Oral Solitary Fibrous Tumor: A Retrospective Clinico-Pathological Study and Long-Term Follow-Up
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klemperer, P.; Rabin, C.B. Primary neoplasms of the pleura: A report of five cases. Arch. Pathol. 1931, 11, 385–412. [Google Scholar] [CrossRef] [PubMed]
- Suster, S.; Nascimento, A.G.; Miettinen, M.; Sickel, J.Z.; Moran, C.A. Solitary fibrous tumors of soft tissue. A clinicopathologic and immunohistochemical study of 12 cases. Am. J. Surg. Pathol. 1995, 19, 1257–1266. [Google Scholar] [CrossRef]
- Westra, W.H.; Gerald, W.L.; Rosai, J. Solitary fibrous tumor. Consistent CD34 immunoreactivity and occurrence in the orbit. Am. J. Surg. Pathol. 1994, 18, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Zukerberg, L.R.; Rosenberg, A.E.; Randolph, G.; Pilch, B.Z.; Goodman, M.L. Solitary fibrous tumor of the nasal cavity and paranasal sinuses. Am. J. Surg. Pathol. 1991, 15, 126–130. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Zhu, Q.; Xia, W.; Bhagat, S.K. Solitary fibrous tumor of the salivary gland: A case report. Oncol. Lett. 2016, 11, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Benlyazid, A.; Lescanne, E.; Lefrancq, T.; Fetissoff, F.; Beutter, P. Solitary fibrous tumour of the larynx: Report of a case. J. Laryngol. Otol. 1998, 112, 286–289. [Google Scholar] [CrossRef]
- Rodriguez, I.; Ayala, E.; Caballero, C.; De Miguel, C.; Matias-Guiu, X.; Cubilla, A.L.; Rosai, J. Solitary fibrous tumor of the thyroid gland: Report of seven cases. Am. J. Surg. Pathol. 2001, 25, 1424–1428. [Google Scholar] [CrossRef]
- Greig, A.V. Solitary fibrous tumour of the deep soft tissues of the neck. J. Laryngol. Otol. 1998, 112, 503–505. [Google Scholar] [CrossRef]
- Nunes, F.B.; Sant’Ana, M.S.P.; Silva, A.M.B.; Agostini, M.; Silva Canedo, N.H.; de Andrade, B.A.B.; Romañach, M.J.; Corrêa, D.L.; Tomasi, R.A.; Radhakrishnan, R.; et al. Solitary fibrous tumour of the oral cavity: An update. J. Oral. Pathol. Med. 2020, 49, 14–20. [Google Scholar] [CrossRef]
- WHO. Classification of Tumours Editorial Board. Soft tissue and bone tumours. Lyon (France): International Agency for Research on Cancer. In WHO Classification of Tumours Series, 5th ed.; WHO: Geneva, Switzerland, 2020; Volume 3, p. 104. [Google Scholar]
