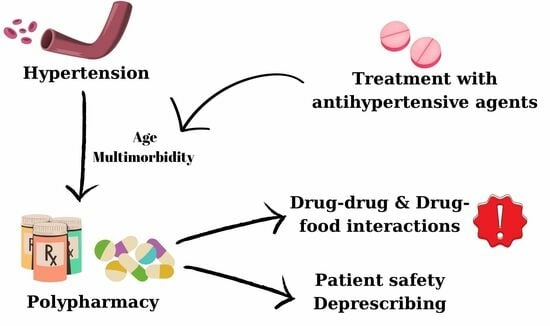
Polypharmacy in the Management of Arterial Hypertension—Friend or Foe?
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Participants
2.2. Data Sources
2.3. Variables of Interest
2.4. Statistics
3. Results
- -
- Amiodarone/atorvastatin/repaglinide–grapefruit/grapefruit juice (which can lead to an increase in the drug’s plasmatic concentration);
- -
- Metformin/rosiglitazone/sitagliptin–alcohol (risk of lactic acidosis);
- -
- Perindopril/valsartan/candesartan/ramipril–foods rich in potassium (risk of hyperkalemia);
- -
- Acenocoumarol–foods rich in vitamin K (in particular “beef liver, broccoli, Brussels sprouts, cabbage, lettuce, soy beans, spinach, watercress, and other green leafy vegetables”).
4. Discussion
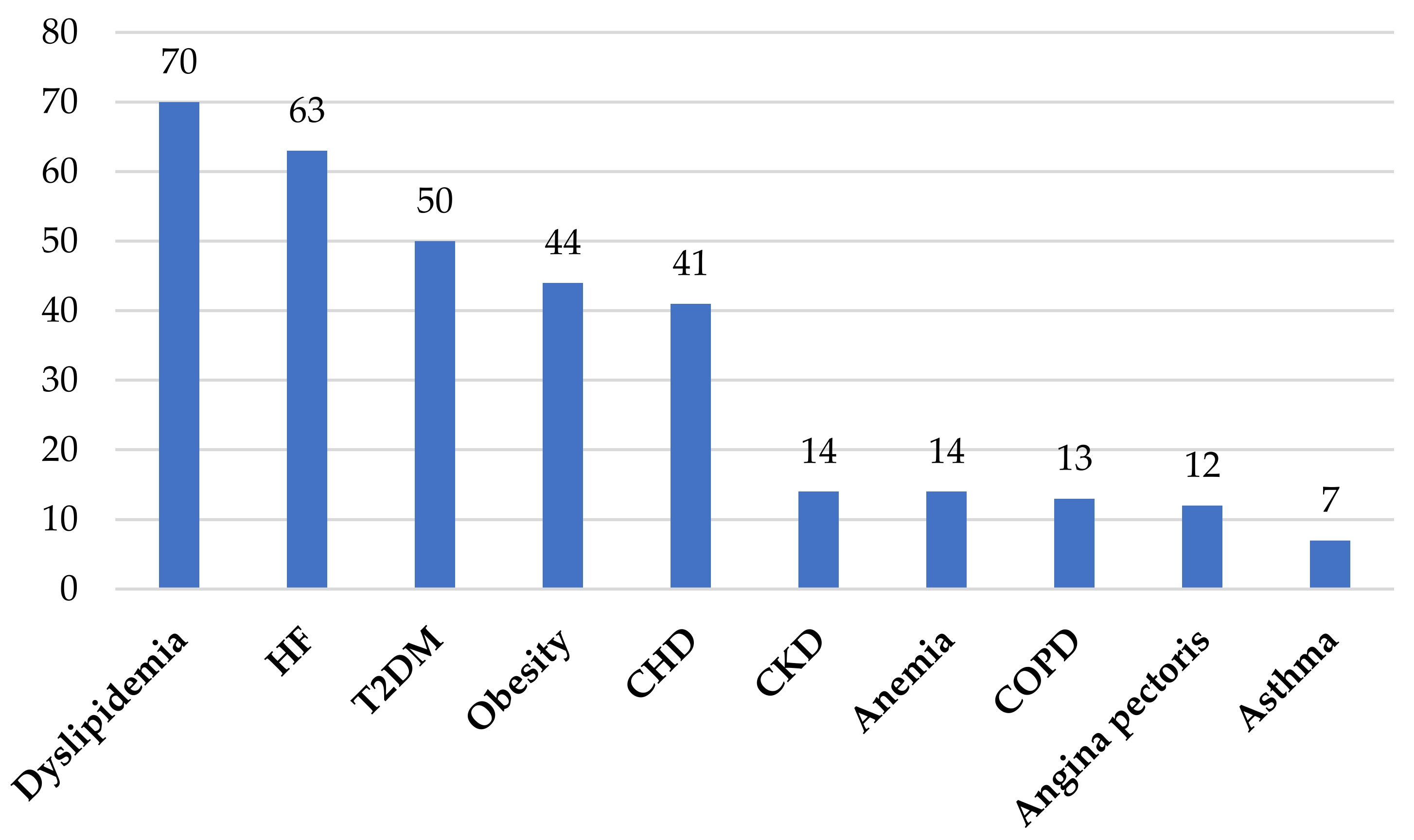
4.1. Comorbidities Frequently Associated with Hypertension
4.2. Polypharmacy in Patients with Hypertension
4.3. Antihypertensive Drugs Interactions (Drug–Drug and Food–Drug Interactions)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mills, K.T.; Stefanescu, A.; He, J. The Global Epidemiology of Hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies from 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J. Epidemiology of Hypertension. Clin. Queries Nephrol. 2013, 2, 56–61. [Google Scholar] [CrossRef]
- Reuter, H.; Jordan, J. Status of Hypertension in Europe. Curr. Opin. Cardiol. 2019, 34, 342–349. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Hypertension Fact Sheets. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 23 May 2021).
- Munger, M.A. Polypharmacy and Combination Therapy in the Management of Hypertension in Elderly Patients with Co-Morbid Diabetes Mellitus. Drugs Aging 2010, 27, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef] [PubMed]
- Dorobantu, M.; Bartos, D.; Apetrei, E.; Arsenescu-Georgescu, C.; Pop, D.; Ghiorghe, S.; Tanasescu, R.; Craiu, E.; Manitiu, I.; Tautu, O. Hypertension in Romania: Where are we and what can we do? Results from SEPHAR II study. Rom. J. Cardiol. 2021, 22, 285–292. [Google Scholar]
