Evaluation of Obstructive Sleep Apnea Phenotypes Treatment Effectiveness
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Phenotypes
3.2.1. Positional OSA
3.2.2. Severe OSA
3.2.3. OSA and PLM
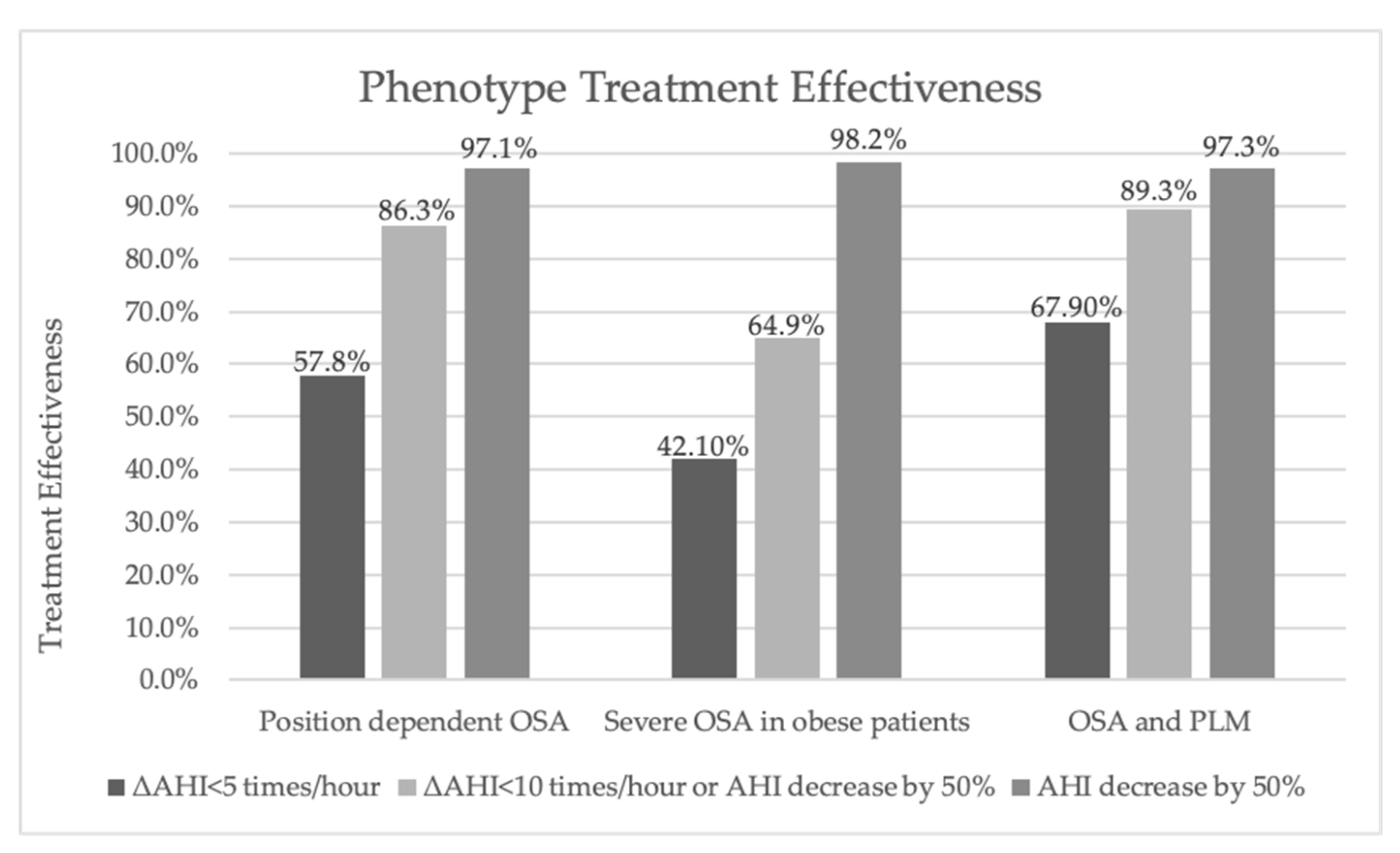
3.3. The Effectiveness of Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, H.P.; Chen, Y.F.; Du, J.K. Obstructive sleep apnea treatment in adults. Kaohsiung J. Med. Sci. 2020, 36, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Maciel Dias de Andrade, F.; Pinto Pedrosa, R. The role of physical exercise in obstructive sleep apnea. J. Bras. Pneumol. 2016, 42, 457–464. [Google Scholar] [CrossRef]
- Khattak, H.K.; Hayat, F.; Pamboukian, S.V.; Hahn, H.S.; Schwartz, B.P.; Stein, P.K. Obstructive sleep apnea in heart failure: Review of prevalence, treatment with continuous positive airway pressure, and prognosis. Tex. Hear. Inst. J. 2018, 45, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Muraki, I.; Wada, H.; Tanigawa, T. Sleep apnea and type 2 diabetes. J. Diabetes Investig. 2018, 9, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Laratta, C.R.; Ayas, N.T.; Povitz, M.; Pendharkar, S.R. Diagnosis and treatment of obstructive sleep apnea in adults. Can. Med. Assoc. J. 2017, 189, E1481–E1488. [Google Scholar] [CrossRef]
- Gabryelska, A.; Sochal, M.; Wasik, B.; Bialasiewicz, P. Patients with obstructive sleep apnea are over four times more likely to suffer from psoriasis than the general population. J. Clin. Sleep Med. 2018, 14, 153. [Google Scholar] [CrossRef]
- Damianaki, A.; Vagiakis, E.; Sigala, I.; Pataka, A.; Rovina, N.; Vlachou, A.; Krietsepi, V.; Zakynthinos, S.; Katsaounou, P. Τhe Co-Existence of Obstructive Sleep Apnea and Bronchial Asthma: Revelation of a New Asthma Phenotype? J. Clin. Med. 2019, 8, 1476. [Google Scholar] [CrossRef]
- Kuczyński, W.; Gabryelska, A.; Mokros, Ł.; Białasiewicz, P. Obstructive sleep apnea syndrome and hypothyroidism—Merely concurrence or causal association? Adv. Respir. Med. 2016, 84, 302–306. [Google Scholar] [CrossRef]
