Vitamin D Enhanced the Osteogenic Differentiation of Cell Spheroids Composed of Bone Marrow Stem Cells
Abstract
1. Introduction
2. Materials and Methods
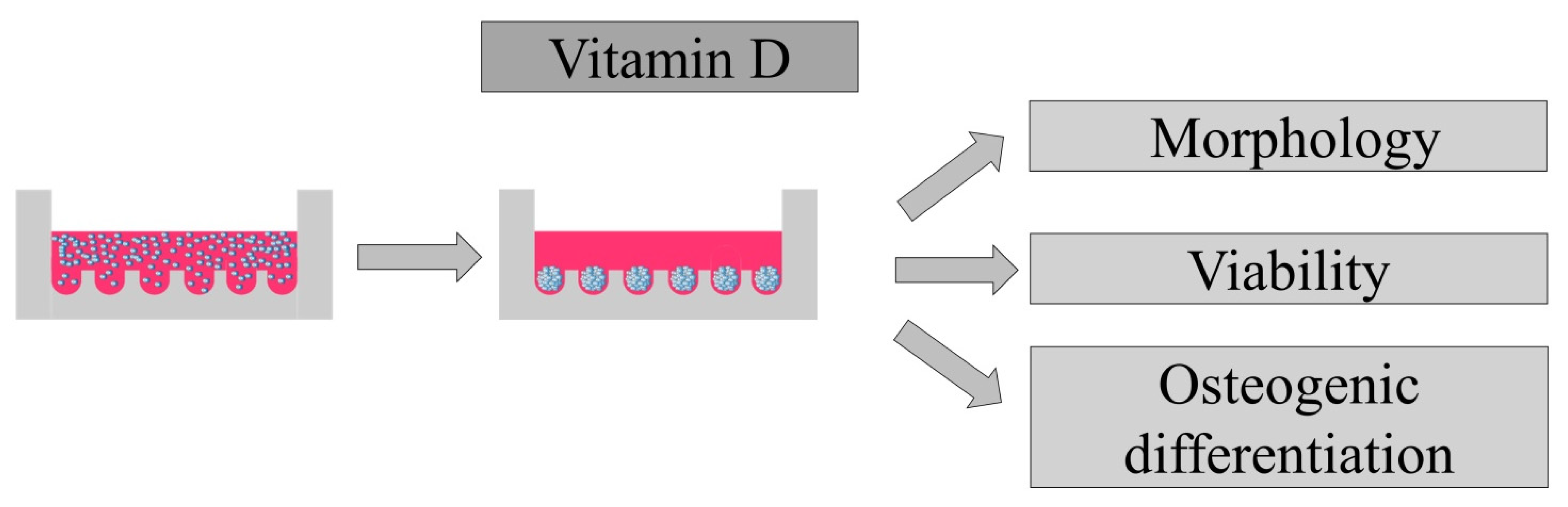
2.1. Design of the Present Study