- Maher, R.L.; Hanlon, J.; Hajjar, E.R. Clinical Consequences of Polypharmacy in Elderly. Expert Opin. Drug Saf. 2014, 13, 57–65. [Google Scholar] [CrossRef]
- Riaz, M.H. Potential drug-drug interactions and strategies for their detection and prevention. Farmacia 2019, 67, 572–579. [Google Scholar] [CrossRef]
- Midão, L.; Giardini, A.; Menditto, E.; Kardas, P.; Costa, E. Polypharmacy Prevalence among Older Adults Based on the Survey of Health, Ageing and Retirement in Europe. Arch. Gerontol. Geriatr. 2018, 78, 213–220. [Google Scholar] [CrossRef]
- Mortazavi, S.S.; Shati, M.; Keshtkar, A.; Malakouti, S.K.; Bazargan, M.; Assari, S. Defining Polypharmacy in the Elderly: A Systematic Review Protocol. BMJ Open 2016, 6, e010989. [Google Scholar] [CrossRef] [PubMed]
- Kirchmayer, U.; Mayer, F.; Basso, M.; De Cristofaro, R.; Mores, N.; Cappai, G.; Agabiti, N.; Fusco, D.; Davoli, M.; Gambassi, G. Polypharmacy in the Elderly: A Population Based Cross-Sectional Study in Lazio, Italy. Eur. Geriatr. Med. 2016, 7, 484–487. [Google Scholar] [CrossRef]
- Crismaru, I.; Pantea Stoian, A.; Bratu, O.G.; Găman, M.A.; Stanescu, A.M.A.; Bacalbasa, N.; Diaconu, C.C. Low-density lipoprotein cholesterol lowering treatment: The current approach. Lipids Health Dis. 2020, 19, 85. [Google Scholar] [CrossRef] [PubMed]
- Parulekar, M.S.; Rogers, C.K. Polypharmacy and Mobility. In Geriatric Rehabilitation; Cifu, D.X., Lew, H.L., Oh-Park, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–129. [Google Scholar]
- Lalic, S.; Sluggett, J.K.; Ilomäki, J.; Wimmer, B.C.; Tan, E.C.K.; Robson, L.; Emery, T.; Bell, J.S. Polypharmacy and Medication Regimen Complexity as Risk Factors for Hospitalization among Residents of Long-Term Care Facilities: A Prospective Cohort Study. J. Am. Med. Dir. Assoc. 2016, 17, 1067.e1–1067.e6. [Google Scholar] [CrossRef]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What Is Polypharmacy? A Systematic Review of Definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Bohm, M. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [PubMed]
- Drug Interactions Checker. Available online: https://www.drugs.com/drug_interactions.html (accessed on 23 May 2021).
