Abstract
Background and objectives: Acquired brain injury (ABI) is the first cause of disability and physical activity (PA) is a key element in functional recovery and health-related quality of life (HRQoL) during the subacute and chronic phases. However, it is necessary to develop PA programs that respond to the heterogeneity and needs of this population. The aim of this study was to assess the effectiveness of a PA program on the HRQoL in this population. Materials and Methods: With regard to recruitment, after baseline evaluations, participants were assigned to either the intervention group (IG, n = 38) or the control group (CG, n = 35). Functional capacity, mood, quality of life and depression were measured pre- and post-intervention. The IG underwent the “Physical Activity and Sport for Acquired Brain Injury” (PASABI) program, which was designed to improve HRQoL (1-h sessions, two to four sessions/week for 18 weeks). The CG underwent a standard rehabilitation program without PA. Results: Results for the IG indicated significant differences and large effect sizes for the physical and mental dimensions of quality of life, as well as mood and functional capacity, indicating an increase in HRQoL. No significant differences were found for the CG across any variables. Conclusions: The PASABI program was feasible and beneficial for improving physiological and functionality variables in the IG. The wide range of the activities of the PASABI program allow its application to a large number of people with ABI, promoting health through PA, especially in the chronic phase.
1. Introduction
Acquired brain injury (ABI) requires specific training programs based in neurorehabilitation with the aim of achieving the highest possible level of autonomy and participation during the subacute and chronic phases [1]. The consequences of an ABI are different in each person, and it is usual to find deficits in the physical, cognitive, behavioral, and sensorial dimensions. As a result, a multidisciplinary approach is fundamental for the treatment of ABI [2]. The incidence of ABI increases each year, with the two most common etiologies being traumatic brain injury (TBI) and stroke [3,4].
Health-related quality of life (HRQoL) includes the physical, psychological, and social dimensions, which requires the evaluation of different variables to know this multidimensional concept [5]. Literature points out that HRQoL is lower in people with an ABI compared to that in people without an ABI due to the sedentary lifestyle, generating secondary problems [6,7], and social leisure activities are recommended to promote the highest HRQoL [6]. In this line, physical activity (PA) has demonstrated being an important tool to improve not only physical affectation [8,9] but also cognitive [10,11] and behavioral sequels [12], being a key to socialize, to increase reintegration into the community, and improve HRQoL [13]. It seems in the literature that there is no consensus on the type of PA program that should be proposed to people with chronic ABI, whose main goal should be to improve HRQoL, coinciding with interest in group activities [14,15].
The International Classification of Functioning (ICF) developed by the World Health Organization (WHO) [16] is the bio-psycho-social framework to design and implement PA programs for people with disability and specifically used for functional evaluation and PA participation in people with ABI [17]. For all of the above mentioned, this study aimed to assess the efficacy of the “Physical Activity and Sport Brain Injury” (PASABI) program in the HRQoL of people with ABI during the subacute and chronic phases. It was hypothesized that involvement in the PASABI program would improve mood, depression, quality of life, functional capacity, and participation.
As the systematic review carried out by Jones et al. [18] points out, intervention programs are generally not described in depth, thus preventing their reproducibility. Most of the research includes specific proposals for stroke or TBI, although people with ABI in rehabilitation centers receive the same therapies. The two most practiced PA programs in the literature are the treadmill and exercise bike, which are not group activities [14,15].
2. Materials and Methods
2.1. Participants
A total of 73 subjects were recruited into this study according to the following inclusion criteria: (a) being over 18 years of age; (b) not having language problems that make oral and written comprehension impossible; (c) being able to walk independently or with auxiliary material; (d) having had at least 1 year of evolution since the ABI; (e) having received at least 3 months of rehabilitation for the ABI; (f) residing at home and not in a rehabilitation center; (g) not having resumed working life; and (h) having a sufficient level of cognitive functioning to answer questionnaires coherently.
With regard to recruitment (Figure 1), after baseline evaluations, participants were assigned, using a mixed paired design, to either the intervention group (IG) or the control group (CG). The assignment of each group was done according to the preference of the participants for ethics and feasibility conditions.
