Diagnostic Workflow in Competitive Athletes with Ventricular Arrhythmias and Suspected Concealed Cardiomyopathies
Abstract
1. Introduction
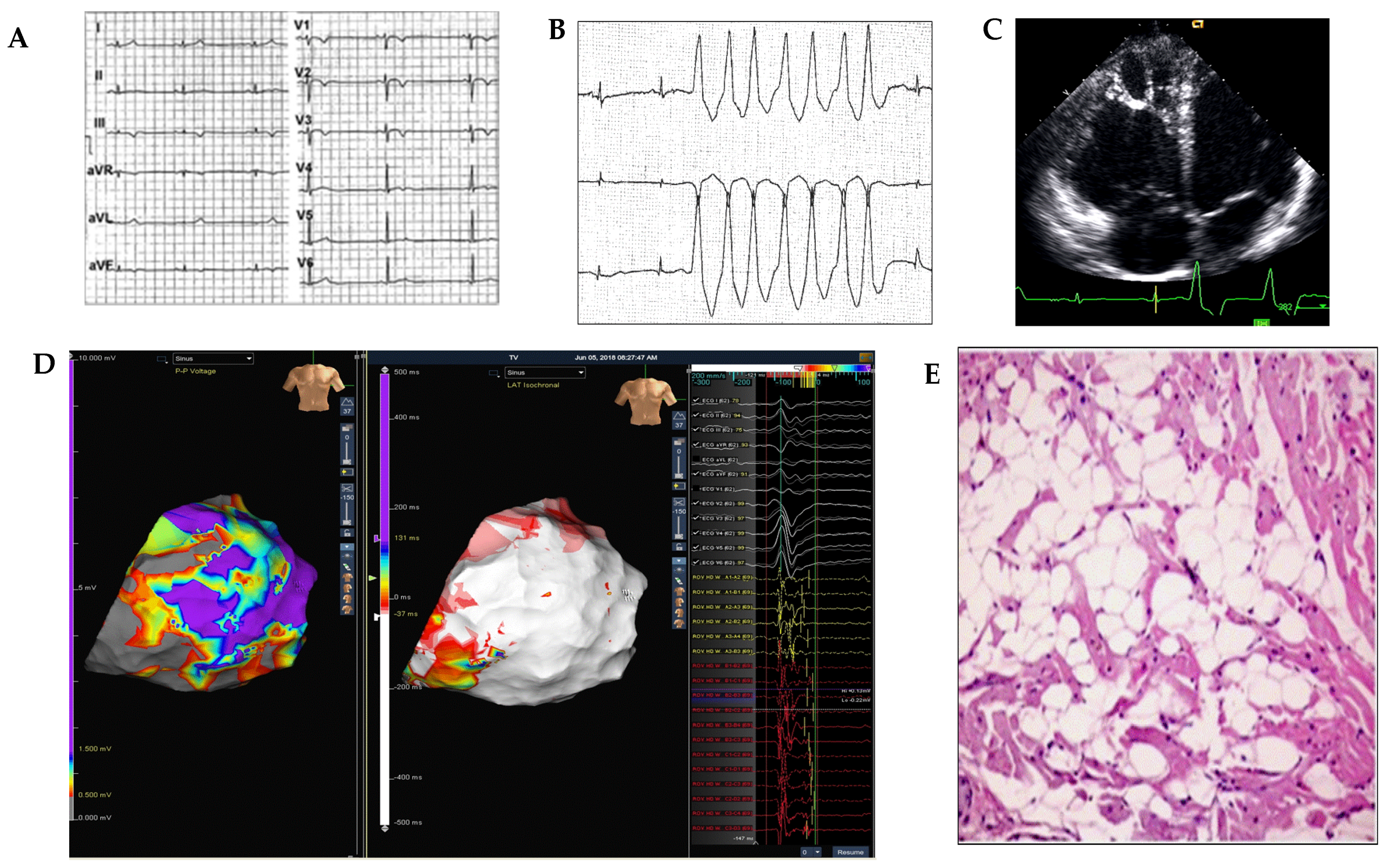
2. First Level Cardiological Tools (Baseline ECG, Holter ECG, Stress Test, Transthoracic Echocardiography)
3. Second-Level Diagnostic Tools (Cardiac MRI, Electroanatomic Mapping, Endomyocardial Biopsy)
4. Third-Level Diagnostic Tools (Genetic Screening)
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basso, C.; Rizzo, S.; Carturan, E.; Pilichou, K.; Thiene, G. Cardiac arrest at rest and during sport activity: Causes and prevention. Eur. Heart J. Suppl. 2020, 22, E20–E24. [Google Scholar] [CrossRef]
- Peterson, D.F.; Kucera, K.; Thomas, L.C.; Maleszewski, J.; Siebert, D.; Lopez-Anderson, M.; Zigman, M.; Schattenkerk, J.; Harmon, K.G.; Drezner, J.A. Aetiology and incidence of sudden cardiac arrest and death in young competitive athletes in the USA: A 4-year prospective study. Br. J. Sports Med. 2020. [Google Scholar] [CrossRef]
- Jouven, X.; Bougouin, W.; Narayanan, K.; Marijon, E. Sudden cardiac death and sports. Eur. Heart J. 2017, 38, 232–234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corrado, D.; Basso, C.; Rizzoli, G.; Schiavon, M.; Thiene, G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J. Am. Coll. Cardiol. 2003, 42, 1959–1963. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, G.; Papadakis, M.; Robertus, J.L.; Dhutia, H.; Steriotis, A.K.; Tome, M.; Mellor, G.; Merghani, A.; Malhotra, A.; Behr, E.; et al. Etiology of Sudden Death in Sports: Insights From a United Kingdom Regional Registry. J. Am. Coll. Cardiol. 2016, 67, 2108–2115. [Google Scholar] [CrossRef]
- MacLachlan, H.; Drezner, J.A. Cardiac evaluation of young athletes: Time for a risk-based approach? Clin. Cardiol. 2020, 43, 906–914. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gat, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease: The Task Force on sports cardiology and exercise in patients with cardiovascular disease of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef] [PubMed]
- Vessella, T.; Zorzi, A.; Merlo, L.; Pegoraro, C.; Giorgiano, F.; Trevisanato, M.; Viel, M.; Formentini, P.; Corrado, D.; Sarto, P. The Italian preparticipation evaluation programme: Diagnostic yield, rate of disqualification and cost analysis. Br. J. Sports Med. 2019, 54, 231–237. [Google Scholar] [CrossRef]
- Corrado, D.; Drezner, J.A.; D’Ascenzi, F.; Zorzi, A. How to evaluate premature ventricular beats in the athlete: Critical review and proposal of a diagnostic algorithm. Br. J. Sports Med. 2019, 54, 1142–1148. [Google Scholar] [CrossRef]
- Pelliccia, A.; Solberg, E.E.; Papadakis, M.; Adami, P.E.; Biffi, A.; Caselli, S.; La Gerche, A.; Niebauer, J.; Pressler, A.; Schmied, C.M.; et al. Recommendations for participation in competitive and leisure time sport in athletes with cardiomyopathies, myocarditis, and pericarditis: Position statement of the Sport Cardiology Section of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2019, 40, 19–33. [Google Scholar] [CrossRef]
