Clinical Impact of Supraclavicular Lymph Node Involvement of Stage IIIC Non-Small Cell Lung Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment and Evaluation
2.2. Statistical Analysis
2.3. Radiotherapy Procedure
3. Results
3.1. Patient Characteristics
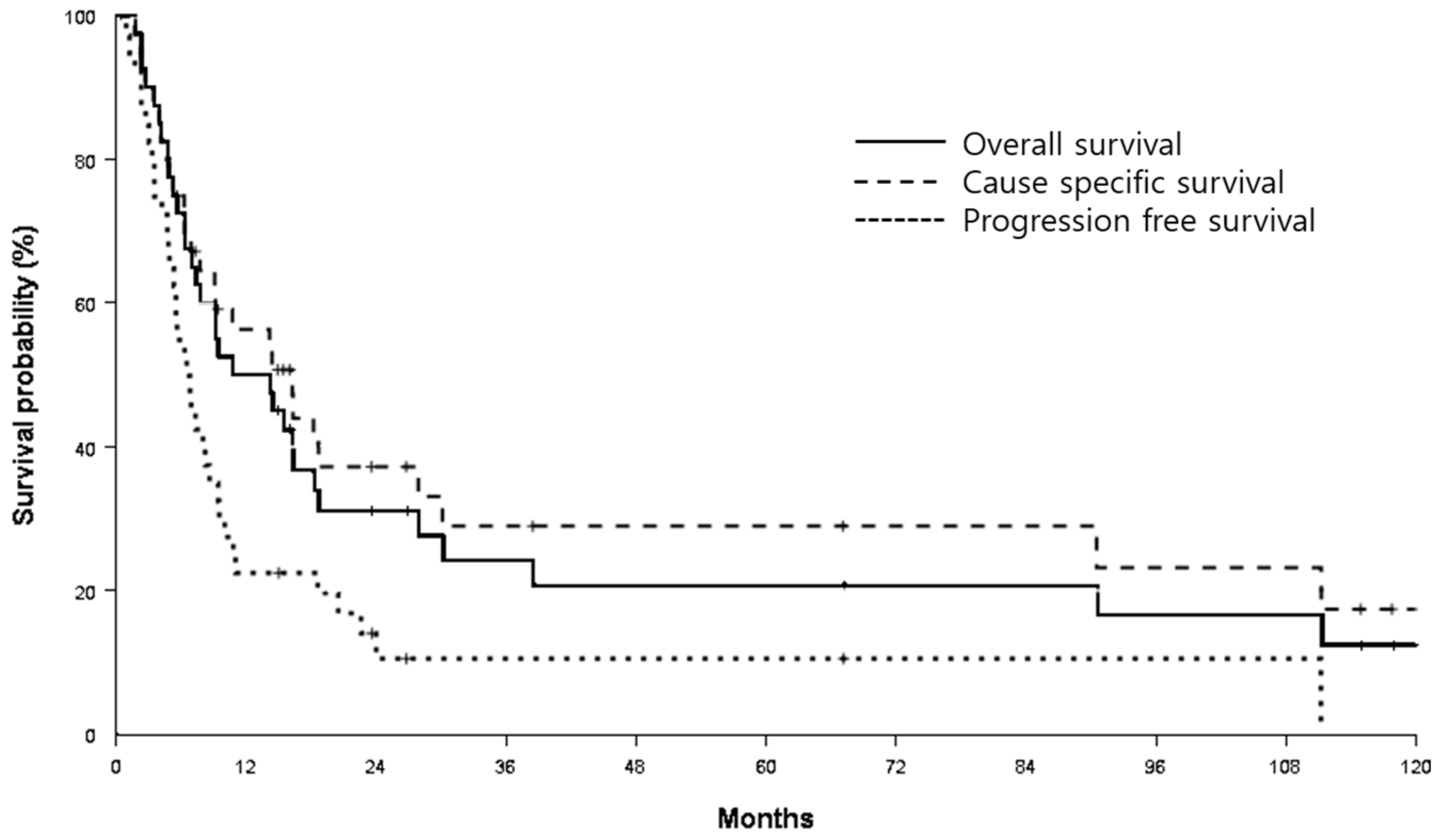
3.2. Survival and Local Control Rates
3.3. Treatment-Related Toxicities
3.4. Patterns of Failure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NCCN. NCCN Guidelines Version 2.2021. Non-Small Cell Lung Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 6 January 2021).