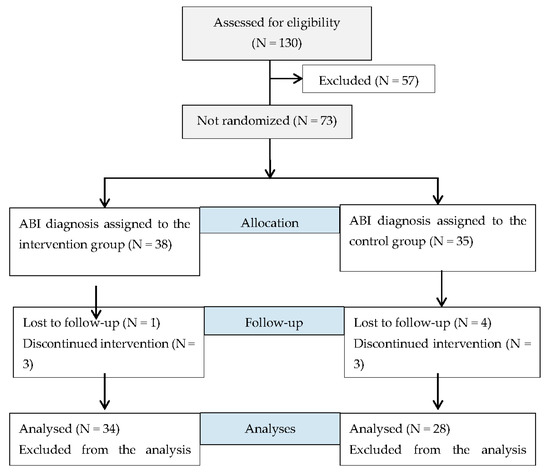
Figure 1.
Consort flow diagram for participants. (Acquired brain injury—ABI).
2.2. Study Design
The present study was a non-randomized clinical trial. Pre-post intervention design, with measurements at baseline and after 18 weeks of intervention.
2.3. Outcome Measures
Different instruments, according to the dimensions of the ICF, were used to assess the program’s effects on the HRQoL of participants. Table 1 shows the variables of study of HRQoL that have been established. The measurements were made at baseline and after 18 weeks of intervention in both the IG and CG.

Table 1.
Study variables of health-related quality of life (HRQoL) based on International Classification of Functioning (ICF) domains.
For demographic data, a collection form was used with the sociodemographic data referring to date of birth, gender, housing, profession prior to the ABI, etiology of the injury, months since the injury, type of displacement, mobility, prior PA practice, and whether the participant was currently receiving therapies in a rehabilitation center or not. Additionally, the Spanish version of the Beck Depression Inventory II (BDI-II) (Sanz and Vázquez, 1998) was used to evaluate depressive symptoms. Mood with respect to factors of a negative (anger, fatigue, tension, and depressed state) and positive (vigor and friendliness) nature were evaluated using the Spanish version of the Profile of Mood States (POMS) [28].
Furthermore, the SF-36 version 1.4 was used to assess quality of life [29,30]. This tool evaluates the self-perception of health and quality of life. In addition, the level of activity was measured through the Global Physical Activity Questionnaire (GPAQ) [31]. This questionnaire contains the dimensions work, travel, and free time. Finally, the 6 Minute Walk Test (6MWT) was selected to assess functional capacity [32].
2.4. Intervention
First, participants were provided with general information about the study and informed consent, in addition to recording demographic data. Then, general cognitive functioning, depressive symptoms, mood, quality of life, activity level, and functional ability were assessed before and after 18 weeks of the PASABI program. The 18-week exercise program was developed in six municipal sports centers of Madrid in people with ABI in the chronic phase, with participants performing two to four sessions per week at 1 h per session.
Physical Activity, Sport and Acquired Bran Injury (PASABI)
The PASABI program aims to increase autonomy in the conduct of DLAs, generate social relationships, and achieve greater reintegration into the community. It defines the type of activity to be carried out related to the level of affectation and proposes specific tasks focused on key aspects of improving the HRQoL of people with ABI. It includes games as a key element, because they are a resource that involves fun, socialization, interactions that incite instinctive learning, organization, and the development of complex skills not only for the child but also for the adult. Furthermore, diverse activities designed according to abilities based on the proposal of García-Hernández and Pérez-Rodríguez [33], as well as previous studies that established the benefits of PA on the health of people with ABI [18,33,34,35]. The activities of the PASABI program include water activity, swimming, paddle tennis, initiation to football and initiation to athletics. Within the characteristics of the structure of the session. The proposed PASABI program included a first phase with a warm-up exercise (5–10 min of joint mobility tasks) and a second phase with at least three specific analytical exercises and three specific AFA exercises. Moreover, an interactive part with at least one game. Finally, return to the calm with 5 min of breathing control tasks and 10 min stretching the muscle groups involved. Two adapted PA professionals supervised the intervention.