- Carlos, R.; de Andrade, B.A.; Canedo, N.H.; Abrahão, A.C.; Agostini, M.; de Almeida, O.P.; Romañach, M.J. Clinicopathologic and immunohistochemical features of five new cases of solitary fibrous tumor of the oral cavity. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2016, 121, 390–395. [Google Scholar] [CrossRef]
- Kao, Y.C.; Lin, P.C.; Yen, S.L.; Huang, S.C.; Tsai, J.W.; Li, C.F.; Tai, H.C.; Lan, J.; Chuang, I.C.; Yu, S.C.; et al. Clinicopathological and genetic heterogeneity of the head and neck solitary fibrous tumours: A comparative histological, immunohistochemical and molecular study of 36 cases. Histopathology 2016, 68, 492–501. [Google Scholar] [CrossRef]
- Alawi, F.; Stratton, D.; Freedman, P.D. Solitary fibrous tumor of the oral soft tissues: A clinicopathologic and immunohistochemical study of 16 cases. Am. J. Surg. Pathol. 2001, 25, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Salas, S.; Resseguier, N.; Blay, J.Y.; Le Cesne, A.; Italiano, A. Prediction of local and metastatic recurrence in solitary fibrous tumor: Construction of a risk calculator in a multicenter cohort from the French Sarcoma Group (FSG) database. Ann. Oncol. 2017, 28, 1979–1987. [Google Scholar] [CrossRef]
- Iorio, B.; Ronchi, A.; Montella, M.; Cozzolino, I.; De Luca, R.; Rusciano, M.; Tartaro, G.; Colella, G.; Franco, R. Malignant extrapleural solitary fibrous tumor arising in the sublingual gland: A case report and review of literature. Oral. Oncol. 2019, 90, 141–144. [Google Scholar] [CrossRef]
- O’Regan, E.M.; Vanguri, V.; Allen, C.M.; Eversole, L.R.; Wright, J.M.; Woo, S.B. Solitary fibrous tumor of the oral cavity: Clinicopathologic and immunohistochemical study of 21 cases. Head Neck Pathol. 2009, 3, 106–115. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Iorio-Siciliano, V.; Alibrandi, A.; Ramaglia, L.; Leonardi, R. Effectiveness of a nutraceutical agent in the non-surgical periodontal therapy: A randomized, controlled clinical trial. Clin. Oral. Investig. 2020. