Updating Perspectives on Meta-Analyses in the Field of Radiation Oncology
Abstract
1. Introduction
2. Utility of Meta-Analyses in the Field of Radiation Oncology
3. Heterogeneity and the Effects Model
4. Meta-Analyses of Observational Studies
5. Direction for Future Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berlin, J.A.; Golub, R.M. Meta-analysis as evidence: Building a better pyramid. JAMA 2014, 312, 603–606. [Google Scholar] [CrossRef]
- Abubakar, I.; Tillmann, T.; Banerjee, A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Shin, H.-Y.; Lee, J.-Y.; Song, J.; Lee, S.; Lee, J.; Lim, B.; Kim, H.; Lee, Y.-S. Cause-of-death statistics in the Republic of Korea, 2014. J. Korean Med. Assoc. 2016, 59, 221–232. [Google Scholar] [CrossRef]
- Cheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jamel, A.; He, J. Cancer statistics in China 2015. CA A Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol. Biomarkers Prev. 2015, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Benzies, K.M.; Premji, S.; Hayden, K.A.; Serrett, K. State-of-the-Evidence Reviews: Advantages and Challenges of Including Grey Literature. Worldviews Evid. Based Nurs. 2006, 3, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Mahood, Q.; Van Eerd, D.; Irvin, E. Searching for grey literature for systematic reviews: Challenges and benefits. Res. Synth. Methods 2014, 5, 221–234. [Google Scholar] [CrossRef]
- Landoni, F.; Maneo, A.; Colombo, A.; Placa, F.; Milani, R.; Perego, P.; Favini, G.; Ferri, L.; Mangioni, C. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997, 350, 535–540. [Google Scholar] [CrossRef]
- Nigro, N.D.; Vaitkevicius, V.; Considine, B. Combined therapy for cancer of the anal canal: A preliminary report. Dis. Colon Rectum 1974, 17, 354–356. [Google Scholar] [CrossRef]
- Cummings, B.; Keane, T.; O’Sullivan, B.; Wong, C.; Catton, C. Epidermoid anal cancer: Treatment by radiation alone or by radiation and 5-fluorouracil with and without mitomycin C. Int. J. Radiat. Oncol. 1991, 21, 1115–1125. [Google Scholar] [CrossRef]
- Fraunholz, I.; Rabeneck, D.; Weis, C.; Rödel, C. Combined-Modality Treatment for Anal Cancer. Strahlenther. Onkol. 2010, 186, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Park, H.S.; An, Y.; Yarbrough, W.G.; Contessa, J.N.; Decker, R.; Mehra, S.; Judson, B.L.; Burtness, B.; Husain, Z.A. Upfront surgery versus definitive chemoradiotherapy in patients with human Papillomavirus-associated oropharyngeal squamous cell cancer. Oral Oncol. 2018, 79, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Warner, L.; Chudasama, J.; Kelly, C.G.; Loughran, S.; McKenzie, K.; Wight, R.; Dey, P. Radiotherapy versus open surgery versus endolaryngeal surgery (with or without laser) for early laryngeal squamous cell cancer. Cochrane Database Syst. Rev. 2014, 2014, CD002027. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Treutwein, M.; Hipp, M.; Koelbl, O.; Bogner, L. IMRT of prostate cancer. Strahlenther. Onkol. 2009, 185, 379–383. [Google Scholar] [CrossRef][Green Version]
- Cao, C.; D’Amico, T.; Demmy, T.; Dunning, J.; Gossot, D.; Hansen, H.; He, J.; Jheon, S.; Petersen, R.H.; Sihoe, A.; et al. Surgery versus SABR for resectable non-small-cell lung cancer. Lancet Oncol. 2015, 16, e370–e371. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; Ch 20. In Meta-Regression; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Rim, C.H.; Kim, C.Y.; Yang, D.S.; Yoon, W.S. Comparison of radiation therapy modalities for hepatocellular carcinoma with portal vein thrombosis: A meta-analysis and systematic review. Radiother. Oncol. 2018, 129, 112–122. [Google Scholar] [CrossRef]
- Rim, C.H.; Kim, C.Y.; Yang, D.S.; Yoon, W.S. External beam radiation therapy to hepatocellular carcinoma involving inferior vena cava and/or right atrium: A meta-analysis and systemic review. Radiother. Oncol. 2018, 129, 123–129. [Google Scholar] [CrossRef]