2.5. Statistical Data
For descriptive statistics, data were checked for normality using the Kolmogorov–Smirnov test, which indicated the need for non-parametric tests. A descriptive analysis (by group) of the variables studied was carried out using, as reference values, the means and standard deviations for continuous variables and frequencies and percentages for categorical variables. The chi-square test was used for categorical variables, and the Mann–Whitney U test was used for continuous variables. After the intervention, the Wilcoxon W test of related samples was used to establish intragroup differences, and the Mann–Whitney U test of independent samples was used to analyse the differences between groups. Cliff’s Delta was calculated for the estimation of the non-parametric effect size in the comparisons. An r-value higher than 0.1, 0.3 or 0.5 was considered as a small, medium, or large effect size, respectively [36]. The significance level was set at p ≤ 0.05, and the SPSS 22.0 statistical package was used.
2.6. Ethics
All participants signed the informed consent form. The design of the research was supervised and approved by the Ethics Committee of the leading institution of the study, and the recommendations of the Declaration of Helsinki were followed at all times [37]. This protocol is registered in Clinical Trial.gov (NCT03162484).
3. Results
Demographic characteristics of all the participants in the IG and CG at baseline are presented in Table 2. Of the 38 people with ABI assigned to the IG, only 34 were included in the intervention. One participant was lost to follow-up. Additionally, three participants did not complete the 18-week intervention for personal reasons. At 18 weeks, more than 80% of this sample showed adherence to the program. On other hand, in the CG, seven participants were included—four participants did not pass the neuropsychological assessment and three participants did not complete the planning program.

Table 2.
Baseline demographic characteristics of participants.
Table 3 shows the results for each variable at baseline and after 18 weeks of intervention according to group. After the intervention, significant differences were found in the IG for the variables sedentary, functional capacity, physical function (p < 0.001), and mental health (p < 0.05), producing an increase in the scores, as well as a generalized decrease in the scores of the variables, depression-B, depression, anger (p < 0.05), and stress (p < 0.01). Regarding the QA score, significant differences were found in the variables sedentary (p < 0.01), friendship and fatigue (p < 0.05), producing a general increase in the scores, also in the IG.

Table 3.
SF-36, POMS, GPAQ, BDI-II, and 6MWT at baseline and post-intervention assessments.
With respect to the comparison of scores after the intervention, differences were found between the QA and IG in the variables sedentary (U = 359.50, p < 0.05, r = 0.28), functional capacity (U = 360.00, p < 0.05, r = 0.28), physical function (U = 402.00, p < 0.05, r = 0.32), showing the CG with the highest score in the sedentary variable, and the IG in the functional capacity and physical function variables.
4. Discussion
This study assessed the efficacy of a group PA program for people with ABI in the chronic phase, proposing a new approach and a line of work to be explored in the future for this population. The PASABI program has proven to be feasible. Furthermore, the adherence to the program was very high. Out of 39 participants, none of them left the program, and all exceeded 80% attendance at the sessions. In our opinion, the design based on the ICF framework succeeds in not only responding to the functional characteristics but also contextual factors near their place of residence and minimizing barriers to practice [38]. This along with the professionalism of the staff team in the referral of each participant to the most suitable activity was critical to the success of the program in terms of participation and effectiveness [33].
No adverse events occurred during the sessions, and no participants reported secondary problems or complications arising from practice. The length of the intervention with regard to the session time (1 h) and the 18 weeks of intervention could have been decisive in the positive results of the study. Likewise, a frequency of two to four sessions per week allowed participants to adapt to their possibilities and led to a transfer beyond the study. Most participants in the IG continued to engage in the program after the completion of the study.
Previous studies considered age as a predictor of PA practice; however, the mean age between the two groups evaluated was similar, and the IG had greater age differences between the minimum and the maximum. Likewise, the results revealed that the severity of the injury and the sport practice before the injury could be indicators for participation after ABI in PA programs [39].