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Williams, R.C.; Leonardi, R. Independent impact of periodontitis and cardiovascular disease on elevated soluble urokinase-type plasminogen activator receptor (suPAR) levels. J. Periodontol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.P.; Daniels, T.; Jordan, R.C. Solitary fibrous tumor of the head and neck. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2010, 110, 79–84. [Google Scholar] [CrossRef]
- Chan, J.K. Solitary fibrous tumour--everywhere, and a diagnosis in vogue. Histopathology 1997, 31, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Gengler, C.; Guillou, L. Solitary fibrous tumour and haemangiopericytoma: Evolution of a concept. Histopathology 2006, 48, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Olson, N.J.; Linos, K. Dedifferentiated Solitary Fibrous Tumor: A Concise Review. Arch. Pathol. Lab. Med. 2018, 142, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Davanzo, B.; Emerson, R.E.; Lisy, M.; Koniaris, L.G.; Kays, J.K. Solitary fibrous tumor. Transl. Gastroenterol. Hepatol. 2018, 3, 94. [Google Scholar] [CrossRef] [PubMed]
- Demicco, E.G.; Park, M.S.; Araujo, D.M.; Fox, P.S. Solitary fibrous tumor: A clinicopathological study of 110 cases and proposed risk assessment model. Mod. Pathol. 2012, 25, 1298–1306. [Google Scholar] [CrossRef] [PubMed]

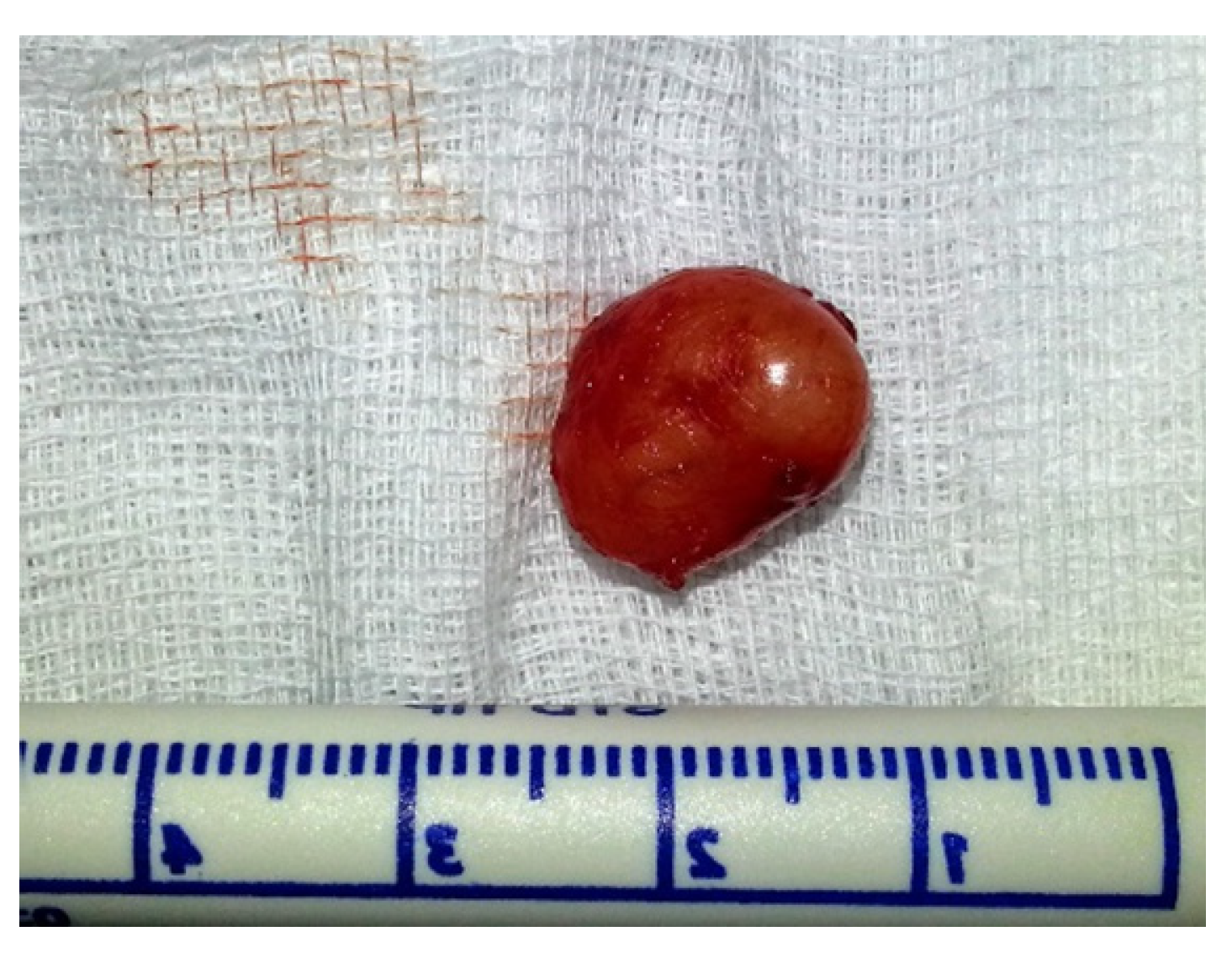

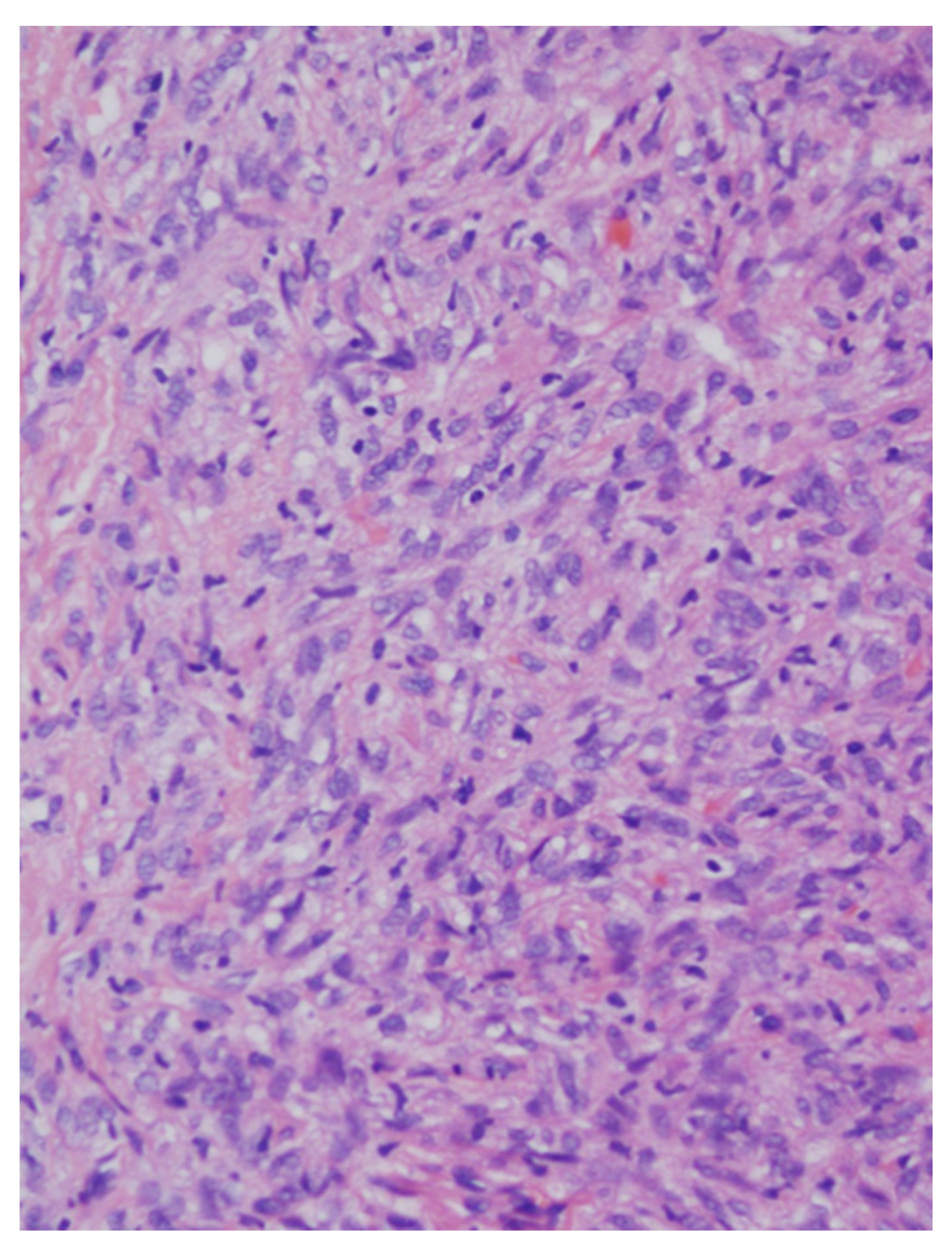
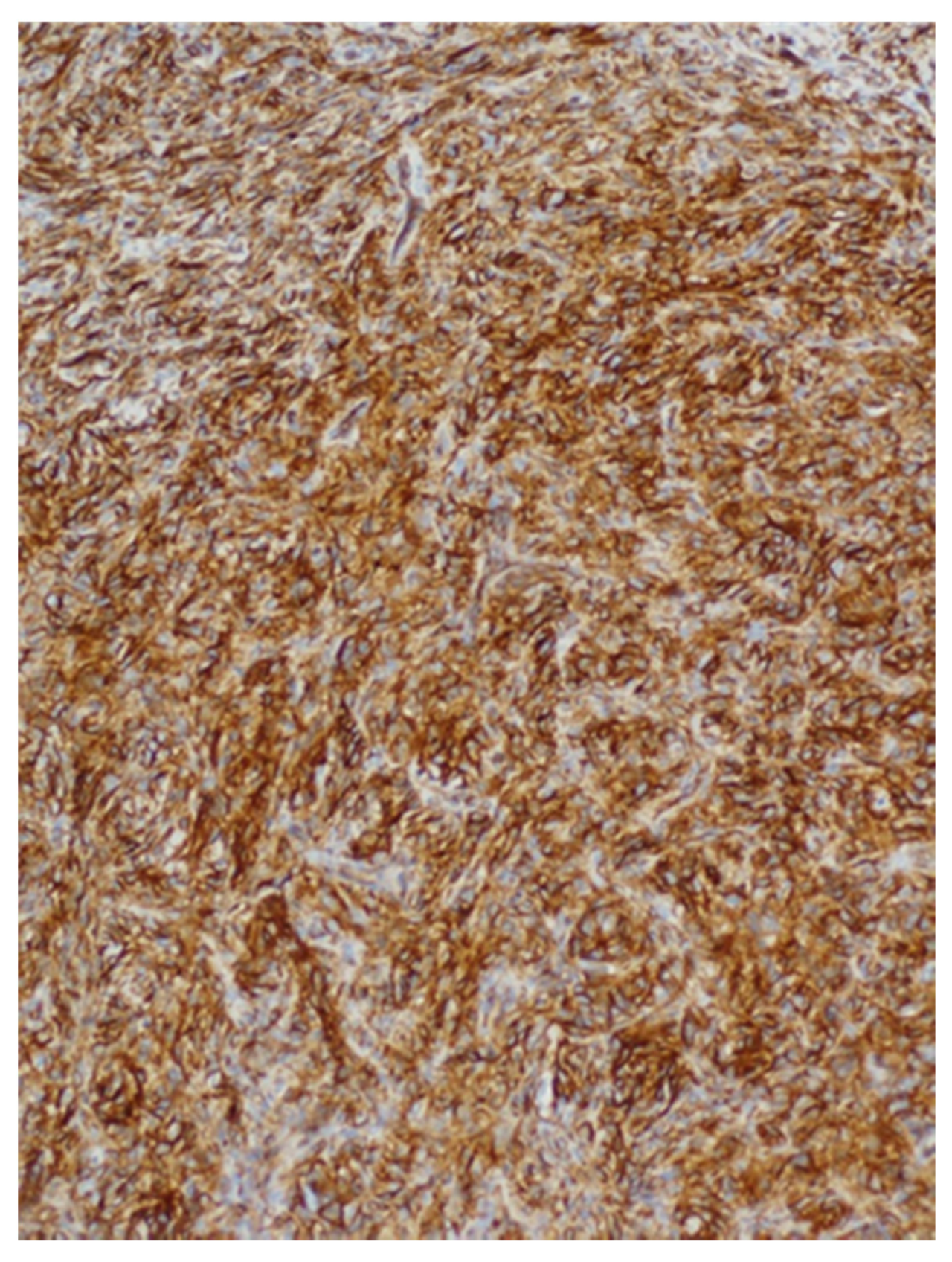
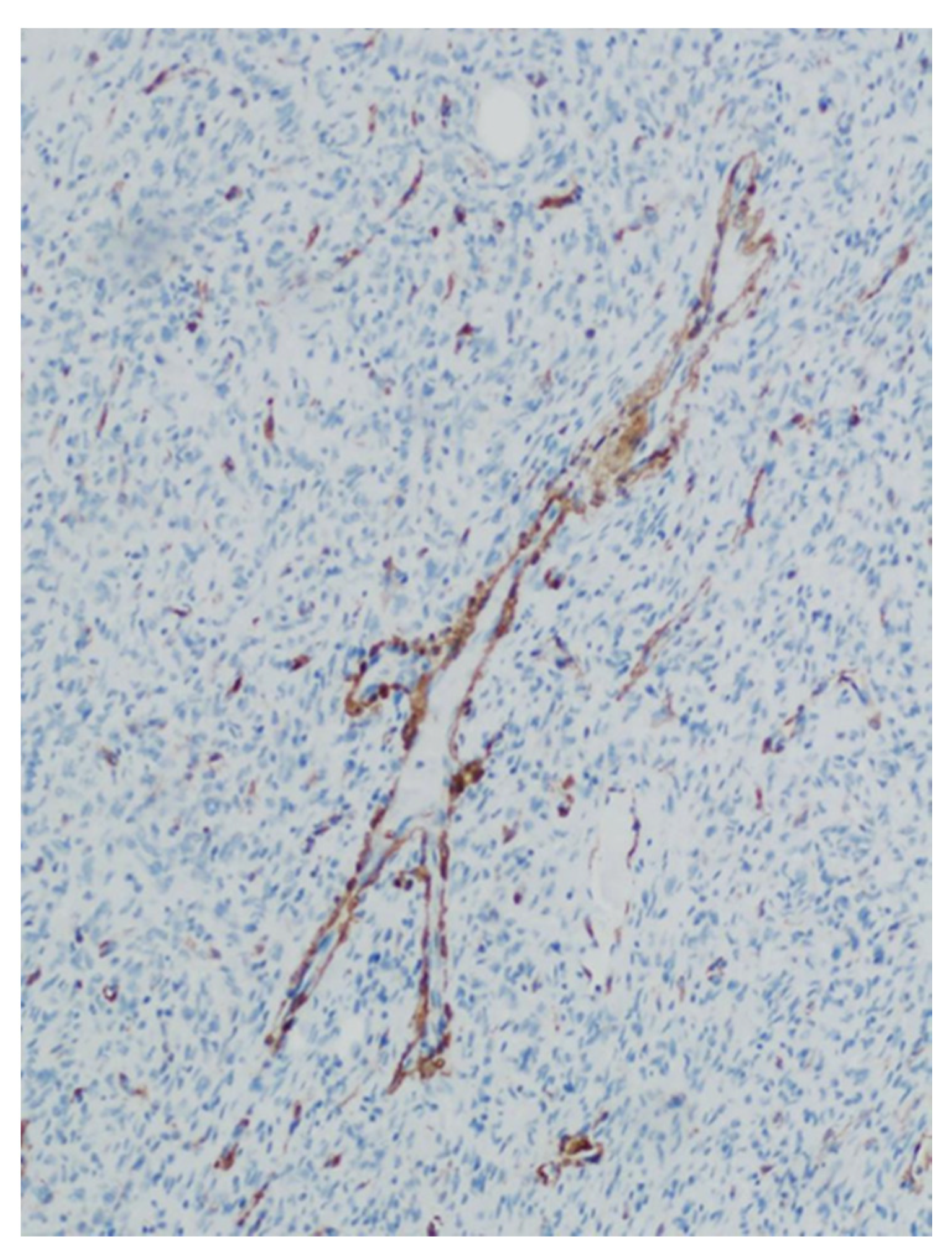
| Patients | Age (years) | Sex | Location | Size (mm) | Surgical Procedure | Follow-Up (months) | Recurrence |
|---|---|---|---|---|---|---|---|
| 1 | 43 | M | Buccal mucosa | 25 | Local excision | 30 | No |
| 2 | 24 | F | Upper labial mucosa | 14 | Local excision | 2 | No |
| 3 | 41 | F | Buccal mucosa | 17 | Local excision | 60 | No |
| 4 | 60 | M | Floor of mouth | 35 | Local excision | 74 | No |
| 5 | 63 | M | Lower labial mucosa | 3 | Local excision | NA | NA |
| 6 | 60 | F | Buccal mucosa | 50 | Local excision+ Embolization | 66 | No |
| 7 | 43 | F | Buccal mucosa | 14 | Local excision | 15 | No |
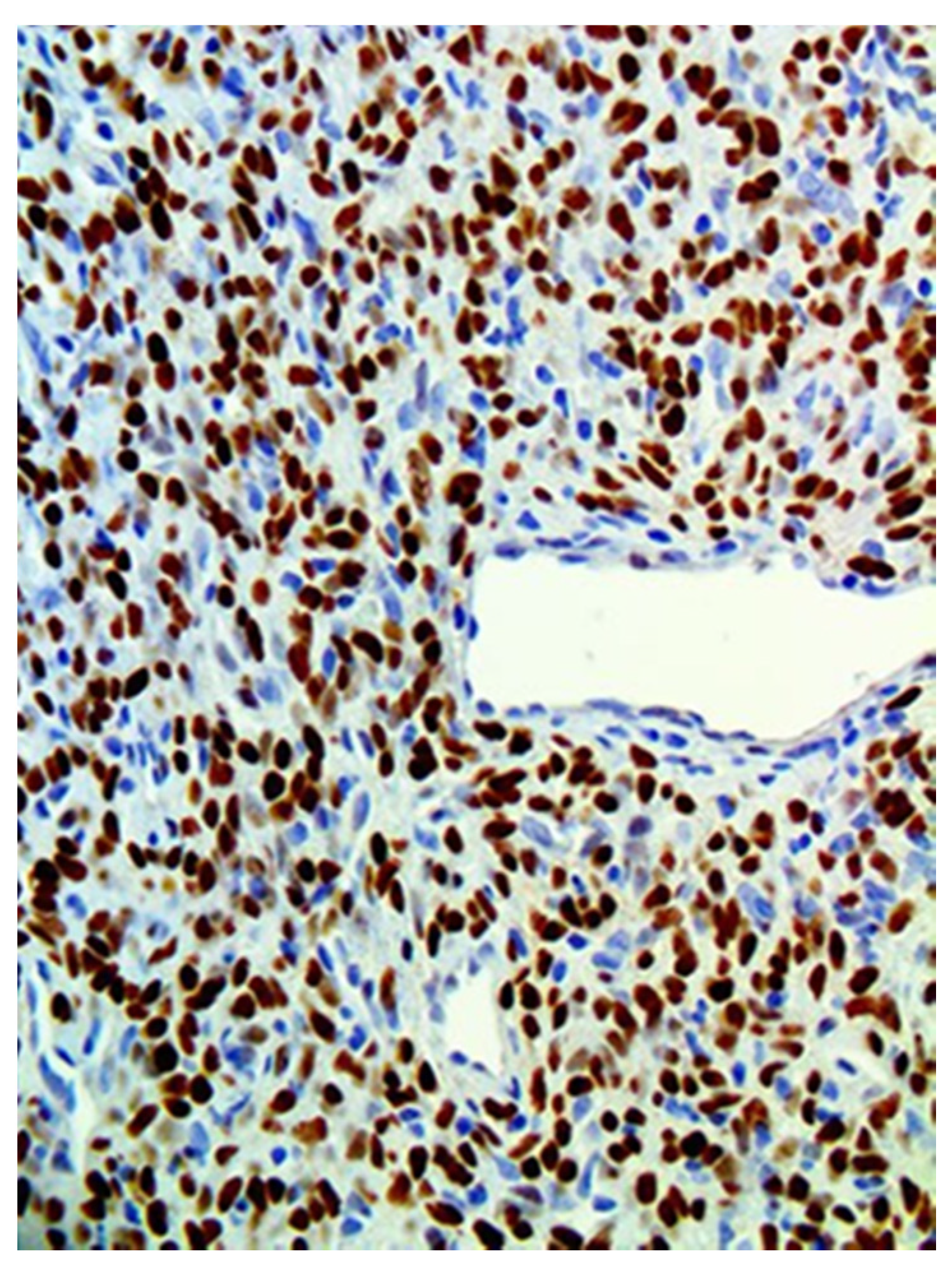
| Patients | CD34 | CD99 | STAT6 | Bcl-2 |
|---|---|---|---|---|
| 1 | + | + | + | NA |