2.2. Fabrication of Stem Cell Spheroids
2.3. Determination of Qualitative and Quantitative Cell Viability
2.4. Evaluation of Alkaline Phosphatase Activity
2.5. Total RNA Extraction and Quantification of RUNX2, BSP, OCN and COL1A1 mRNA by Real-Time Quantitative Polymerase Chain Reaction (qPCR)
2.6. Statistical Analysis
3. Results
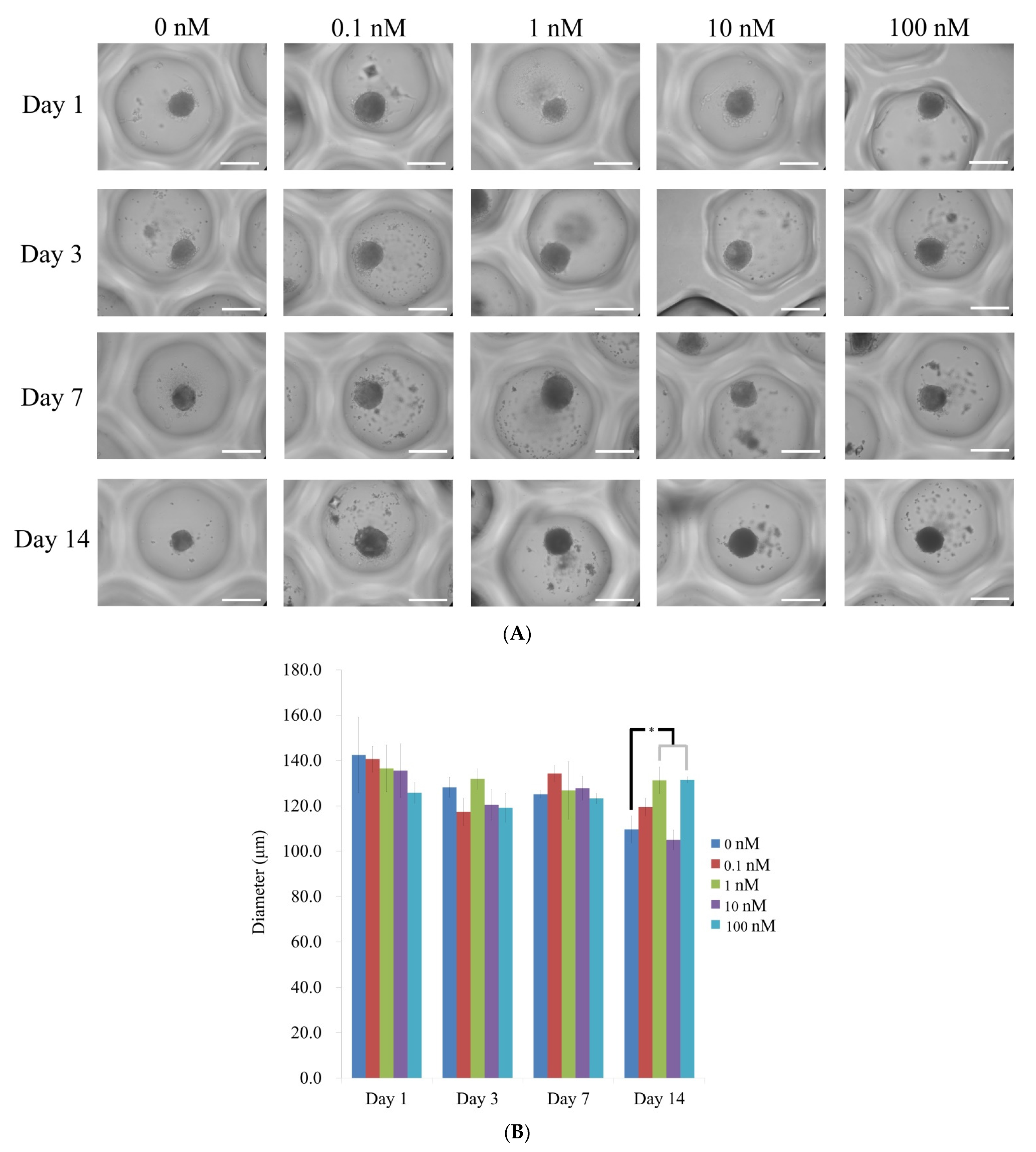
3.1. Cell Spheroids of Human Bone Marrow-Derived Mesenchymal Stem Cells
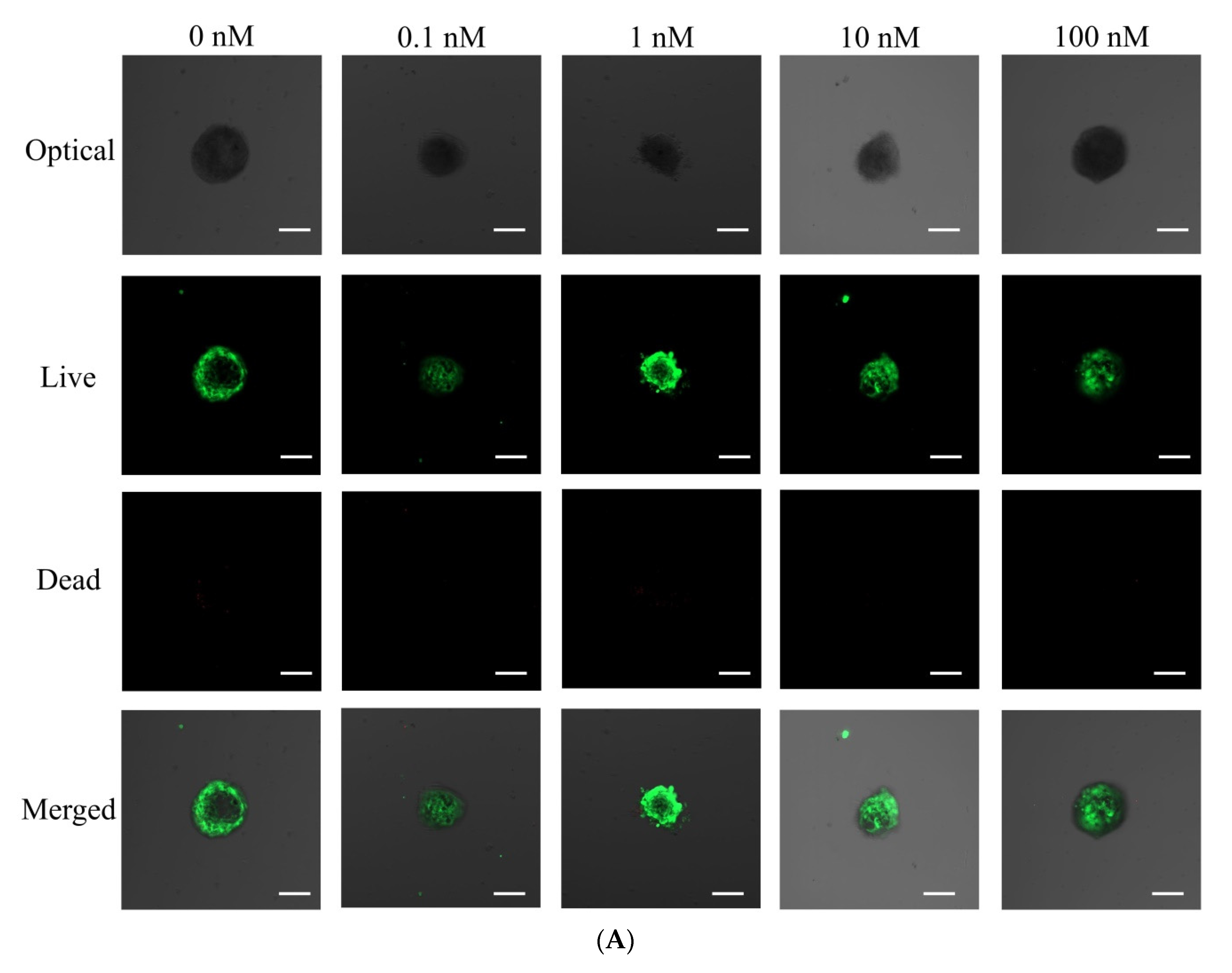