With respect the content of the PASABI program, it seems that group activities like those provided by the program are efficient and allow participation and high rates of engagement, as mentioned. Regarding aquatic activities, we agree with authors in affirming the effectiveness of this type of exercise to improve HRQoL [34,40].
In relation to the improvement of the quality of life measured with the SF-36, the results indicated significant improvements of the IG in the physical and mental dimensions, which corroborates the capacity of a group PA to generate benefits in the different areas, coinciding with authors who also point this out [41,42]. The results on mood indicate a significant improvement in the dimensions of depression, cholera, and tension, coinciding with expert authors on this topic [25,43]. With respect to fatigue, the dimensions included in the POMS increased significantly in the CG after the 18-week intervention period. This could be justified by the low physical condition of the CG that leads to lower physical levels and a greater sense of fatigue at any effort.
Due to the importance of depression in the functional recovery of people with ABI, in this study, the BDI-II was used to assess depressive symptoms. A tendency to decrease in the IG and increase in the CG after the intervention was observed, coinciding with other authors who found improvement in depressive symptoms after an intervention [12,44] and stroke.
Moreover, the findings show that there was no improvement in the level of activity after the intervention. It should be noted that the increase in the score in the sedentary variable was greater in the CG, although it increased in both groups. This data may be due to the fact that 56% of the IG did not receive therapy in rehabilitation centers or return to work, compared to 100% of the CG, who did receive therapy during this time, providing more active hours. Nonetheless, it is remarkable that the IG has increased physical function and functional capacity; although, the hours of activity did not increase. So, in order to achieve an adequate physical condition and an adequate mood, it seems essential to perform PA as an indispensable complement to the standard rehabilitation programs in the chronic phase [45].
All studies confirm the impact of functional capacity on HRQoL in people with ABI [23]. Studies show improvement in functional capacity after a PA program, either individual or group from a minimum of eight weeks [9,46]. The 6MWT appears to be a reliable and valid indicator of functional capacity [47,48].
As limitations of this research, we can indicate that the study sample was limited to voluntarily participating individuals, leading to a possible selection bias. The sample consisted of participants mildly to moderately affected by ABI, not including people with more affectation given the characteristics of the PASABI program. The positive results of the study in a population with ABI open a fruitful line of research in the role of PA-oriented health in this population, with application to other ABI levels of impairment. In this regard, this study could be a starting point for future investigations with a randomized clinical trial design.
5. Conclusions
The bio-psycho-social framework proposed by the ICF is adequate to encompass all aspects to be taken into account in the design of a group PA program in people with chronic ABI.
Having professional staff, minimizing barriers, and selecting schedules and facilities close to the place of residence are critical to achieving a high level of attendance and adherence to the program.
