity because of their less impressive long-term results. From our modern perspective it is not intuitive why this might be. At the time these shortcomings were attributed to impracticality of the concept of skin grafting itself, but in fact the reason had to do with the unintentional introduction of homografting—the use of skin grafts across members of the same species. Reverdin, along with numerous other surgeons of his age, held the conviction that homografts were for all intents and purposes interchangeable with autografts. In fact, in many publications from that period the authors failed to bother specifying what type of graft was even used. Reverdin’s reputation in burn surgery hinges on reports that he used his own skin to treat injured patients. In 1872, he wrote about the care of a burn victim:
“In my first grafts I used skin from the patient himself, but I soon became convinced that the results was the same when I used skin from another individual. This has been demonstrated with certainty.”
—Jacques-Louis Reverdin [
64].
Although the idea of a surgeon using their own skin seems remarkably philanthropic by our modern cultural norms, during Reverdin’s time many surgeons would excise fragments of their own skin to demonstrate to patients who were particularly scared that the procedure was in fact not as painful as the patient might imagine. The modern surgeon might even equate it to a form of informed consent. Surgeons also reported that many patient’s relatives were more than willing to donate fragments of their own skin to aid in the healing of a loved one [
65]. Going one step further, in the Berlin Military Hospital a skin graft donor could be found for as little as the price of a beer [
66]. Donor tissue were also collected in more creative ways—for example from amputated extremities and circumcised foreskin [
67,
68]. The timing between graft harvest and placement, as well as the temperature that the collected tissue was stored at, was a matter of great debate in those days. Some surgeons arguing that the procedure needed to occur as soon as possible and that the graft must be kept warm, while others reported success with grafts that had been collected four days prior and were stored at 10 °C [
69,
70]. This dichotomy resulted in a number or remarkable scenarios. One surgeon described placing the soon-to-be amputees in the same operative theater adjacent to the anticipated skin graft recipient. Another surgeon described his method of keeping grafts warm by placing them in his armpit while transporting them from donor to recipient [
70,
71]. In addition, despite amputations being routinely performed in those days, disease like smallpox, syphilis and tuberculosis were also equally commonplace. Therefore, the sudden appearance of numerous cases of infectious diseases transmitted through homografts in the literature should come as no surprise to the modern reader [
64,
72,
73]. Surgeons also described progressive graft degradation and failure in a time when the mechanism of graft rejection was not understood. Therefore, for a time homografting was abandoned by most practitioners, and considered to be an inherently unsuccessful enterprise.
In the 20th century, however, with advances in critical care and medical technology, there was an unexpected revival of cadaveric homografting with the introduction of the skin bank and cryopreservation. In 1903, the German physician Johann Wentscher (1852–1913), a contemporary of Thiersch, was the first to document the viability of refrigerated skin grafts at 0 °C for 14 days [
74]. Long-term cryopreservation was not possible until the 1930s when effective, reproducible, standardized storage methods were established. It was during World War II that serious investment went into skin banking due to the large number or wounded soldiers that were returning from the war. The sheer burden of injury produced by World War II spurred the development of skin banks and trauma-specific research centers, such as the United States Institute of Surgical Research [
75]. Researchers found that harvested skin could be preserved in a glycerol-based cryopreservative for up to up to four months at −79 °C [
76]. Such skin banks allowed wartime hospitals to have a read supply of homograft skin for severe burn victim with insufficient donor site.
Around the same time, across the Atlantic, the Battle of Britain (1940) raged and a plane crashed in Oxford near the home of British immunologist and Zoologist, Sir Peter Medawar (1915–1987). The physician caring for the horribly burned pilot consulted Medawar for advice. Although Medawar had no experience caring for burn victims, he believed that skin grafting would afford the airman the best chance of survival. Unfortunately, despite Medawar’s efforts, the pilot would not survive. The experience, however, would instill in Medawar a life-long curiosity about the immunological intricacies of skin grafting. During the remainder of the war Medawar collaborated with the Scottish Plastic surgeon Thomas Gibson (1915–1993) to perform homografts and autografts on other soldiers [
77]. The two observed that homografts, though initially appearing to incorporate, would go on to be rejected within two weeks’ time. In contrast, autografts were often successfully engrafted during the same time frame. Medawar also noticed that if a second homograft from the same donor was re-attempted, graft rejection occurred more quickly. This affirmed Medawar’s suspicions that the etiology of graft failure was immune-mediated. He reported his findings to the War Wounds Committee of the Medical Research Council in 1944, The Behaviour and Fate of Skin Autografts and Skin Homografts in Rabbits [
78].