- Thomas, F.; Bean, K.; Guize, L.; Quentzel, S.; Argyriadis, P.; Benetos, A. Combined effects of systolic blood pressure and serum cholesterol on cardiovascular mortality in young (<55 years) men and women. Eur. Heart J. 2002, 23, 528–535. [Google Scholar]
- Málek, F. Arterial hypertension and chronic heart failure. Cor Et Vasa 2013, 55, e259–e263. [Google Scholar] [CrossRef]
- He, J.; Ogden, L.G.; Bazzano, L.A.; Vupputuri, S.; Loria, C.; Whelton, P.K. Risk factors for congetive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch. Intern. Med. 2001, 161, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Sowers, J.J.R. Diabetes mellitus and vascular disease. Hypertension 2013, 61, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Kim, H.C.; Shin, A.; Yeom, H.; Jang, S.-Y.; Lee, J.H.; Kim, C.; Suh, I. Prevalence of comorbidity among people with hypertension: The Korea National Health and Nutrition Examination Survey 2007–2013. Korean Circ. J. 2016, 46, 672–680. [Google Scholar] [CrossRef]
- Pavlou, D.I.; Paschou, S.A.; Anagnostis, P.; Spartalis, M.; Spartalis, E.; Vryonidou, A.; Tentolouris, N.; Siasos, G. Hypertension in patients with type 2 diabetes mellitus: Targets and management. Maturitas 2018, 112, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Schumacher, H.; Redon, J.; Verdecchia, P.; Schmieder, R.; Jennings, G.; Yusoff, K.; Ryden, L.; Liu, G.L.; Teo, K.; et al. Blood pressure targets recommended by guidelines and incidence of cardiovascular and renal events in the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET). Circulation 2011, 124, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef]
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharmacol. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef]
- The Long-term Intervention with Pravastatin in Ischemic Disease (LIPID) study group. Prevention of cardiovascular events and death with pravastatin inpatients with coronary heart disease and a broad range of initial cholesterol levels. N. Engl. J. Med. 1998, 339, 1349–1357. [Google Scholar] [CrossRef]
- Escobar, E. Hypertension and coronary heart disease. J. Hum. Hypertens. 2002, 16, S61–S63. [Google Scholar] [CrossRef]
- Phan, O.; Burnier, M.; Wuerzner, G. Hypertension in chronic kidney disease—Role of arterial calcification and impact on treatment. Eur. Cardiol. 2014, 9, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Goodman, W.G.; Goldin, J.; Kuizon, B.D.; Yoon, C.; Gales, B.; Sider, D.; Wang, Y.; Chung, J.; Emerick, A.; Greaser, L.; et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N. Engl. J. Med. 2000, 342, 1478–1483. [Google Scholar] [CrossRef]
- Wallace, K.; Zhao, X.; Misra, R.; Sambamoorthi, U. The humanistic and economic burden associated with anxiety and depression among adults with comorbid diabetes and hypertension. J. Diabetes Res. 2018, 2018, 4842520. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.M. Anxiety and hypertension: Is there a link? A literature review of the comorbidity relationship between anxiety and hypertension. Curr. Hypertens. Rep. 2019, 21, 66. [Google Scholar] [CrossRef] [PubMed]
- Khezrian, M.; McNeil, C.J.; Murray, A.D.; Myint, P.K. An overview of prevalence, determinants and health outcomes of polypharmacy. Ther. Adv. Drug Saf. 2020, 11, 2042098620933741. [Google Scholar] [CrossRef] [PubMed]
- Marengoni, A.; Winblad, B.; Karp, A.; Fratiglioni, L. Prevalence of chronic diseases and multimorbidity among the elderly population in Sweden. Am. J. Public Health 2008, 98, 1198–1200. [Google Scholar]