This research has shown that the PASABI program, with content based on different sports modalities and adapted activities designed specifically for people with ABI in the chronic phase to achieve the highest autonomy and participation, has positive effects on HRQoL.
Author Contributions
Conceptualization, M.P.-R. and J.J.G.-H.; methodology, J.C.; software, S.G.-G.; validation, J.C. and J.P.-T.; formal analysis, S.G.-G.; investigation, M.P.-R.; data curation, J.J.G.-H.; writing—original draft preparation, M.P.-R.; writing—review and editing, J.P.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of University Polytechnic of Madrid (19 December 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jolliffe, L.; Lannin, N.A.; Cadilhac, D.A.; Hoffmann, T. Systematic Review of Clinical Practice Guidelines to Identify Recommendations for Rehabilitation After Stroke and Other Acquired Brain Injuries. BMJ Open 2018, 8, e018791:1–e018791:14. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Pick, A.; Nair, A.; Disler, P.B.; Wade, D.T. Multi-Disciplinary Rehabilitation for Acquired Brain Injury in Adults of Working Age. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the Global Incidence of Traumatic Brain Injury. J. Neurosurg. 2018, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Von Steinbuechel, N.; Richter, S.; Morawetz, C.; Riemsma, R. Assessment of Subjective Health and Health-Related Quality of Life in Persons with Acquired or Degenerative Brain Injury. Curr. Opin. Neurol. 2005, 18, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Proctor, C.J.; Best, L.A. Social and Psychological Influences on Satisfaction with Life after Brain Injury. Disabil. Health J. 2019, 12, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Stiekema, A.P.M.; Winkens, I.; Ponds, R.; De Vugt, M.E.; Van Heugten, C.M. Finding a New Balance in Life: A Qualitative Study on Perceived Long-Term Needs of People With Acquired Brain Injury and Partners. Brain Inj. 2020, 34, 421–429. [Google Scholar] [CrossRef] [PubMed]
- García-Hernández, J.J.; Mediavilla-Saldaña, L.; Pérez-Rodríguez, P.R.; Pérez-Tejero, J.; González-Alted, C. Analysis of the effect of physical group activities in patients with acquired brain injury in the subacute phase. Rev. Neurol. 2013, 5, 64–70. [Google Scholar]
- Luo, L.; Meng, H.; Wang, Z.; Zhu, S.; Yuan, S.; Wang, Y.; Wang, Q. Effect of High-Intensity Exercise on Cardiorespiratory Fitness in Stroke Survivors: A Systematic Review and Meta-Analysis. Ann. Phys. Rehabil. Med. 2020, 63, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.M.; Keyser, R.E.; Dsurney, J.; Chan, L. Improved Cognitive Performance Following Aerobic Exercise Training in People With Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2015, 96, 754–759. [Google Scholar] [CrossRef]
- Marzolini, S.; Oh, P.; McIlroy, W.; Brooks, D. The Effects of an Aerobic and Resistance Exercise Training Program on Cognition Following Stroke. Neurorehabil. Neural Repair 2013, 27, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, M.; Pérez-Tejero, J.; García-Hernández, J.J.; Franco, E.; Coterón, J. Physical activity and acquired brain damage, chronic phase: Influence on quality of life. J. Sport Psychol. 2020, 29, 16–23. [Google Scholar]
- Vanderbeken, I.; Kerckhofs, E. A Systematic Review of the Effect of Physical Exercise on Cognition in Stroke and Traumatic Brain Injury Patients. NeuroRehabilitation 2017, 40, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, P.; Miele, A.; Gallè, F.; Liguori, G. Adapted Physical Activity and Stroke: A Systematic Review. J. Sports Med. Phys. Fitness 2018, 58, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Merali, S.; Cameron, J.I.; Barclay, R.; Salbach, N.M. Characterising Community Exercise Programmes Delivered by Fitness Instructors for People With Neurological Conditions: A Scoping Review. Health Soc. Care Community 2016, 24, E101–E116. [Google Scholar] [PubMed]