In 1945, Ray David Owens (1915–2014) introduced the concept of chimerism while studying dizygotic cattle twins. Owens observed the presence of “mixed blood types” that were the result of in utero genetic exposure [
79]. Frank Macfarlane Burnet (1899–1985) an Australian Virologist built upon these findings and proposed the theory of immune tolerance, suggesting that immunologic self-awareness could be influenced, particularly during embryogenesis. Medawar tested this theory by crossing allografts between dizygotic cattle twins. He observed that grafts remained intact for several weeks longer than would typically be expected. Medawar took the experiment one step further using a mouse model. He inoculated fetal mice with splenic cells from a donor (second) mouse strain. After eight weeks, he performed allografts using the donor strain of mice and observed that the transplanted skin was tolerated. As a control, skin from a previously unexposed (third) strain was also grafted and was expectantly rejected [
80]. This experiment is credited as the foundation for modern transplant immunology and both Medawar and Burnet were awarded the Nobel Prize in Medicine for their discovery of immune tolerance.
Prior to World War II, Colonel James Barrett Brown (1899–1971) had postulated that homograft rejection was due to the genetic disparity between donor and host. In 1937, he performed the first successful “homograft” in which both the donor and recipient of a skin graft were identical twins. During the war as the Chief of plastic surgery at Valley Forge General Hospital (Phoenixville, Pennsylvania), Brown took Joseph E. Murray (1919–2012), then a surgical intern and First Lieutenant, under his wing. This act of charity spared Murray overseas deployment and afforded him experience caring for wartime victims of burn-related trauma. Similar to Medawar, Murray’s first-hand exposure to burn victims would inspire an academic career in tissue transplantation. Murray would lay the foundation for our modern understanding of skin’s enhanced antigenicity [
81,
82]. In the decades that followed World War II, a significant amount of research on the immune response to skin grafts was performed. In the 1950s, major histocompatibility complexes (MHC) were discovered. However, Rupert Everett Billingham (1921–2002) demonstrated that both MHC-matched and mismatched donor homografts resulted graft failure [
83]. The 1960s saw a wave of animal experiments with immunosuppressants like phenothiazine derivatives, methylhydrazine derivatives, anti-lymphocyte biologics, steroids, anti-metabolites, and even x-ray irradiation—none of which were effective in reducing skin graft rejection rates or were practical for use in human use [
84]. In the 1970s, transplanted skin was found to generate a more robust immune response than solid organs due to its higher antigenicity. Although the exact mechanisms for cell-mediate (T-cell) and innate (Natural Killer cell) mediated acute rejection was not better understood till more recent decades, surgeons have come to understand that homograft do not replace the need for autograft, but serves as temporary bridge to autograft application. Of note, Murray replicated the work of him predecessor, Brown, performing the first living donor kidney transplant between identical twins, and was awarded the Nobel Prize in Medicine for his achievement in 1990 [
81].
In 1954, Douglas MacGilchrist Jackson (1916–2002), a contemporary of William Heneage Ogilvie, was a British surgeon from the Birmingham Accident Hospital who is remembered for his “alternate strip method,” which involved placing half-inch strips of autograft and homograft in an alternating sequence to cover large posterior thoracic burn wounds. Jackson credited the idea to Rainsford Mowlem (1902–1986), a New Zealander who was appointed to presidency of the British Association of Plastic Surgeons. Jackson performed this method on 16 patients and reported successful results with many patients being able to return to their lives without significant functional disability within a year. Jackson also coined the term “creeping substitution”, an observation he noted when epithelialization from the autograft strips boarders would grow underneath the homograft strips and eventually connect adjacent autograft strips. Subsequently, the strips of homograft would separate and reveal an epithelialized wound bed underneath [
85].