- Zinchuk, A.V.; Gentry, M.J.; Concato, J.; Yaggi, H.K. Phenotypes in obstructive sleep apnea: A definition, examples and evolution of approaches. Sleep Med. Rev. 2017, 35, 113–123. [Google Scholar] [CrossRef]
- Eckert, D.J.; White, D.P.; Jordan, A.S.; Malhotra, A.; Wellman, A. Defining Phenotypic Causes of Obstructive Sleep Apnea Identification of Novel Therapeutic Targets. Am. J. Respir. Crit. Care Med. 2013, 188, 996–1004. Available online: www.atsjournals.org (accessed on 12 January 2021). [CrossRef]
- Gabryelska, A.; Białasiewicz, P. Association between excessive daytime sleepiness, REM phenotype and severity of obstructive sleep apnea. Sci. Rep. 2020, 10, 1–6. [Google Scholar]
- Pien, G.W.; Ye, L.; Keenan, B.T.; Maislin, G.; Björnsdóttir, E.; Arnardottir, E.S.; Benediktsdottir, B.; Gislason, T.; Pack, A.I. Changing Faces of Obstructive Sleep Apnea: Treatment Effects by Cluster Designation in the Icelandic Sleep Apnea Cohort. Sleep 2018, 41, 1–13. Available online: https://academic.oup.com/sleep/article/41/3/zsx201/4782667 (accessed on 12 January 2021). [CrossRef] [PubMed]
- Sutherland, K.; Takaya, H.; Qian, J.; Petocz, P.; Ng, A.T.; Cistulli, P.A. Oral Appliance Treatment Response and Polysomnographic Phenotypes of Obstructive Sleep Apnea. J. Clin. Sleep Med. 2015, 11, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Schütz, S.G.; Lisabeth, L.D.; Shafie-Khorassani, F.; Case, E.; Sanchez, B.N.; Chervin, R.D.; Brown, D.L. Clinical phenotypes of obstructive sleep apnea after ischemic stroke: A cluster analysis. Sleep Med. 2019, 60, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Sateia, M.J. International classification of sleep disorders-third edition highlights and modifications. Chest 2014, 146, 1387–1394. Available online: https://pubmed.ncbi.nlm.nih.gov/25367475/ (accessed on 12 January 2021). [CrossRef]