- Tragni, E.; Sala, F.; Casula, M. Elders with multiple chronic conditions: Epidemiology and drug use. G Ital. Farm. Farm. 2014, 6, 5–14. [Google Scholar]
- Vrettos, I.; Voukelatou, P.; Katsoras, A.; Theotoka, D.; Kalliakmanis, A. Diseases linked to polypharmacy in elderly patients. Curr. Gerontol. Geriatr. Res. 2017, 2017, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bueno, D.R.; Monteiro, H.L.; Rosa, C.S.C.; Codogno, J.S.; Fernandes, R.A.; Marucci, M.F.N. Associação entre níveis de atividade física e polifarmácia em pacientes hipertensos. Medicina 2016, 49, 240. [Google Scholar] [CrossRef][Green Version]
- Santos, A.N.M.D.; Nogueira, D.R.C.; Gutierrez, B.A.O.; Chubaci, R.Y.S.; de Borja Oliveira, C.R. Cardiometabolic diseases and active aging-polypharmacy in control. Rev. Bras. Enferm. 2020, 73, e20180324. [Google Scholar] [CrossRef]
- Beckett, N.S.; Peters, R.; Fletcher, A.E. Treatment of hy pertension in patients 80 years of age or older. N. Engl. J. Med. 2008, 358, 1887–1898. [Google Scholar] [CrossRef]
- Benetos, A.; Labat, C.; Rossignol, P.; Fay, R.; Rolland, Y.; Valbusa, F.; Salvi, P.; Zamboni, M.; Manckoundia, P.; Hanon, O.; et al. Treatment with multiple blood pressure medications, achieved blood pressure, and mortality in older nursing home residents: The PARTAGE study. JAMA Intern. Med. 2015, 175, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: Final results of the systolic hypertension in the elderly program (SHEP). JAMA 1991, 265, 3255. [CrossRef]
- Piccoliori, G.; Mahlknecht, A.; Sandri, M.; Valentini, M.; Vögele, A.; Schmid, S.; Deflorian, F.; Engl, A.; Sönnichsen, A.; Wiedermann, C. Epidemiology and associated factors of polypharmacy in older patients in primary care: A northern Italian cross-sectional study. BMC Geriatr. 2021, 21, 197. [Google Scholar] [CrossRef] [PubMed]
- Burnier, M.; Polychronopoulou, E.; Wuerzner, G. Hypertension and drug adherence in the elderly. Front. Cardiovasc. Med. 2020, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Adhimoolam, M.; Kannan, S. Study of drug–Drug interactions among the hypertensive patients in a tertiary care teaching hospital. Perspect Clin. Res. 2018, 9, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Osterhoudt, K.C.; Penning, T.M. Chapter 4: Drug Toxicity and Poisoning. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutic; McGraw-Hill Medical: New York, NY, USA, 2011; p. 12e. [Google Scholar]
- Becker, M.L.; Kallewaard, M.; Caspers, P.W.J.; Visser, L.E.; Leufkens, H.G.M.; Stricker, B.H.C. Hospitalisations and emergency department visits due to drug-drug interactions: A literature review. Pharmacoepidemiol. Drug Saf. 2007, 16, 641–651. [Google Scholar] [CrossRef]
- Patel, P.S.; Rana, D.A.; Suthar, J.V.; Malhotra, S.D.; Patel, V.J. A study of potential adverse drug-drug interactions among prescribed drugs in medicine outpatient department of a tertiary care teaching hospital. J. Basic Clin. Pharm. 2014, 5, 44–48. [Google Scholar]
- Kothari, N.; Ganguly, B. Potential drug-drug interactions among medications prescribed to hypertensive patients. J. Clin. Diagn. Res. 2014, 8, HC01–HC04. [Google Scholar] [CrossRef] [PubMed]
- Chelkeba, L.; Alemseged, F.; Bedada, W. Assessment of potential drug-drug interactions among outpatients receiving cardiovascular medications at Jimma University specialized hospital, South West Ethiopia. Int. J. Basic Clin. Pharmacol. 2013, 2, 144–152. [Google Scholar] [CrossRef]