- Ramnath, N.; Dilling, T.J.; Harris, L.J.; Kim, A.W.; Michaud, G.C.; Balekian, A.A.; Diekemper, R.; Detterbeck, F.C.; Arenberg, D.A. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. 5), e314S–e340S. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, F.A.; Crowley, J.; Van Houtte, P.; Postmus, P.E.; Carney, D.; Chansky, K.; Shaikh, Z.; Goldstraw, P. The International Association for the Study of Lung Cancer lung cancer staging project: Proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J. Thorac. Oncol. 2007, 2, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.M.; Kim, J.M.; Ahn, Y.C.; Pyo, H.; Kim, B.; Oh, D.; Ju, S.G.; Shin, J.S.; Hong, C.-S.; Park, H.; et al. Effect of Radiation Therapy Techniques on Outcome in N3-positive IIIB Non-small Cell Lung Cancer Treated with Concurrent Chemoradiotherapy. Cancer Res. Treat. 2016, 48, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Healy, B.; Pantarotto, J.; Laurie, S.A.; Macrae, R.; Sabri, E.; Wheatley-Price, P. Prognostic factors in the radical nonsurgical treatment of stage IIIB non–small-cell lung cancer. Clin. Lung Cancer 2014, 15, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Asamura, H.; Chansky, K.; Crowley, J.; Goldstraw, P.; Rusch, V.W.; Vansteenkiste, J.F.; Watanabe, H.; Wu, Y.L.; Zielinski, M.; Ball, D.; et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2015, 10, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Aupérin, A.; Le Péchoux, C.; Rolland, E.; Curran, W.J.; Furuse, K.; Fournel, P.; Belderbos, J.; Clamon, G.; Ulutin, H.C.; Paulus, R.; et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J. Clin. Oncol. 2010, 28, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.; Choy, H.; Bradley, J.; Rosenzweig, K.E.; Bogart, J.; Curran, W.J., Jr.; Gore, E.; Langer, C.; Louie, A.V.; Lutz, S.; et al. Definitive radiation therapy in locally advanced non-small cell lung cancer: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based clinical practice guideline. Pract. Radiat. Oncol. 2015, 5, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.D.; Ieumwananonthachai, N.; Purdy, J.A.; Wasserman, T.H.; Lockett, M.A.; Graham, M.V.; Perez, C.A. Gross tumor volume, critical prognostic factor in patients treated with three-dimensional conformal radiation therapy for non-small-cell lung carcinoma. Int. J. Radiat. Oncol. 2002, 52, 49–57. [Google Scholar] [CrossRef]
- Wiersma, T.G.; Dahele, M.; Verbakel, W.F.; Van De Ven, P.M.; De Haan, P.F.; Smit, E.F.; Van Reij, E.J.; Slotman, B.J.; Senan, S. Concurrent chemoradiotherapy for large-volume locally-advanced non-small cell lung cancer. Lung Cancer 2013, 80, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Machtay, M.; Seiferheld, W.; Komaki, R.; Cox, J.D.; Sause, W.T.; Byhardt, R.W. Is prolonged survival possible for patients with supraclavicular node metastases in non-small cell lung cancer treated with chemoradiotherapy?: Analysis of the Radiation Therapy Oncology Group experience. Int. J. Radiat. Oncol. 1999, 44, 847–853. [Google Scholar] [CrossRef]
- Oh, D.; Ahn, Y.C.; Park, H.C.; Lim, D.H.; Noh, J.M.; Cho, W.K.; Pyo, H. The prognostic impact of supraclavicular lymph node in N3-IIIB stage non-small cell lung cancer patients treated with definitive concurrent chemo-radiotherapy. Oncotarget 2017, 8, 35700–35706. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Lin, D.Y. Cox regression analysis of multivariate failure time data: The marginal approach. Stat. Med. 1994, 13, 2233–2247. [Google Scholar] [CrossRef] [PubMed]