- Rim, C.H.; Kim, H.J.; Seong, J. Clinical feasibility and efficacy of stereotactic body radiotherapy for hepatocellular carcinoma: A systematic review and meta-analysis of observational studies. Radiother. Oncol. 2019, 131, 135–144. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Rim, C.H.; Kim, Y.; Kim, C.Y.; Yoon, W.S.; Yang, D.S. Is stereotactic body radiotherapy for ultra-central lung tumor a feasible option? A systemic review and meta-analysis. Int. J. Radiat. Biol. 2019, 95, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Lovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Page, M.J.; Shamseer, L.; Altman, D.G.; Tetzlaff, J.; Sampson, M.; Tricco, A.C.; Catalá-López, F.; Li, L.; Reid, E.K.; Sarkis-Onofre, R.; et al. Epidemiology and Reporting Characteristics of Systematic Reviews of Biomedical Research: A Cross-Sectional Study. PLoS Med. 2016, 13, e1002028. [Google Scholar] [CrossRef]
- Hasan, H.; Muhammed, T.; Yu, J.S.; Taguchi, K.; Samargandi, O.A.; Howard, A.F.; Lo, A.C.; Olson, R.; Goddard, K. Assessing the methodological quality of systematic reviews in radiation oncology: A systematic review. Cancer Epidemiol. 2017, 50, 141–149. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Identifying and Quantifying Heterogeneity. In Introduction to Meta-Analysis; Wiley: Hoboken, NJ, USA, 2009; pp. 107–125. [Google Scholar]
- Liver, E.A. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar]
- Petereit, D.G.; Coleman, C.N. Global Challenges in radiation oncology. Front. Oncol. 2015, 5, 103. [Google Scholar] [CrossRef]
- Iagsi, R.; Sheets, N.; Jankovic, A.; Motomura, A.R.; Amarnath, S.; Ubel, P.A. Frequency, nature, effects, and correlates of conflicts of interest in published clinical cancer research. Cancer 2009, 115, 2783–2791. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis. Chapter 40. In When Does It Make Sense to Perform a Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis. Chapter 43. In Criticisms of Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Cochran, W.G. The Combination of Estimates from Different Experiments. Biometrics 1954, 10, 101. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Fixed-Effect Versus Random-Effects Models; Wiley: Hoboken, NJ, USA, 2009; pp. 77–86. [Google Scholar]
- Reeves, B. Chapter 24: Including Non-Randomized Studies on Intervention Effects. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Chichester, UK, 2019; pp. 595–620. [Google Scholar]
- Frieden, T.R. Evidence for Health Decision Making—Beyond Randomized, Controlled Trials. N. Engl. J. Med. 2017, 377, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B.; et al. Meta-analysis of Observational Studies in EpidemiologyA Proposal for Reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, C.Y.; Koom, W.S.; Rim, C.H. Practical effectiveness of re-irradiation with or without surgery for locoregional recurrence of rectal cancer: A meta-analysis and systematic review. Radiother. Oncol. 2019, 140, 10–19. [Google Scholar] [CrossRef]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.-R.; Villanueva, M.-T.; Witzigmann, H.; et al. Preoperative Versus Postoperative Chemoradiotherapy for Locally Advanced Rectal Cancer: Results of the German CAO/ARO/AIO-94 Randomized Phase III Trial After a Median Follow-Up of 11 Years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-K.; Bhosale, P.R.; Crane, C.H.; Iyer, R.B.; Skibber, J.M.; Rodriguez-Bigas, M.A.; Feig, B.W.; Chang, G.J.; Eng, C.; Wolff, R.A.; et al. Patterns of Locoregional Recurrence After Surgery and Radiotherapy or Chemoradiation for Rectal Cancer. Int. J. Radiat. Oncol. 2008, 71, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Concato, J.; Shah, N.; Horwitz, R.I. Randomized, Controlled Trials, Observational Studies, and the Hierarchy of Research Designs. N. Engl. J. Med. 2000, 342, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- MacLehose, R. A Systematic Review of Comparisons of Effect Sizes Derived from Randomised and Non-Randomised Studies; Health Technology Assessment: Winchester, UK, 2000; Volume 4, pp. 1–154. [Google Scholar]
- Wells, G. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non Randomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 October 2020).