- Drezner, J.A.; Sharma, S.; Baggish, A.; Papadakis, M.; Wilson, M.G.; Prutkin, J.M.; La Gerche, A.; Ackerman, M.J.; Borjesson, M.; Salerno, J.C.; et al. International criteria for electrocardiographic interpretation in athletes: Consensus statement. Br. J. Sports Med. 2017, 51, 704–731. [Google Scholar] [CrossRef] [PubMed]
- Delise, P.; Sitta, N.; Lanari, E.; Berton, G.; Centa, M.; Allocca, G.; Cati, A.; Biffi, A. Long-term effect of continuing sports activity in competitive athletes with frequent ventricular premature complexes and apparently normal heart. Am. J. Cardiol. 2013, 112, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A.; Vessella, T.; De Lazzari, M.; Cipriani, A.; Menegon, V.; Sarto, G.; Spagnol, R.; Merlo, L.; Pegoraro, C.; Marra, M.P.; et al. Screening young athletes for diseases at risk of sudden cardiac death: Role of stress testing for ventricular arrhythmias. Eur. J. Prev. Cardiol. 2020, 27, 311–320. [Google Scholar] [CrossRef]
- Zorzi, A.; De Lazzari, M.; Mastella, G.; Niero, A.; Trovato, D.; Cipriani, A.; Peruzza, F.; Portolan, L.; Berton, G.; Sciacca, F.; et al. Ventricular Arrhythmias in Young Competitive Athletes: Prevalence, Determinants, and Underlying Substrate. J. Am. Heart Assoc. 2018, 7, e009171. [Google Scholar] [CrossRef]
- Cipriani, A.; Zorzi, A.; Sarto, P.; Donini, M.; Rigato, I.; Bariani, R.; De Lazzari, M.; Pilichou, K.; Thiene, G.; Iliceto, S.; et al. Predictive value of exercise testing in athletes with ventricular ectopy evaluated by cardiac magnetic resonance. Heart Rhythm. 2019, 16, 239–248. [Google Scholar] [CrossRef]
- Gimeno, J.R.; Tomé-Esteban, M.; Lofiego, C.; Hurtado, J.; Pantazis, A.; Mist, B.; Lambiase, P.; McKenna, W.J.; Elliott, P.M. Exercise-Induced ventricular arrhythmias and risk of sudden cardiac death in patients with hypertrophic cardiomyopathy. Eur. Heart J. 2009, 30, 2599–2605. [Google Scholar] [CrossRef]
- Morshedi-Meibodi, A.; Evans, J.; Levy, D.; Larson, M.; Vasan, R. Clinical correlates and prognostic significance of exercise-induced ventricular premature beats in the community. The Framingham Heart Study. ACC Curr. J. Rev. 2004, 13, 47. [Google Scholar] [CrossRef]
- Selzman, K.A.; Gettes, L.S. Exercise-Induced premature ventricular beats: Should we do anything differently? Circulation 2004, 109, 2374–2375. [Google Scholar] [CrossRef]
- Priori, S.G.; Napolitano, C.; Memmi, M.; Colombi, B.; Drago, F.; Gasparini, M.; DeSimone, L.; Coltorti, F.; Bloise, R.; Keegan, R.; et al. Clinical and Molecular Characterization of Patients with Catecholaminergic Polymorphic Ventricular Tachycardia. Circulation 2002, 106, 69–74. [Google Scholar] [CrossRef]
- Biffi, A.; Pelliccia, A.; Verdile, L.; Fernando, F.; Spataro, A.; Caselli, S.; Santini, M.; Maron, B.J. Long-term clinical significance of frequent and complex ventricular tachyarrhythmias in trained athletes. J. Am. Coll. Cardiol. 2002, 40, 446–452. [Google Scholar] [CrossRef]