- Stammnitz, A.; Jerrentrup, A.; Penzel, T.; Peter, J.H.; Vogelmeier, C.; Becker, H.F. Automatic CPAP titration with different self-setting devices in patients with obstructive sleep apnoea. Eur. Respir. J. 2004, 24, 273–278. Available online: https://erj.ersjournals.com/content/24/2/273 (accessed on 12 January 2021). [CrossRef]
- Meoli, A.L. Positional Therapy: An Easy Suggestion, but Often Not the Right Answer Point-Counterpoint: Is Avoidance of Supine Sleep an Adequate Treatment for OSA? J. Clin. Sleep Med. 2015, 11, 91–92. [Google Scholar] [CrossRef]
- Mokros, L.; Kuczynski, W.; Gabryelska, A.; Franczak, L.; Spalka, J.; Bialasiewicz, P. High negative predictive value of normal body mass index for obstructive sleep apnea in the lateral sleeping position. J. Clin. Sleep Med. 2018, 14, 985–990. [Google Scholar] [CrossRef]
- Clebak, K.T.; Demetriou, T.J.; Carey, S. Positional therapy for obstructive sleep apnea. T. 101, American Family Physician. 2020. Available online: www.cochranelibrary.com (accessed on 12 January 2021).
- Joseph, V.; Nagalli, S. Periodic Limb Movement Disorder. StatPearls. StatPearls Publishing; 2020. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32809562 (accessed on 12 January 2021).
- Mancebo-Sosa, V.; Mancilla-Hernández, V.; Miranda-Ortiz, J.; Hernández-Torres, A.; Gutierrez-Escobar, R.; Poblano, A.; Jiménez-Correa, U. Sleep architecture alterations in patients with periodic limb movements disorder during sleep and sleep breathing disorders. Sleep Sci. 2016, 9, 84–88. [Google Scholar] [CrossRef]
- Martynas, Z.; Vanda, L.; Ramune, G.; Raminta, M.; Osvaldas, R. Arousals and macrostructure of sleep: Importance of NREM stage 2 reconsidered. Sleep Sci. 2013, 6, 91–97. Available online: http://sleepscience.org.br/about-the-authors/14/en-US (accessed on 12 January 2021).
- Kim, J.-W.; Won, T.-B.; Rhee, C.-S.; Park, Y.M.; Yoon, I.-Y.; Cho, S.-W. Polysomnographic phenotyping of obstructive sleep apnea and its implications in mortality in Korea. Sci. Rep. 2020, 10, 1–12. Available online: www.nature.com/scientificreports (accessed on 12 January 2021).