- Light, R.J.; Pillemer, D.B. Summing up: The Science of Reviewing Research; Harvard University Press: Cambridge, MA, USA, 1984. [Google Scholar]
- Hedges, L.V.; Olkin, I. Statistical Methods for Meta-Analysis; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Shea, B.J.; Grimshaw, J.M.; Wells, G.; Boers, M.; Andersson, N.; Hamel, C.; Porter, A.C.; Tugwell, P.; Moher, D.; Bouter, L.M. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 2007, 7, 10. [Google Scholar] [CrossRef]
- Shin, I.-S. Recent Research Trends in Meta-analysis. Asian Nurs. Res. 2017, 11, 79–83. [Google Scholar] [CrossRef]
- Lane, P.; Higgins, J.P.T.; Anagnostelis, B.; Anzures-Cabrera, J.; Baker, N.F.; Cappelleri, J.C.; Haughie, S.; Hollis, S.; Lewis, S.C.; Moneuse, P.; et al. Methodological quality of meta-analyses: Matched-pairs comparison over time and between industry-sponsored and academic-sponsored reports. Res. Synth. Methods 2013, 4, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Orwin, R.G. A fail-safe N for effect size in meta-analysis. J. Educ. Stat. 1983, 8, 157–159. [Google Scholar] [CrossRef]
- Easterbrook, P.; Gopalan, R.; Berlin, J.; Matthews, D. Publication bias in clinical research. Lancet 1991, 337, 867–872. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; Ch 19. In Subgroup Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
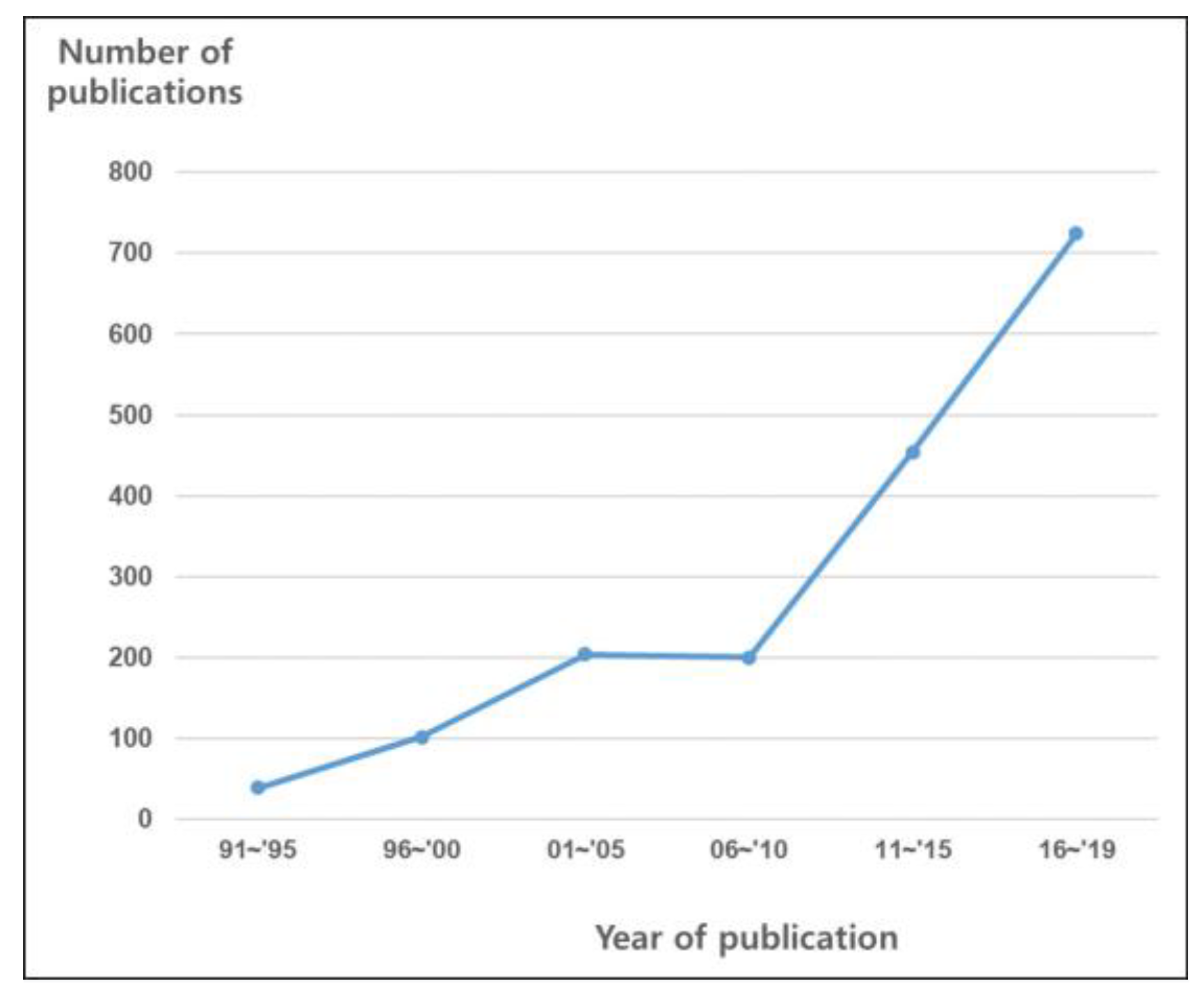

| Conventional Viewpoints | New Viewpoints for Radiation Oncology | |
|---|---|---|
| Utility of meta-analysis | To build the highest level of evidence and more robust conclusions | To provide practical information serving clinical decisions for intractable diseases |
| Issues of heterogeneity | Avoid if possible, to draw firm and undisputed conclusions | Heterogeneity is inevitable and reflects real-life clinical situations Dispersion of effects sizes are subjects of clinical discussion |
| Analysis of observational studies | Prefer to analyze only RCTs, reducing biases and heterogeneity among studies | Important to fill the gaps between RCT results and clinical decisions Efforts to select high-quality studies and reduce biases are encouraged |
| Problems Identified | Future Directions |
|---|---|
| Low methodological quality (low score without formal meta-analysis) | Conducting a formal meta-analysis despite heterogeneities Interpret heterogeneities based on a combination of clinical expertise and statistical methods (e.g., subgroup comparison or meta-regression, sensitivity analysis) rather than avoid such interpretations and limit discussion to narrative descriptions |
| Scarce information of CoI (vast majority of meta-analyses did not document CoIs in the included studies) | Document CoI of studies included in meta-analyses and discuss as relevant, because academic studies might have better qualities than studies with industrial CoI (majority of radiation oncology studies have a merit of being free from CoI) |
| Lack of consideration for study inclusion according to publication status or publication bias | Inclusion of unpublished materials could not be uniformly suggested Thorough discussions by clinical and statistical experts are essential, including the addressing of methods to reduce possible biases |
| Category | Concept | Definition | Common Usages or Interpretation |
|---|---|---|---|