- Niwano, S.; Wakisaka, Y.; Niwano, H.; Fukaya, H.; Kurokawa, S.; Kiryu, M.; Hatakeyama, Y.; Izumi, T. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009, 95, 1230–1237. [Google Scholar] [CrossRef]
- Ventura, R.; Steven, D.; Klemm, H.U.; Lutomsky, B.; Müllerleile, K.; Rostock, T.; Servatius, H.; Risius, T.; Meinertz, T.; Kuck, K.-H.; et al. Decennial follow-up in patients with recurrent tachycardia originating from the right ventricular outflow tract: Electrophysiologic characteristics and response to treatment. Eur. Heart J. 2007, 28, 2338–2345. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A.; Mastella, G.; Cipriani, A.; Berton, G.; Del Monte, A.; Gusella, B.; Nese, A.; Portolan, L.; Sciacca, F.; Tikvina, S.; et al. Burden of ventricular arrhythmias at 12-lead 24-hour ambulatory ECG monitoring in middle-aged endurance athletes versus sedentary controls. Eur. J. Prev. Cardiol. 2018, 25, 2003–2011. [Google Scholar] [CrossRef]
- Zeppilli, P.; Russo, A.D.; Santini, C.; Palmieri, V.; Natale, L.; Giordano, A.; Frustaci, A. In Vivo Detection of Coronary Artery Anomalies in Asymptomatic Athletes by Echocardiographic Screening. Chest 1998, 114, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.; Gervasi, S.; Bianco, M.; Cogliani, R.; Poscolieri, B.; Cuccaro, F.; Marano, R.; Mazzari, M.; Basso, C.; Zeppilli, P. Anomalous origin of coronary arteries from the “wrong” sinus in athletes: Diagnosis and management strategies. Int. J. Cardiol. 2018, 252, 13–20. [Google Scholar] [CrossRef]
- Nucifora, G.; Muser, D.; Masci, P.G.; Barison, A.; Rebellato, L.; Piccoli, G.; Daleffe, E.; Toniolo, M.; Zanuttini, D.; Facchin, D.; et al. Prevalence and prognostic value of concealed structural abnormalities in patients with apparently idiopathic ventricular arrhythmias of left versus right ventricular origin: A magnetic resonance imaging study. Circ. Arrhythmia Electrophysiol. 2014, 7, 456–462. [Google Scholar] [CrossRef]
- Mavrogeni, S.I.; Bacopoulou, F.; Apostolaki, D.; Chrousos, G.P. Sudden cardiac death in athletes and the value of cardiovascular magnetic resonance. Eur. J. Clin. Investig. 2018, 48, e12955. [Google Scholar] [CrossRef]
- Narducci, M.L.; Pelargonio, G.; La Rosa, G.; Inzani, F.; D’Amati, G.; Novelli, V.; Marano, R.; Perna, F.; Bencardino, G.; Pinnacchio, G.; et al. Role of extensive diagnostic workup in young athletes and nonathletes with complex ventricular arrhythmias. Heart Rhythm. 2020, 17, 230–237. [Google Scholar] [CrossRef]
- Russo, A.D.; Pieroni, M.; Santangeli, P.; Bartoletti, S.; Casella, M.; Pelargonio, G.; Smaldone, C.; Bianco, M.; Di Biase, L.; Bellocci, F.; et al. Concealed cardiomyopathies in competitive athletes with ventricular arrhythmias and an apparently normal heart: Role of cardiac electroanatomical mapping and biopsy. Heart Rhythm. 2011, 8, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A.; Marra, M.P.; Rigato, I.; De Lazzari, M.; Susana, A.; Niero, A.; Pilichou, K.; Migliore, F.; Rizzo, S.; Giorgi, B.; et al. Nonischemic Left Ventricular Scar as a Substrate of Life-Threatening Ventricular Arrhythmias and Sudden Cardiac Death in Competitive Athletes. Circ. Arrhythmia Electrophysiol. 2016, 9, e004229. [Google Scholar] [CrossRef]