- Zinchuk, A.V.; Jeon, S.; Koo, B.B.; Yan, X.; Bravata, D.M.; Qin, L.; Selim, B.J.; Strohl, K.P.; Redeker, N.S.; Concato, J.; et al. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea. Thorax 2018, 73, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Topîrceanu, A.; Udrescu, L.; Udrescu, M.; Mihaicuta, S. Gender Phenotyping of Patients with Obstructive Sleep Apnea Syndrome Using a Network Science Approach. J. Clin. Med. 2020, 9, 4025. Available online: www.mdpi.com/journal/jcm (accessed on 12 January 2021). [CrossRef]
- Vavougios, G.D.; Natsios, G.; Pastaka, C.; Zarogiannis, S.G.; Gourgoulianis, K.I. Phenotypes of comorbidity in OSAS patients: Combining categorical principal component analysis with cluster analysis. J. Sleep Res. 2016, 25, 31–38. [Google Scholar] [CrossRef]
- Bailly, S.; Destors, M.; Grillet, Y.; Richard, P.; Stach, B.; Vivodtzev, I.; Timsit, J.-F.; Lévy, P.; Tamisier, R.; Pépin, J.-L.; et al. Obstructive sleep apnea: A cluster analysis at time of diagnosis. PLoS ONE 2016, 11, e0157318. Available online: www.osfp.fr (accessed on 12 January 2021). [CrossRef] [PubMed]
- Mihaicuta, S.; Udrescu, M.; Topirceanu, A.; Udrescu, L. Network science meets respiratory medicine for OSAS phenotyping and severity prediction. PeerJ 2017, 5, e3289. [Google Scholar] [CrossRef][Green Version]

| Data | Mean ± SD or Median (IQR) | Minimum-Maximum |
|---|---|---|
| Age, years | 55.6 ± 10.4 | 30.0–84.0 |
| Sex: | ||
| Male, % | 75.6 | |
| Female, % | 24.4 | |
| Height, cm | 174.3 ± 9.4 | 149.0–198.0 |
| Weight, kg | 118.2 ± 24.3 | 68.0–209.0 |
| BMI, kg/m2 | 39.0 ± 7.8 | 23.5–63.1 |
| Neck circumference, cm | 45.2 ± 5.1 | 31.0–63.0 |
| Waist circumference, cm | 126.3 ± 15.5 | 87.0–178.0 |
| Neck to waist ratio | 0.4 ± 0.1 | 0.3–0.1 |
| Current smoking, % | 24.5 | |
| Systolic BP, mmHg | 136.1 ± 15.3 | 98.0–190.0 |
| Diastolic BP, mmHg | 83.1 ± 9.9 | 60.0–120.0 |
| ESS | 10.4 ± 5.2 | 1.0–24.0 |
| EDS, % | 76.8 | |
| pH | 7.4 ± 0.1 | 7.3–7.5 |
| SaO2, % | 95.5 (93.7–96.6) | 66.0–100.0 |
| PaCO2, mmHg | 42.2 (39.6–44.9) | 27.5–68.5 |
| PaO2, mmHg | 72.5 (66.6–80.7) | 40.9–146.0 |
| HCO3 | 26.4 ± 2.5 | 21.0–36.0 |
| Data | Mean ± SD or Median (IQR) | Minimum-Maximum |
|---|---|---|
| AHI, times/hour | 50.0 ± 22.9 | 9.8–112.2 |
| ARI, times/hour | 66.1 ± 51.6 | 0.5–173.2 |
| Mean SpO2, % | 91.0 (87.6–92.1) | 65.0–96.0 |
| Nadir SpO2, % | 69.0 ± 2.0 | 27.0–90.0 |
| ODI, times/hour | 42.3 ± 24.4 | 0.6–110.9 |
| Snoring percentage, % | 34.4 ± 19.6 | 1.6–87.3 |
| PLMS, times/hour | 28.7 (8.18–67.9) | 0.0–212.8 |
| Sleep efficiency, % | 81.4 (66.7–93.9) | 15.7–99.9 |
| Mean respiratory rate, times/hour | 20.1 ± 4.7 | 11.4–39.6 |
| Supine AHI, times/hour | 56.6 ± 25.3 | 0.0–122.0 |