| Effects models for pooled analyses | Fixed effects model | A model based on the assumption that all studies in the analysis are functionally identical and that there is a common effect size | For RCTs with very similar design; repetitive lab study samples |
| Random effects model | A model assumes that the true effect size varies among studies and is used to estimate the mean distribution of the effects | For studies from different institutions, meta-analysis including observational studies | |
| Heterogeneity analysis | Cochran’s Q test | The test of null hypothesis Q that all studies have a common effect size | Commonly interpreted in practice as, that to reject Q if p-value < 0.1; I2 interpretation (Higgins et al. [57].): 25%, 50%, and 75% denote borderlines of low, moderate, and high heterogeneities. However, the values should not be interpreted only in a categorical way but also clinically and quantitatively. |
| I2 value (%) | A concept reflecting the proportion of variance between studies divided by total variance | ||
| Analysis of heterogeneity | Subgroup analysis | Comparison among included study subgroups categorized by its characteristics, regarding effect sizes | z-test (same logic as t-test between two groups); analysis of variance (Q test to partition the variance and test the proportion of between-subgroups variance divided by within-studies variance) |
| Meta-regression | Quantitative regression analysis using effect size as dependent variable and moderator of included studies’ characteristics as independent variable (Similar to regression analysis of primary studies) | Useful to identify dose–response relationship | |
| Sensitivity analysis | Analysis of whether the findings robust to the decision made in the process of obtaining them; for example, analysis with outliers and analysis without outliers | Robustness of clinical and methodological decision making (e.g., analysis with only RCT among included studies vs. analysis with RCT + observational studies) | |
| Publication bias | Publication bias | The bias whereby statistically significant results are more likely to be published than null or non-significant results | |
| Funnel plot | A scatterplot of the effect estimates from studies included against some measure of size or precision of each study (powerful studies locate toward top of the plot shaped as a reversed funnel, while smaller studies scatter more widely at the bottom) | Visually inspected asymmetry suggests possible publication bias. Quantitative statistical methods, such as Egger’s test, yield p-value, which is more familiar to clinicians. Trim and fill methods can estimate adjusted effect size considering publication biases from missing studies. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, I.-S.; Rim, C.H. Updating Perspectives on Meta-Analyses in the Field of Radiation Oncology. Medicina 2021, 57, 117. https://doi.org/10.3390/medicina57020117
Shin I-S, Rim CH. Updating Perspectives on Meta-Analyses in the Field of Radiation Oncology. Medicina. 2021; 57(2):117. https://doi.org/10.3390/medicina57020117
Chicago/Turabian StyleShin, In-Soo, and Chai Hong Rim. 2021. "Updating Perspectives on Meta-Analyses in the Field of Radiation Oncology" Medicina 57, no. 2: 117. https://doi.org/10.3390/medicina57020117
APA StyleShin, I.-S., & Rim, C. H. (2021). Updating Perspectives on Meta-Analyses in the Field of Radiation Oncology. Medicina, 57(2), 117. https://doi.org/10.3390/medicina57020117