- Casella, M.; Pizzamiglio, F.; Russo, A.D.; Carbucicchio, C.; Al-Mohani, G.; Russo, E.; Notarstefano, P.; Pieroni, M.; D’Amati, G.; Sommariva, E.; et al. Feasibility of Combined Unipolar and Bipolar Voltage Maps to Improve Sensitivity of Endomyocardial Biopsy. Circ. Arrhythmia Electrophysiol. 2015, 8, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.; Exome Aggregation Consortium; Thomson, K.L.; Ware, J.S.; Funke, B.H.; Woodley, J.; McGuire, K.J.; Mazzarotto, F.; Blair, E.; Seller, A.; et al. Reassessment of Mendelian gene pathogenicity using 7855 cardiomyopathy cases and 60,706 reference samples. Genet. Med. 2017, 19, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Mazzarotto, F.; Tayal, U.; Buchan, R.J.; Midwinter, W.; Wilk, A.; Whiffin, N.; Govind, R.; Mazaika, E.; De Marvao, A.; Dawes, T.J.; et al. Reevaluating the Genetic Contribution of Monogenic Dilated Cardiomyopathy. Circulation 2020, 141, 387–398. [Google Scholar] [CrossRef]
- Ackerman, M.J.; Priori, S.G.; Willems, S.; Berul, C.; Brugada, R.; Calkins, H.; Camm, A.J.; Ellinor, P.T.; Gollob, M.H.; Hamilton, R.M.; et al. HRS/EHRA Expert Consensus Statement on the State of Genetic Testing for the Channelopathies and Cardiomyopathies: This document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 2011, 13, 1077–1109. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narducci, M.L.; Cammarano, M.; Novelli, V.; Bisignani, A.; Pavone, C.; Perna, F.; Bencardino, G.; Pinnacchio, G.; Bianco, M.; Zeppilli, P.; et al. Diagnostic Workflow in Competitive Athletes with Ventricular Arrhythmias and Suspected Concealed Cardiomyopathies. Medicina 2021, 57, 182. https://doi.org/10.3390/medicina57020182
Narducci ML, Cammarano M, Novelli V, Bisignani A, Pavone C, Perna F, Bencardino G, Pinnacchio G, Bianco M, Zeppilli P, et al. Diagnostic Workflow in Competitive Athletes with Ventricular Arrhythmias and Suspected Concealed Cardiomyopathies. Medicina. 2021; 57(2):182. https://doi.org/10.3390/medicina57020182
Chicago/Turabian StyleNarducci, Maria Lucia, Michela Cammarano, Valeria Novelli, Antonio Bisignani, Chiara Pavone, Francesco Perna, Gianluigi Bencardino, Gaetano Pinnacchio, Massimiliano Bianco, Paolo Zeppilli, and et al. 2021. "Diagnostic Workflow in Competitive Athletes with Ventricular Arrhythmias and Suspected Concealed Cardiomyopathies" Medicina 57, no. 2: 182. https://doi.org/10.3390/medicina57020182
APA StyleNarducci, M. L., Cammarano, M., Novelli, V., Bisignani, A., Pavone, C., Perna, F., Bencardino, G., Pinnacchio, G., Bianco, M., Zeppilli, P., Palmieri, V., & Pelargonio, G. (2021). Diagnostic Workflow in Competitive Athletes with Ventricular Arrhythmias and Suspected Concealed Cardiomyopathies. Medicina, 57(2), 182. https://doi.org/10.3390/medicina57020182