- Marquito, A.B.; Fernandes, N.M.D.S.; Colugnati, F.A.B.; de Paula, R.B. Identifying potential drug interactions in chronic kidney disease patients. J. Bras. Nefrol. 2014, 36, 26–34. [Google Scholar] [CrossRef]
- Wrenger, E.; Müller, R.; Moesenthin, M.; Welte, T.; Frölich, J.C.; Neumann, K.H. Interaction of spironolactone with ACE inhibitors or angiotensin receptor blockers: Analysis of 44 cases. BMJ 2003, 327, 147–149. [Google Scholar] [CrossRef]
- Goineau, S.; Pape, D.; Guillo, P.; Ramée, M.-P.; Bellissant, E. Combined effects of metoprolol and spironolactone in dilated cardiomyopathic hamsters. J. Cardiovasc. Pharmacol. 2002, 40, 543–553. [Google Scholar] [CrossRef]
- Patibandla, S.; Heaton, J.; Kyaw, H. Spironolactone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ilieşiu, A.M.; Cordoș, I.; Pârvu, I.; Verde, I.; Liteanu, A.S.; Hodorogea, A.S.; Rădăvoi, G.D. Antihypertensive drugs and blood pressure variability. Farmacia 2021, 69, 200–207. [Google Scholar] [CrossRef]
- May, M.; Schindler, C. Clinically and pharmacologically relevant interactions of antidiabetic drugs. Ther. Adv. Endocrinol. Metab. 2016, 7, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Ayo, J.A.; Agu, H.; Madaki, I. Food and drug interactions: Its side effects. Nutr. Food Sci. 2005, 35, 243–252. [Google Scholar] [CrossRef]
- Dragoi, C.M.; Morosan, E.; Dumitrescu, I.B.; Nicolae, A.C.; Arsene, A.L.; Draganescu, D.; Lupuliasa, D.; Ionita, A.C.; Stoian, A.P.; Nicolae, C.; et al. Insights into chrononutrition: The innermost interplay amongst nutrition, metabolism and the circadian clock, in the context of epigenetic reprogramming. Farmacia 2019, 67, 4. [Google Scholar] [CrossRef]
- Nekvindová, J.; Anzenbacher, P. Interactions of food and dietary supplements with drug metabolizing cytochrome P450 enzymes. Ceska Slov Farm. 2007, 56, 165–173. [Google Scholar]
- Katzung, B.G. Basic and Clinical Pharmacology, 10th ed.; McGraw-Hill Medical: New York, NY, USA, 2006. [Google Scholar]
- Bushra, R.; Aslam, N.; Khan, A.Y. Food-drug interactions. Oman Med. J. 2011, 26, 77–83. [Google Scholar] [CrossRef]
- Uesawa, Y.; Mohri, K. Hesperidin in orange juice reduces the absorption of celiprolol in rats. Biopharm. Drug Dispos. 2008, 29, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Størmer, F.C.; Reistad, R.; Alexander, J. Glycyrrhizic acid in liquorice—Evaluation of health hazard. Food Chem. Toxicol. 1993, 31, 303–312. [Google Scholar] [CrossRef]
- Găman, M.A.; Dobrică, E.C.; Cozma, M.A.; Antonie, N.I.; Stănescu, A.M.A.; Găman, A.M.; Diaconu, C.C. Crosstalk of Magnesium and Serum Lipids in Dyslipidemia and Associated Disorders: A Systematic Review. Nutrients 2021, 13, 1411. [Google Scholar] [CrossRef]
- Błeszyńska, E.; Wierucki, Ł.; Zdrojewski, T.; Renke, M. Pharmacological Interactions in the Elderly. Medicina 2020, 56, 320. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Moreno, J.A.; Navarro-Rodríguez, S.A.; Ruiz-Pérez, S.; Godínez-Reyes, J.C.; Mendoza-Rojo, M. Hypertension Awareness, Treatment, and Control in Mexico: An Opportunistic Medical Student-led Blood Pressure Screening Campaign—A Cross-Sectional Study. Int. J. Med. Students 2020, 8, 263–272. [Google Scholar] [CrossRef]
- Monkman, H.; Kushniruk, A.; Borycki, E.; Sheets, D.; Barnett, J.; Nøhr, C. The Medium Is the Message: How Do Canadian University Students Want Digital Medication Information? Life 2020, 10, 339. [Google Scholar] [CrossRef] [PubMed]