| 2 | + | NA | + | NA |
| 3 | + | NA | + | + |
| 4 | + | NA | + | + |
| 5 | + | NA | + | NA |
| 6 | + | NA | + | + |
| 7 | + | NA | + | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shmuly, T.; Ben Zvi, Y.; Chaushu, G.; Kaplan, I. Oral Solitary Fibrous Tumor: A Retrospective Clinico-Pathological Study and Long-Term Follow-Up. Medicina 2021, 57, 152. https://doi.org/10.3390/medicina57020152
Shmuly T, Ben Zvi Y, Chaushu G, Kaplan I. Oral Solitary Fibrous Tumor: A Retrospective Clinico-Pathological Study and Long-Term Follow-Up. Medicina. 2021; 57(2):152. https://doi.org/10.3390/medicina57020152
Chicago/Turabian StyleShmuly, Tom, Yehonatan Ben Zvi, Gabriel Chaushu, and Ilana Kaplan. 2021. "Oral Solitary Fibrous Tumor: A Retrospective Clinico-Pathological Study and Long-Term Follow-Up" Medicina 57, no. 2: 152. https://doi.org/10.3390/medicina57020152
APA StyleShmuly, T., Ben Zvi, Y., Chaushu, G., & Kaplan, I. (2021). Oral Solitary Fibrous Tumor: A Retrospective Clinico-Pathological Study and Long-Term Follow-Up. Medicina, 57(2), 152. https://doi.org/10.3390/medicina57020152