In 1986, Ming-liang Zhang, a Chinese surgeon from Beijing Jishuitan Hospital, and colleagues further developed this technique by slicing autografts into one millimeter micrografts, which they would place onto a larger sheet of homograft. They called this autograft–homograft composite “Microskin”. Sheets of Microskin were then applied to the wound bed and much like in the alternate strip method, autograft epithelial cells proliferated via creeping substitution and eventually separated from the homograft. Over multiple publications, Zhang described the successful use of Microskin grafting in 32 burn patients (ranging from 2.5 to 45% TBSA) and reported expansion ratios as high as 15:1 to 18:1 [
86]. Despite its advantages, however, Microskin remains technically challenging and requires specialized equipment to apply routinely. J. Wesley Alexander (1934–2018) and colleagues from the Shriners Burns Institute in Cincinnati, Ohio (now called Shriners Children’s Ohio) utilized a method in which 6:1 meshed autograft was reinforced with 3:1 meshed homograft. Alexander noted that by layering homograft on top of the more friable autograft the latter would be protected during the critical period of incorporation. In the 14 patients this method was initially described in, there was a 99% graft take of the underlying autograft with no reported loss at follow-up. The overlying homograft, in contrast, had a 95% initial incorporation within the first three days and a subsequent near-total rejection over the subsequent 30-day period [
87]. The Alexander method or, as it is more commonly called, the “Sandwich technique” has been modified since being introduced, but is still widely utilized today at almost every large burn center today for its simplicity and efficiency.
6.5. Skin Substitutes
Bioengineered skin substitutes have also emerged as an area of interest. Research into skin substitutes dates back to 1975 when Ioannis V. Yannas of the Massachusetts Institute of Technology (MIT) and John Francis Burke (1922–2011), the Chief of Staff at Shriners Burns Institute in Boston (now called Shriners Hospital for Children©—Boston) and the Massachusetts General Hospital Burn Service, collaborated to develop the first bio-synthetic skin substitute called Integra
® (Integra LifeSciences Corp., Plainsboro, NJ, USA). In recognition for their achievement, Yannas and Burke were inducted into the National Inventors Hall of Fame in 2015. Integra
® consists of a layer of cross-linked bovin collagen and shark chondroitin (glycosaminoglycan) with a silicon top-layer. The collagen-chondroitin matrix facilitates the recapitulation of a reticular dermis, while the silicone acts as a temporary protective pseudo-epidermis. After the excision of a full-thickness burn, Integra
® allows the wound bed to regenerate a layer equivalent to the dermis. After approximately three weeks, when the dermis has regenerated, the silicon layer is physically removed and replaced with a standard autograft [
88]. Products like Integra
® are acellular skin substitutes. In contrast, cellular skin substitutes like Transcyte
® (Advanced Tissue Sciences, La Jolla, CA, USA) consist of a synthetic scaffolding seeded with living human cells (fibroblasts).
Cell-based therapies aim to replace lost tissue with cultured skin cells, which was not considered feasible until 1975, when Howard Green (1925–2015) and his graduate student James G. Rheinwald at MIT successfully cultured human keratinocytes [
82]. Green and Rheinwald actually made their discovery by accident while they were trying to replicate a teratoma, an altogether different tumor [
89]. Their discovery called Cultured Epithelial Autografts (CEA) involves harvesting stems cells from the patient’s skin and growing a culture of these cells into an autograft sheet, which can then be applied to burn wounds and was particularly useful when donor sites are limited. In 1983, the mettle of CEA was put to the test when a five-year-old, Jamie Selby, and his seven-year-old brother, Glen Selby, suffered 97% TBSA third-degree burns after playing with flammables in an abandoned building. Both children from Casper, Wyoming were airlifted to Shriners Hospitals for Children©—Boston where Green and his colleagues performed over 350 grafts grown from small patches of donor tissue. Having only produced CEA on a small scale prior to this occasion, Green restructured his laboratory at Harvard University in order to produce CEA around the clock for the two boys. He later went on to found the company BioSurface Technology Inc. (Cambridge, MA, USA). The survival of the two boys, gained CEA national recognition from the medical community. In 2010, Rajiv Sood and his colleagues from Indiana University in Indianapolis, Indiana described their experience with the use of CEA in 88 victims of major burns, ranging from 28 to 98% TBSA over a period of 18-years. They found an overall graft success of 72.7%, and a survival rate of 91%. Sood wrote that “[such results] gives much optimism for continuing to use CEA in critically burned patients” [
90]. Despite its significant benefits, the primary barrier to its routine use in the critically ill burn patient is the two to three weeks of incubation-time needed to produce it.