- Thompson, F.T.; Levine, D.U. Examples of easily explainable suppressor variables in multiple regression research. Mult. Linear Regres. Viewp. 1997, 24, 11–13. [Google Scholar]
- Topkan, E.; Ozdemir, Y.; Guler, O.C.; Kucuk, A.; Besen, A.A.; Mertsoylu, H.; Sezen, D.; Akdemir, E.Y.; Sezer, A.; Bolukbasi, Y.; et al. Comparison of Involved Field Radiotherapy versus Elective Nodal Irradiation in Stage IIIB/C Non-Small-Cell Lung Carcinoma Patients Treated with Concurrent Chemoradiotherapy: A Propensity Score Matching Study. J. Oncol. 2020, 2020, 7083149. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Men, Y.; Feng, L.; Kang, J.; Sun, X.; Yuan, M.; Jiang, W.; Hui, Z. A current review of dose-escalated radiotherapy in locally advanced non-small cell lung cancer. Radiol. Oncol. 2019, 53, 6–14. [Google Scholar] [CrossRef]
- Brotons, M.L.; Bolca, C.; Fréchette, É.; Deslauriers, J. Anatomy and physiology of the thoracic lymphatic system. Thorac. Surg. Clin. 2012, 22, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Chesney, P.J. Lymphatic system and generalized lymphadenopathy. In Principles and Practice of Pediatric Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2012; pp. 127–135.e1. [Google Scholar]
- Mizutani, M.; Nawata, S.; Hirai, I.; Murakami, G.; Kimura, W. Anatomy and histology of Virchow’s node. Anat. Sci. Int. 2005, 80, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Faivre-Finn, C.; Vicente, D.; Kurata, T.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.; Spigel, D.; Garassino, M.; Reck, M.; Senan, S.; et al. LBA49 Durvalumab after chemoradiotherapy in stage III NSCLC: 4-year survival update from the phase III PACIFIC trial. Ann. Oncol. 2020, 31, S1178–S1179. [Google Scholar] [CrossRef]
| Variables | No. of Patients (%) (Total = 40) |
|---|---|
| Sex | |
| Male | 32 (80.0%) |
| Female | 8 (20.0%) |
| Age (years) | |
| Median (range) | 67.5 (44–89) |
| ECOG PS | |
| 0 or 1 | 35 (87.5%) |
| 2 or higher | 5 (12.5%) |
| Smoking history | |
| Non-smoker | 7 (17.5%) |
| Smoker (including ex-smoker) | 33 (82.5%) |
| Current smoking | |
| Non-current | 28 (70.0%) |
| Current smoker | 12 (30.0%) |
| Histology | |
| Squamous cell carcinoma | 26 (65%) |
| Adenocarcinoma | 8 (20.0%) |
| Not specified | 6 (15%) |
| T stage | |
| T1 | 3 (7.5%) |
| T2 | 14 (35.0%) |
| T3 | 8 (20.0%) |
| T4 | 15 (37.5%) |
| Nodal status | |
| Supraclavicular lymph node | 17 (42.5%) |
| Contralateral lymph node | 23 (57.5%) |
| RT modality | |
| 3DCRT | 21 (52.5%) |
| IMRT | 19 (47.5%) |
| RT dose in EQD2 | |
| 3DCRT | 60.6 (±6.0) Gy |
| IMRT | 64.2 (±7.9) Gy, |
| Clinical target volume (cm3) | |
| Median (range) | 292.0 (77.0–1015.0) |
| n | Median OS (95% CI) | p Value | Median CSS (95% CI) | p Value | Median PFS (95% CI) | p Value | 1-Year LC% (±SE) | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| ECOG PS | |||||||||
| 0 or 1 | 35 | 14.4 (6.5–22.3) | 16.4 (10.7–22.1) | 6.8 (4.8–8.8) | 56.8% (±9.7) | ||||
| 2 or higher | 5 | 9.1 (0–19.8) | 0.213 | 9.1 (0–19.8) | 0.117 | 4.8 (0.7–3.5) | 0.274 | 0% | 0.813 |
| Age, years | |||||||||
| <60 | 9 | 27.9 (14.1–41.8) | 27.9 (14.1–41.8) | 11.0 (2.2–19.8) | 55.6% (±16.6) | ||||
| ≥60 | 31 | 9.1 (6.4–11.9) | 0.107 | 10.8 (2.9–18.6) | 0.277 | 6.3 (4.2–8.4) | 0.043 | 53.4% (±11.5) | 0.635 |
| Sex | |||||||||