- World Health Organization. International Classification of Functionality Disability and Health (ICF); IMSERSO: Madrid, Spain, 2001. [Google Scholar]
- Vander Werff, K.R. The Application of the International Classification of Functioning, Disability and Health to Functional Auditory Consequences of Mild Traumatic Brain Injury. Semin. Hear. 2016, 37, 216–232. [Google Scholar]
- Jones, T.M.; Hush, J.M.; Dear, B.F.; Titov, N.; Dean, C.M. The Efficacy of Self-Management Programmes for Increasing Physical Activity in Community-Dwelling Adults with Acquired Brain Injury (ABI): A Systematic Review. Syst. Rev. 2014, 3, 39:1–39:6. [Google Scholar] [CrossRef]
- Pang, M.Y.; Eng, J.J.; Dawson, A.S.; McKay, H.A.; Harris, J.E. A Community-Based Fitness and Mobility Exercise Program for Older Adults With Chronic Stroke: A Randomized, Controlled Trial. J. Am. Geriatr. Soc. 2005, 53, 1667–1674. [Google Scholar] [CrossRef]
- Liu-Ambrose, T.; Eng, J.J. Exercise Training and Recreational Activities to Promote Executive Functions in Chronic Stroke: A Proof-of-Concept Study. J. Stroke Cerebrovasc. Dis. 2015, 24, 130–137. [Google Scholar] [CrossRef]
- Preston, E.; Dean, C.M.; Ada, L.; Stanton, R.; Brauer, S.; Kuys, S.; Waddington, G. Promoting Physical Activity After Stroke via Self-Management: A Feasibility Study. Top. Stroke Rehabil. 2017, 24, 353–360. [Google Scholar]
- Hoffman, J.M.; Bell, K.R.; Powell, J.M.; Behr, J.; Dunn, E.C.; Dikmen, S.; Bombardier, C.H. A Randomized Controlled Trial of Exercise to Improve Mood after Traumatic Brain Injury. PM R 2010, 2, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Polinder, S.; Haagsma, J.A.; van Klaveren, D.; Steyerberg, E.W.; van Beeck, E.F. Health-Related Quality of Life After TBI: A Systematic Review of Study Design, Instruments, Measurement Properties, and Outcome. Popul. Health Metr. 2015, 13, 4:1–4:12. [Google Scholar] [CrossRef] [PubMed]
- Grauwmeijer, E.; Heijenbrok-Kal, M.H.; Ribbers, G.M. Health-Related Quality of Life 3 Years after Moderate to Severe Traumatic Brain Injury: A Prospective Cohort Study. Arch. Phys. Med. Rehabil. 2014, 95, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Driver, S.; Ede, A. Impact of Physical Activity on Mood after TBI. Brain Inj. 2009, 23, 203–212. [Google Scholar] [CrossRef]
- Haagsma, J.A.; Scholten, A.C.; Andriessen, T.M.; Vos, P.E.; Van Beeck, E.F.; Polinder, S. Impact of Depression and Post-Traumatic Stress Disorder on Functional Outcome and Health-Related Quality of Life of Patients With Mild Traumatic Brain Injury. J. Neurotrauma 2015, 32, 853–862. [Google Scholar] [CrossRef]
- Faulkner, J.; McGonigal, G.; Woolley, B.; Stoner, L.; Wong, L.; Lambrick, D. A Randomized Controlled Trial to Assess the Psychosocial Effects of Early Exercise Engagement in Patients Diagnosed With Transient Ischaemic Attack and Mild, Non-Disabling Stroke. Clin. Rehabil. 2015, 29, 783–794. [Google Scholar] [CrossRef]
- Andrade, E.; Arce, C.; de Francisco, C.; Torrado, J.; Garrido, J. Abbreviated Version in Spanish of the POMS Questionnaire for Adult Athletes and General Population. Rev. Psicol. Deporte 2013, 22, 95–102. [Google Scholar]
- Ware, J.D., Jr.; Sherbourne, C.D. The MOS 36-item Short-Form Health Survey (SF-36). I. Conceptual Framework and Item Selection. Med Care Res. Rev. 1992, 30, 473–483. [Google Scholar]
- Alonso, J. Cuestionario de Salud SF-36 Versión Española 1.4. Barcelona1999. Available online: http://www.chime.ucla.edu/measurement/SF-36%20Spain.pdf (accessed on 23 November 2019).