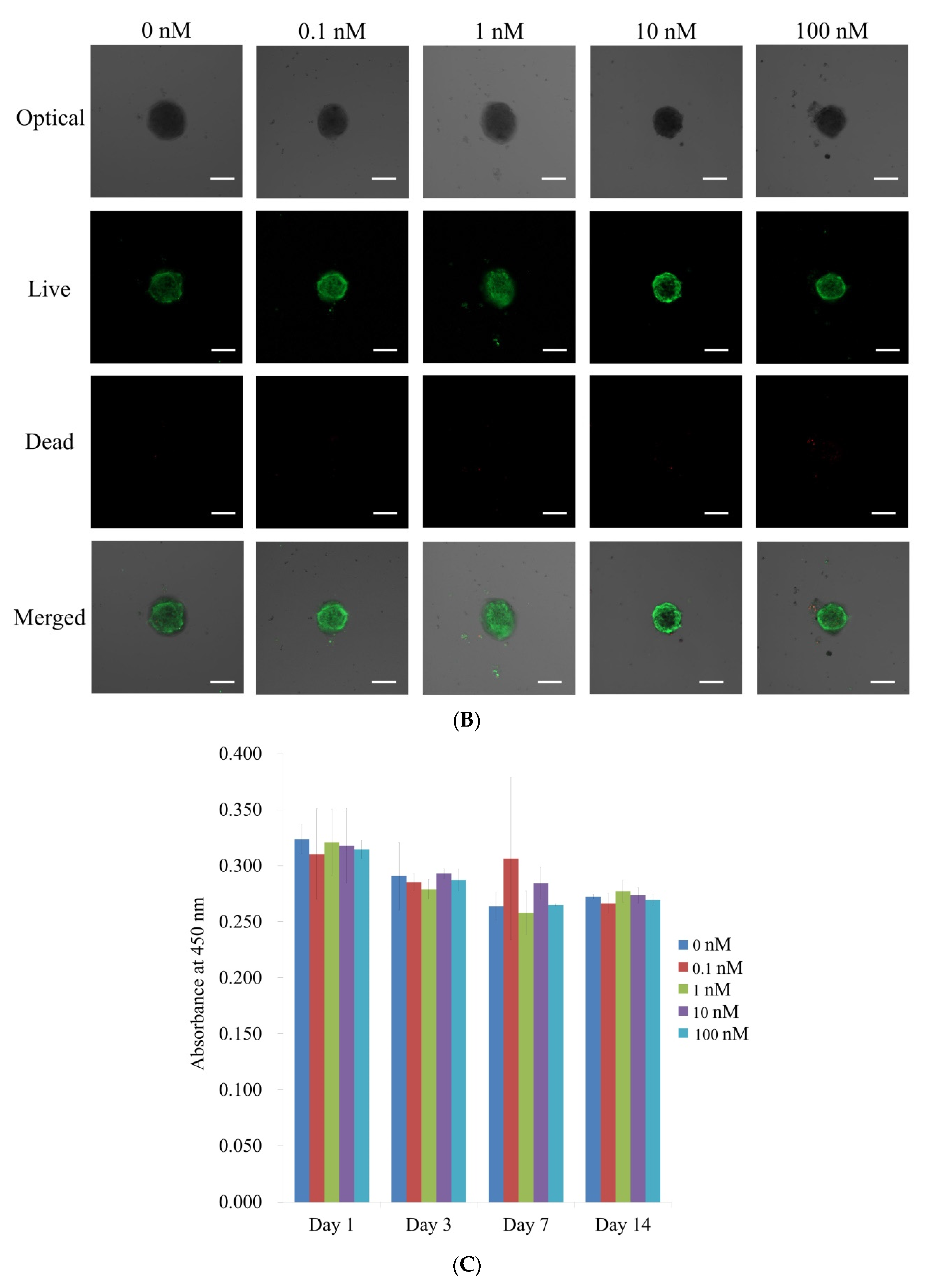
3.2. Qualitative Determination and Quantitative Values for Cell Viability
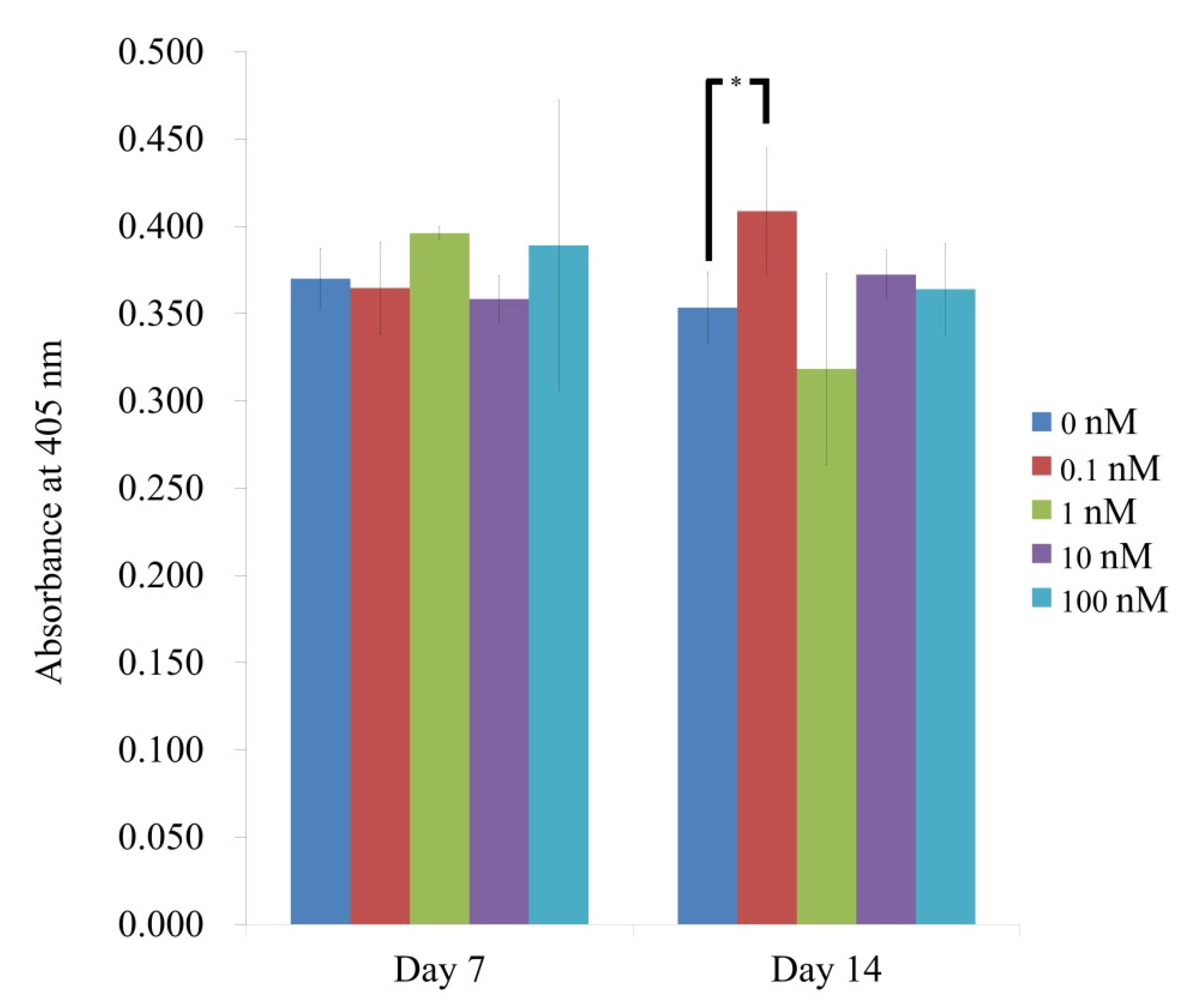
3.3. Evaluation of Alkaline Phosphatase Activity
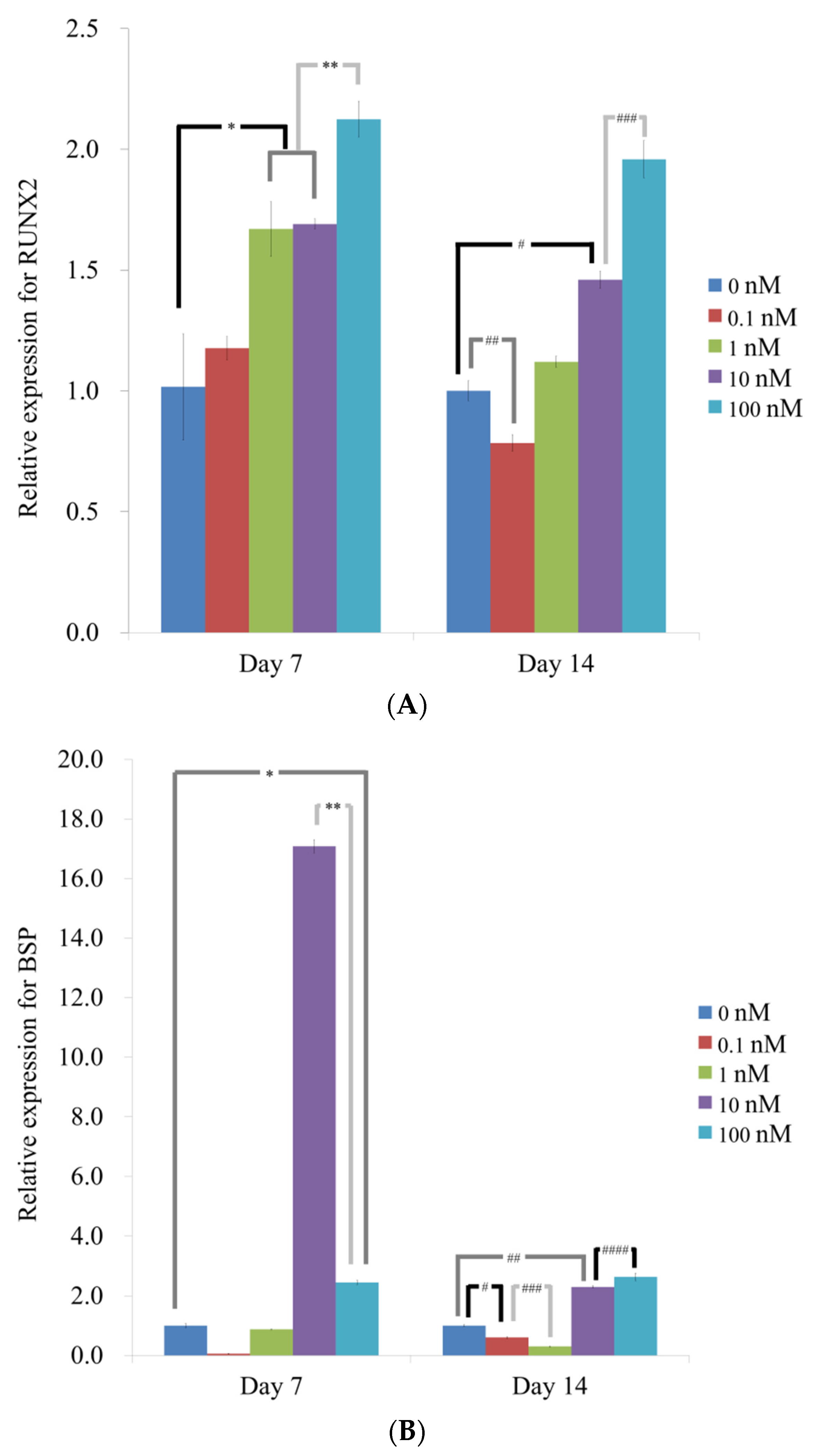