| Male | 32 | 9.4 (2.4–14.5) | 14.4 (3.2–25.6) | 7.3 (4.7–9.9) | 55.2% (±11.1) | ||||
| Female | 8 | 15.5 (6.7–24.2) | 0.776 | 16.3 (2.4–30.2) | 0.801 | 5.3 (2.3–8.3) | 0.151 | 45.0% (±18.8) | 0.376 |
| Smoking status | |||||||||
| Non-smoker | 7 | 16.3 (6.9–25.7) | 16.3 (1.7–30.9) | 5.5 (5.0–6.0) | 51.4% (±20.4) | ||||
| Smoker | 33 | 9.4 (2.2–16.7) | 0.997 | 14.2 (2.9–25.5) | 0.94 | 7.3 (4.4–10.2) | 0.339 | 53.5% (±10.9) | 0.809 |
| Histology | |||||||||
| Squamous | 26 | 9.1 (4.3–13.9) | 10.8 (0–23.8) | 5.6 (3.2–8.0) | 63.6% (±11.7) | ||||
| Others | 14 | 14.4 (12.3–16.6) | 0.91 | 18.7 (9.1–28.4) | 0.959 | 6.9 (5.4–8.4) | 0.809 | 38.6% (±14.8) | 0.399 |
| T stage | |||||||||
| 1 or 2 | 17 | 14.2 (2.7–25.7) | 16.3 (12.3–2.3) | 7.3 (3.3–11.3) | 70.7% (±12.6) | ||||
| 3 or 4 | 23 | 9.4 (1.1–17.7) | 0.937 | 14.4 (2.6–26.3) | 0.715 | 6.3 (4.4–8.2) | 0.359 | 39.8% (±12.8) | 0.672 |
| SCN involvement | |||||||||
| No | 23 | 16.4 (10.0–22.7) | 18.7 (3.2–34.3) | 8.6 (6.4–10.8) | 58.2% (±12.4) | ||||
| Yes | 17 | 7.8 (4.0–11.5) | 0.236 | 7.8 (4.0–11.5) | 0.146 | 4.9 (2.5–7.3) | 0.084 | 47.0% (±14.4) | 0.189 |
| RT modality | |||||||||
| 3DCRT | 21 | 14.2 (1.3–27.1) | 18.3 (10.2–26.4) | 8.0 (5.9–10.1) | 45.9% (±12.8) | ||||
| IMRT | 19 | 10.8 (3.2–18.3) | 0.617 | 14.4 (6.4–22.4) | 0.604 | 5.6 (4.5–6.7) | 0.8 | 62.6% (±13.6) | 0.1 |
| HR (95% CI) | p Value | |
|---|---|---|
| OS | ||
| Age | 2.06 (0.84–5.03) | 0.114 |
| CSS | ||
| ECOG PS | 2.47 (0.91–6.74) | 0.077 |
| SCN involvement | 1.97 (0.88–4.40) | 0.099 |
| PFS | ||
| Age | 2.79 (1.12–6.96) | 0.028 |
| SCN involvement | 2.08 (1.04–4.17) | 0.039 |
| Local control rates | ||
| SCN involvement | 3.05 (1.09–8.50) | 0.034 |
| IMRT use | 0.28 (0.09–0.86) | 0.027 |
| Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|
| Pneumonitis | 15.0% | 7.5% | 7.5% |
| Esophagitis | 50.0% | 7.5% | 0.0% |
| Hemoptysis | 7.5% | 0.0% | 0.0% |
| No Complication (n = 34) | Grade ≥3 Pneumonitis (n = 6) | p Value * | |
|---|---|---|---|
| EQD2 (continuous) | 61.7 ± 7.4 | 66.6 ± 0.9 | 0.001 |
| EQD2 | 0.056 | ||
| <63 Gy | 19 (55.9%) | 1 (16.6%) | |
| ≥63 Gy | 15 (44.1%) | 5 (83.3%) | |
| Radiation therapy modality | 0.042 | ||
| 3DCRT | 20 (58.8%) | 1 (16.7%) | |
| IMRT | 14 (41.2%) | 5 (83.3%) | |
| Volume of CTV | 349.2 ± 226.1 | 531.6 ± 337.0 | 0.2 |
| Mean lung dose | 1436.7 ± 527.7 | 1439.1 ± 618.6 | 0.994 |
| V20 | 28.4 ± 15.0 | 17.2 ± 2.9 | 0.208 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Yoon, W.S.; Jang, M.H.; Rim, C.H. Clinical Impact of Supraclavicular Lymph Node Involvement of Stage IIIC Non-Small Cell Lung Cancer Patients. Medicina 2021, 57, 301. https://doi.org/10.3390/medicina57030301
Park S, Yoon WS, Jang MH, Rim CH. Clinical Impact of Supraclavicular Lymph Node Involvement of Stage IIIC Non-Small Cell Lung Cancer Patients. Medicina. 2021; 57(3):301. https://doi.org/10.3390/medicina57030301
Chicago/Turabian StylePark, Sunmin, Won Sup Yoon, Mi Hee Jang, and Chai Hong Rim. 2021. "Clinical Impact of Supraclavicular Lymph Node Involvement of Stage IIIC Non-Small Cell Lung Cancer Patients" Medicina 57, no. 3: 301. https://doi.org/10.3390/medicina57030301
APA StylePark, S., Yoon, W. S., Jang, M. H., & Rim, C. H. (2021). Clinical Impact of Supraclavicular Lymph Node Involvement of Stage IIIC Non-Small Cell Lung Cancer Patients. Medicina, 57(3), 301. https://doi.org/10.3390/medicina57030301