- OMS. Global Physical Activity Questionnaire (GPAQ); Organización Mundial de la Salud: Geneva, Switzerland, 2006. [Google Scholar]
- Enright, P.L. The Six-Minute Walk Test. Respir. Care 2003, 48, 783–785. [Google Scholar]
- García-Hernández, J.J.; González-Alted, C.; Bilbao, Á.; Croche, L.F.; Pérez-Rodríguez, M.; Bravo, S.; Bize, A. Daño Cerebral Adquirido. Guía de Actividades Físico-Deportivas; IMSERSO: Madrid, Spain, 2011. [Google Scholar]
- Marinho-Buzelli, A.R.; Bonnyman, A.M.; Verrier, M.C. The Effects of Aquatic Therapy on Mobility of Individuals with Neurological Diseases: A Systematic Review. Clin. Rehabil. 2015, 29, 741–751. [Google Scholar] [CrossRef]
- Hasan, S.; Rancourt, S.N.; Austin, M.W.; Ploughman, M. Defining Optimal Aerobic Exercise Parameters to Affect Complex Motor and Cognitive Outcomes after Stroke: A Systematic Review and Synthesis. Neural Plast. 2016, 2016, 2961573:1–2961573:12. [Google Scholar] [CrossRef]
- Cohen, J. Quantitative Methods in Psychology: A Power Primer. Psychol. Bull. 1992, 112, 1155–1159. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [CrossRef]
- Lorenz, L.S.; Charrette, A.L.; O’Neil-Pirozzi, T.M.; Doucett, J.; Fong, J. Healthy Body, Healthy Mind: A Mixed Methods Study of Outcomes, Barriers and Supports for Exercise by People Who Have Chronic Moderate-to-Severe Acquired Brain Injury. Disabil. Health J. 2018, 11, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Morris, T.P.; Tormos Muñoz, J.M.; Cattaneo, G.; Solana-Sànchez, J.; Bartrés-Faz, D.; Pascual-Leone, A. Traumatic Brain Injury Modifies the Relationship Between Physical Activity and Global and Cognitive Health: Results From the Barcelona Brain Health Initiative. Front. Behav. Neurosci. 2019, 13, 135:1–135:7. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de la Cruz, S. Influence of an Aquatic Therapy Program on Perceived Pain, Stress, and Quality of Life in Chronic Stroke Patients: A Randomized Trial. Int. J. Environ. Res. Public Health 2020, 17, 4796:1–4796:12. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.L.; Lee, S.M. Effects of Therapeutic Tai Chi on Balance, Gait, and Quality of Life in Chronic Stroke Patients. Int. J. Rehabil. Res. 2015, 38, 156–161. [Google Scholar] [CrossRef]
- Meester, D.; Al-Yahya, E.; Dennis, A.; Collett, J.; Wade, D.T.; Ovington, M.; Liu, F.; Meaney, A.; Cockburn, J.; Johansen-Berg, H.; et al. A Randomized Controlled Trial of a Walking Training with Simultaneous Cognitive Demand (Dual-Task) in Chronic Stroke. Eur. J. Neurol. 2019, 26, 435–441. [Google Scholar] [CrossRef]
- Weinstein, A.A.; Chin, L.M.K.; Collins, J.; Goel, D.; Keyser, R.E.; Chan, L. Effect of Aerobic Exercise Training on Mood in People With Traumatic Brain Injury: A Pilot Study. J. Head Trauma Rehabil. 2017, 32, E49–E56. [Google Scholar] [CrossRef]
- Wise, E.K.; Hoffman, J.M.; Powell, J.M.; Bombardier, C.H.; Bell, K.R. Benefits of Exercise Maintenance after Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2012, 93, 1319–1323. [Google Scholar] [CrossRef]
- Mala, H.; Rasmussen, C.P. The Effect of Combined Therapies on Recovery after Acquired Brain Injury: Systematic Review of Preclinical Studies Combining Enriched Environment, Exercise, or Task-Specific Training with Other Therapies. Restor. Neurol. Neurosci. 2017, 35, 25–64. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.D.; Wilks, R.; McCaw-Binns, A. Effect of Aerobic Exercise (Walking) Training on Functional Status and Health-Related Quality of Life in Chronic Stroke Survivors: A Randomized Controlled Trial. Stroke 2013, 44, 1179–1181. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, N.; Straudi, S.; Malagoni, A.M.; Argirò, M.; Felisatti, M.; Nardini, E.; Zambon, C.; Basaglia, N.; Manfredini, F. Effects of Low-Intensity Endurance and Resistance Training on Mobility in Chronic Stroke Survivors: A Pilot Randomized Controlled Study. Eur. J. Phys. Rehabil. Med. 2017, 53, 228–239. [Google Scholar] [PubMed]
- Nave, A.H.; Rackoll, T.; Grittner, U.; Bläsing, H.; Gorsler, A.; Nabavi, D.G.; Audebert, H.J.; Klosterman, F.; Müller-Werdan, U.; Steinhagen-Thiessen, E.; et al. Physical Fitness Training in Patients with Subacute Stroke (PHYS-STROKE): Multicentre, Randomised Controlled, Endpoint Blinded Trial. BMJ 2019, 366, l5101:1–l5101:15. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).