| Drug Class | Percentage (%) | Number of Patients |
|---|---|---|
| Stage 1 | 6.62% | 11 |
| Stage 2 | 39.76% | 66 |
| Stage 3 | 43.38% | 72 |
| Unspecified | 10.24% | 17 |
| Total | 100.00% | 166 |
| Drug Class | Percentage (%) | Number of Patients |
|---|---|---|
| Intermediate | 30.12% | 50 |
| High | 25.90% | 43 |
| Very high | 37.96% | 63 |
| Unspecified | 6.02% | 10 |
| Total | 100.00% | 166 |
| Drug Class | Type of Drug | Number of Patients |
|---|---|---|
| Diuretics (n = 126) | Furosemide | 47 |
| Indapamide | 38 | |
| Spironolactone | 36 | |
| Hydrochlorothiazide | 5 | |
| Beta-blockers (n = 94) | Metoprolol | 45 |
| Nebivolol | 21 | |
| Carvedilol | 14 | |
| Bisoprolol | 14 | |
| Angiotensin-converting-enzyme (ACE) inhibitors (n = 82) | Perindopril | 57 |
| Ramipril | 14 | |
| Enalapril | 5 | |
| Captopril | 2 | |
| Lisinopril | 2 | |
| Trandolapril | 2 | |
| Calcium channel blockers (n = 67) | Amlodipine | 50 |
| Diltiazem | 8 | |
| Lercanidipine | 5 | |
| Felodipine | 2 | |
| Verapamil | 1 | |
| Nifedipine | 1 | |
| Angiotensin receptor blockers (n = 46) | Candesartan | 22 |
| Olmesartan | 16 | |
| Irbersartan | 4 | |
| Valsartan | 3 | |
| Telmisartan | 1 |
| Drug Class | Number | Percentage |
|---|---|---|
| Statins | 89 | 53.6% |
| Antiplatelet agents | 69 | 41.5% |
| Proton pump inhibitors | 66 | 39.7% |
| Anticoagulants | 51 | 30.7% |
| Oral antidiabetics | 30 | 18.0% |
| Antianginal agents | 28 | 16.8% |
| Vitamins and minerals | 24 | 14.4% |
| Antibiotics | 21 | 12.6% |
| Insulin | 7 | 4.2% |
| Parameter [per Patient] | Hypertensive | Normotensive | p-Value |
|---|---|---|---|
| Age [years] | 68.46 ± 12.70 | 67.82 ± 14.56 | 0.72 |
| Comorbidities | 9.13 ± 3.52 | 7.90 ± 3.82 | 0.01 |
| Prescribed drugs | 6.72 ± 2.58 | 5.74 ± 3.18 | 0.01 |
| Drug–drug interactions | 6.55 ± 5.82 | 4.93± 5.59 | 0.03 |
| Minor drug–drug interactions | 1.25 ± 1.50 | 1.08 ±1.84 | 0.46 |
| Moderate drug–drug interactions | 4.94 ± 4.75 | 3.54 ± 4.17 | 0.02 |
| Major drug–drug interactions | 0.44 ± 0.77 | 0.37 ±0.73 | 0.52 |
| Food–drug interactions | 2.64 ± 1.29 | 2.02 ± 1.73 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaconu, C.C.; Cozma, M.-A.; Dobrică, E.-C.; Gheorghe, G.; Jichitu, A.; Ionescu, V.A.; Nicolae, A.C.; Drăgoi, C.M.; Găman, M.-A. Polypharmacy in the Management of Arterial Hypertension—Friend or Foe? Medicina 2021, 57, 1288. https://doi.org/10.3390/medicina57121288
Diaconu CC, Cozma M-A, Dobrică E-C, Gheorghe G, Jichitu A, Ionescu VA, Nicolae AC, Drăgoi CM, Găman M-A. Polypharmacy in the Management of Arterial Hypertension—Friend or Foe? Medicina. 2021; 57(12):1288. https://doi.org/10.3390/medicina57121288
Chicago/Turabian StyleDiaconu, Camelia Cristina, Matei-Alexandru Cozma, Elena-Codruța Dobrică, Gina Gheorghe, Alexandra Jichitu, Vlad Alexandru Ionescu, Alina Crenguța Nicolae, Cristina Manuela Drăgoi, and Mihnea-Alexandru Găman. 2021. "Polypharmacy in the Management of Arterial Hypertension—Friend or Foe?" Medicina 57, no. 12: 1288. https://doi.org/10.3390/medicina57121288
APA StyleDiaconu, C. C., Cozma, M.-A., Dobrică, E.-C., Gheorghe, G., Jichitu, A., Ionescu, V. A., Nicolae, A. C., Drăgoi, C. M., & Găman, M.-A. (2021). Polypharmacy in the Management of Arterial Hypertension—Friend or Foe? Medicina, 57(12), 1288. https://doi.org/10.3390/medicina57121288