In 1989, Steven T. Boyce from the Shriners Burns Institute in Cincinnati, Ohio and colleagues from the University of California San Diego Medical Center introduced an alternative product which they expected to replace CEA called Cultured Skin Substitutes (CSS). Consisting of a collagen-glycosaminoglycan sheet inoculated with human dermal fibroblasts on one side and epidermal keratinocytes on the other, CSS could potential to preserve donor sites as it did not rely on autologous cell harvesting [
91]. Boyce prodigiously published the benefits of CSS, showing in vitro that it could be modified in various ways to emulate the anatomic features of autografts—“lipid supplements” could enhance the epidermal barrier, pigmentation could be optimized to the patient with the addition of melanocytes, the addition of growth factors could enhance wound healing and the CSS itself could be genetically enhances to expedite wound closure [
92,
93,
94,
95]. For a time, CSS was used at a number of burn centers, especially within the Shriners system, however, it did not gain sufficient traction from the Food and Drug Administration to be adapted for widespread clinical use.
Around the same time, another emerging technology was gaining attention within the tissue translocation community—cellular suspension. Rupert E. Billingham (1921–2002) and Joyce Reynolds of the University College (London, United Kingdom) first introduced cellular suspension in 1952 using an enzymatic preparation with Trypsin [
96]. Although the suspended cells were viable, this early technique was not successful because the cells failed to adhere to the wound bed. In 1988, János Hunyadi and colleagues revisited the technique by trypsinizing keratinocytes and suspending them in a fibrin glue solution, a process which they named Keratinocytes in Fibrin Glue Suspension (KFGS). Hunyadi was able to use KFGS technology to successfully treat venous stasis ulcers, but did not apply it to the treatment of burn wounds [
97]. In 1994, Hans-Wilhelm Kaiser and colleagues from the University of Bonn Medical School and Burn Centre in Colonge-Merheim, Germany published the use of fibrin glue suspension techniques with cells derived from CEA in the treatment of burn wounds [
98]. Several subsequent studies compared and contrasted aerosolized skin cells with and without fibrin glue, and, in 2003, Lachlan J. Currie and colleagues concluded that there was no observable of histologic difference in outcomes [
99].
The merger of autologous epithelial cell culturing and cellular suspension occurred in the early 1990s by the British-born Australian plastic surgeon, Fiona Melanie Wood. As the Burn Director of the Royal Perth Hospital, Wood had experienced first-hand the morbid consequence of awaiting CEA production in a critically injured burn patient. After several deaths, which Wood believed could have been salvaged if the production time of CEA could be decreased, she began experimenting with the production process of cultured epithelial cells. In her early work, Wood discovered that CEA could be applied after culture growth of as little as 10 days. Not only would the sub-confluent cultured graft adhere to the wound, but Wood observed that the wound healed quicker. The mechanism for this observation was unknown, but Wood suspected that it was a result of cellular signaling pathways within the wound bed that promote proliferation or that sub-confluent cells inherently had improved proliferative potential having spent less time in culture medium [
100]. Edwin A. Deitch and colleagues also found that burn wounds closed after 21 days, independent of the method, incurred a 70% risk of hypertrophic scar formation, while wounds closed within ten days only afforded a 4% risk [
101]. Wood recruited Marie L. Stoner, a cell biologist, to help her reduce cultured graft application time table and the two worked tirelessly—successfully minimizing it to 5 days. In their method, a postage-stamp size piece of uninjured donor epidermis is harvested. Keratinocytes are scrapped off and cultured in a concentrated single-cell suspension for 5 days and collected while in the pre-confluent stage. The cells are then placed in solution and aerosolized onto the burn wound using a standard syringe with spray-nozzle attachment. Wood dubbed this process CellSpray (later referred to as Spray-on-Skin™) and she went on to commercialize the technology in 1993 with the help of the Australian biotechnology company Clinical Cell Culture Pty Ltd. (also referred to as C3) [
102]. Over the next decade the global burn community would regard Spray-on-Skin™ with skepticism, as Wood did not publish any trials demonstrating its comparative efficacy. Wood continued to use the method routinely in her own practice and insisted that the results spoke for themselves. The lack of scientific evidence and Wood’s financial relationship with C3 would raise concerns among her peers and negatively affect global interest in the technology. Therefore, for a time, C3 proved a service, albeit on a humble scale, to burn centers in Australia, New Zealand, and the United Kingdom.