| Non-supine AHI, times/hour | 41.6 ± 28.0 | 0.0–118.1 |
| Data | Positional OSA | Severe OSA | OSA and PLM | p Value |
|---|---|---|---|---|
| Age, years | 56.1 ± 10.1 | 55.3 ± 9.6 | 56.8 ± 9.3 | 0.784 |
| BMI, kg/m2 | 37.6 ± 8.0 | 42.1 ± 6.7 | 38.3 ± 7.2 | 0.001 |
| Neck circumference, cm | 44.5 ± 5.0 | 47.7 ± 4.4 | 44.1 ± 4.6 | <0.001 |
| Waist circumference, cm | 122.8 ± 14.9 | 133.9 ± 14.2 | 127.5 ± 14.5 | <0.001 |
| Neck-waist ratio | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.102 |
| Current smoking, % | 14.6 | 28.3 | 44.0 | 0.041 |
| Systolic BP, mmHg | 135.3 ± 16.6 | 138.9 ± 12.4 | 130.3 ± 12.4 | 0.039 |
| ESS | 10.2 ± 5.2 | 11.2 ± 4.5 | 8.8 ± 5.2 | 0.197 |
| EDS, % | 71.7 | 88.1 | 72.4 | 0.047 |
| SaO2, % | 95.9 (94.6–97.1) | 95.3 (93.0–96.4) | 95.6 (93.3–96.6) | 0.090 |
| PaCO2, mmHg | 41.4 (39.3–44.0) | 43.6 (40.8–47.6) | 41.8 (38.6–42.5) | 0.010 |
| PaO2, mmHg | 76.0 (68.9–83.7) | 68.9 (64.3–78.4) | 72.2 (65.0–80.4) | 0.024 |
| AHI, times/hour | 37.2 ± 15.9 | 74.0 ± 12.9 | 45.2 ± 21.2 | <0.001 |
| ARI, times/hour | 62.3 ± 52.9 | 63.6 ± 47.2 | 92.3 ± 52.1 | 0.021 |
| Mean SpO2, % | 92.0 (90.5–93.0) | 88.1 (84.8–90.8) | 91.0 (87.3–92.0) | <0.001 |
| Nadir SpO2, % | 73.0 ± 10.0 | 63.1 ± 10.2 | 72.7 ± 8.4 | <0.001 |
| ODI, times/hour | 31.2 ± 17.9 | 63.2 ± 22.6 | 41.1 ± 25.3 | <0.001 |
| Snoring percentage, % | 35.0 ± 20.9 | 26.4 ± 15.0 | 42.4 ± 17.9 | 0.007 |
| PLMS, times/hour | 16.5 (5.5–46.6) | 34.4 (13.9–52.2) | 124.9 (105.2–164.1) | <0.001 |
| Sleep efficiency, % | 81.2 (66.0–94.2) | 80.4 (63.9–92.7) | 90.2 (68.5–97.8) | 0.407 |
| Supine AHI, times/hour | 47.9 ± 21.9 | 76.4 ± 19.6 | 47.5 ± 26.9 | <0.001 |
| Non supine AHI, times/hour | 24.8 ± 17.2 | 71.4 ± 17.2 | 39.4 ± 26.1 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charčiūnaitė, K.; Gauronskaitė, R.; Šlekytė, G.; Danila, E.; Zablockis, R. Evaluation of Obstructive Sleep Apnea Phenotypes Treatment Effectiveness. Medicina 2021, 57, 335. https://doi.org/10.3390/medicina57040335
Charčiūnaitė K, Gauronskaitė R, Šlekytė G, Danila E, Zablockis R. Evaluation of Obstructive Sleep Apnea Phenotypes Treatment Effectiveness. Medicina. 2021; 57(4):335. https://doi.org/10.3390/medicina57040335
Chicago/Turabian StyleCharčiūnaitė, Karolina, Rasa Gauronskaitė, Goda Šlekytė, Edvardas Danila, and Rolandas Zablockis. 2021. "Evaluation of Obstructive Sleep Apnea Phenotypes Treatment Effectiveness" Medicina 57, no. 4: 335. https://doi.org/10.3390/medicina57040335
APA StyleCharčiūnaitė, K., Gauronskaitė, R., Šlekytė, G., Danila, E., & Zablockis, R. (2021). Evaluation of Obstructive Sleep Apnea Phenotypes Treatment Effectiveness. Medicina, 57(4), 335. https://doi.org/10.3390/medicina57040335