On 12th October 2002, however, Spray-on-Skin™ would return to the international limelight when militant terrorist bombed the tourist-rich Kuta district of Bali, Indonesia. The Royal Australian Air Force transported over a hundred patients to hospitals throughout Australia, of which 28 were brought to Wood’s burn unit. In preparation for such an event, Wood had coordinated with Woodside Petroleum, a local energy company, to trial a burn catastrophe response plan the year prior. As part of the plan, the production of Spray-on-Skin™ would be upregulated proportionally to meet the acute needs of the patients. By exercising this plan, Wood and her colleagues were able to save all but three of the patients they had received that day [
102]. Naturally, international interest in Spray-on-Skin™ increased. In 2005, Wood introduced another technological achievement in skin transplantation technology—aerosolization of non-cultured epidermal cells. Wood called her novel system ReCell
®; in 2008, C3 would be restructured under a new name, Avita Medical, Inc. (Valencia, California), and ReCell
® would be their primary product. John Harvey, the president of the Australian and New Zealand Burn Association described Wood’s work as “ground-breaking” [
103]. In 2005, for her significant contributions to the field of burn care, Wood would be named Australian of the Year.
The ReCell
® system is available as a single-use kit that combines the extraction and application of cells into a single process. Cells are harvested from the dermal-epidermal junction of a STSG taken at 6–8/1000th of-an-inch thickness. The tissues are then enzymatically (trypsin) and mechanically disrupted. Keratinocytes, melanocytes, fibroblasts, and Langerhans cells are the suspended in a lactate solution. Each square centimeter of donor STSG generates one milliliter of suspension, which in turn can be applied to approximately 80 cm
2 of wound area (80:1 expansion ratio) [
104]. Similar to CellSpray, ReCell
® was introduced with little comparative evidence of efficacy relative to other routinely used skin grafting techniques. In fact, it would be Sood and colleagues who would publish the first phase 2 trial comparing outcomes between ReCell
® and meshed STSG in 10 patients [
105]. Although under-powered, Sood showed that ReCell
® demonstrated similar results and required a small donor site. In 2018, a larger multi-institutional randomized control trial was performed by James Hill Holmes IV, and collaborators comparing ReCell
® with meshed STSG in deep partial thickness wounds in 83 patients. At four weeks, patients treated with ReCell
® demonstrated similar wound closure (98 vs. 100%), pain and scarring compared to controls. In contrast, control donor sites were approximately 40 times larger, incurred more pain, and expectantly took longer to heal than donor sites in the treatment group. Subsequent follow-up one year after treatment revealed that patient’s that received ReCell
® were considerably more satisfaction than controls [
106]. Based on these trails, ReCell
® received approval by the Food and Drug Administration in 2018.
Critics of ReCell
® also raised concern over the viability of extracted cells after applications. In 2012, a study using ReCell
® found 75.5% of cells were viable at the time of harvest and 69.5% survived aerosolization. Critics also argued that application of non-cultured cells result in delayed epithelialization and thin wound coverage, especially when utilized on full thickness wounds that lack existing dermal elements. Initially, Wood responded to these concerns in 2007 by demonstrating the successful combined use of ReCell
® and Integra
® on a porcine model. Wood performed a single-stage repair of ten full thickness wounds on a pair of Yorkshire swine and histologically compared results with controls treated with Integra
® alone and ReCell
® alone. Wood’s results showed that simultaneous treatment enhanced reconstruction of full thickness wounds compared to controls, but once again did not provide a comparison of her technique with what would be considered a standard of care practice [
107]. More than a decade later, in 2019, Holmes IV and collaborators once again performed a second multi-institutional trial comparing the concurrent use of ReCell
® and STSG with in-subject controls receiving STSG alone. The trial was performed on 30 patients for treatment of mixed depth wounds including deep partial thickness and full-thickness burns. The mean TBSA was 21% (+/− 13%) and the average area grafted was 2443 cm
2 (+/− 1675 cm
2). The results for treatment with ReCell
® and STSG showed non-inferiority for wound healing (92 vs. 85%) and a statistically significant reduction in donor site area (32%;
p < 0.001) [
104]. These results suggest that ReCell
® combined with STSG can be a safe and effective treatment for deep burn wounds and can help minimize the amount of donor surface area utilized. As of December 2020, ReCell
® is being used at 83 of the nations 132 burn centers and is considered one of the most promising advance in skin translocation technology [
108].