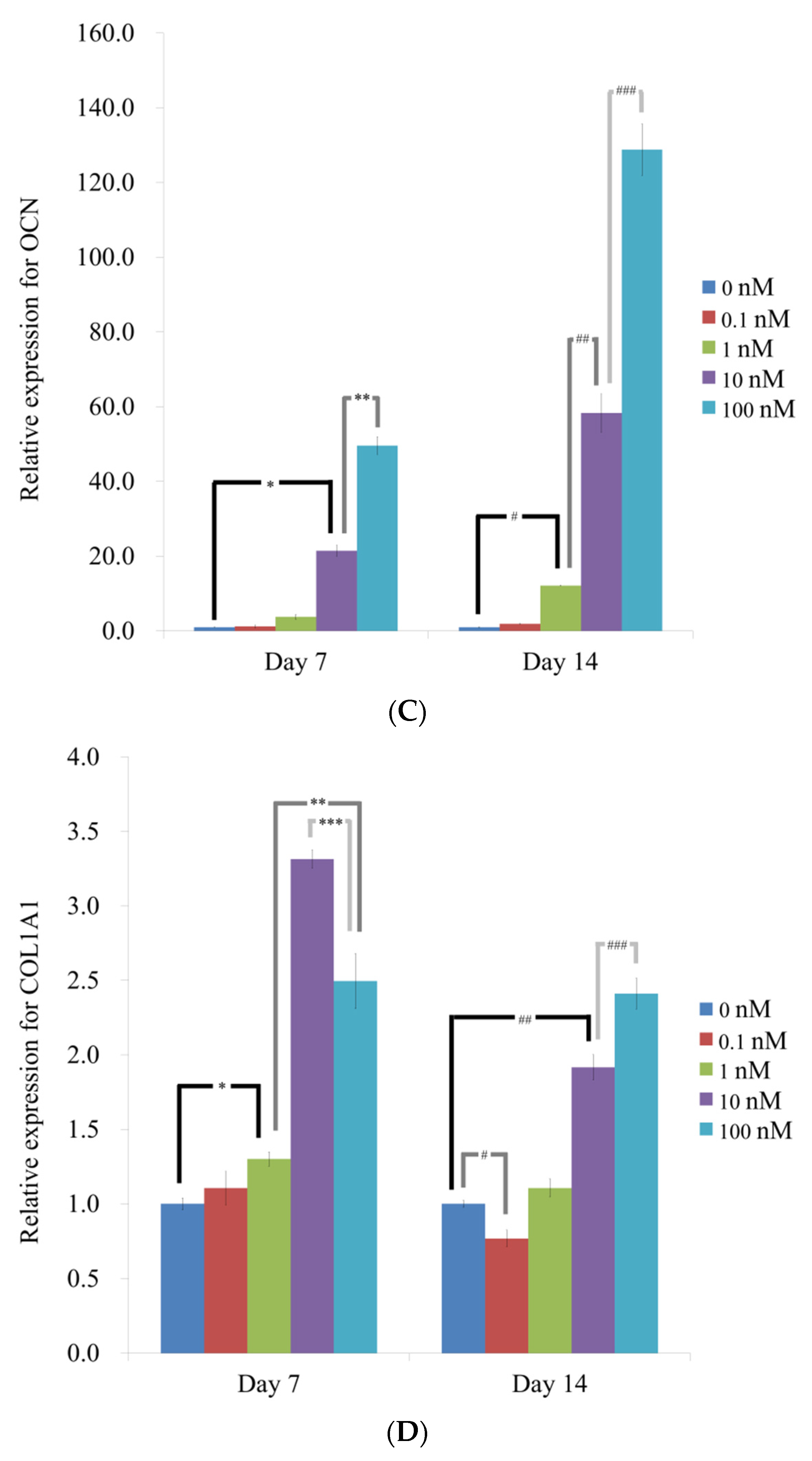
3.4. Evaluation of RUNX2, BSP, OCN, and COL1A1 by qPCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, A.A.; Kheur, S.; Badhe, R.V.; Raj, A.T.; Bhonde, R.; Jaisinghani, A.; Vyas, N.; Patil, V.R.; Alhazmi, Y.A.; Parveen, S.; et al. Assessing the potential use of chitosan scaffolds for the sustained localized delivery of vitamin D. Saudi J. Biol. Sci. 2021, 28, 2210–2215. [Google Scholar] [CrossRef]
- Costantini, E.; Sinjari, B.; Piscopo, F.; Porreca, A.; Reale, M.; Caputi, S.; Murmura, G. Evaluation of Salivary Cytokines and Vitamin D Levels in Periodontopathic Patients. Int. J. Mol. Sci. 2020, 21, 2669. [Google Scholar] [CrossRef]
- Lieberherr, M. Effects of vitamin D3 metabolites on cytosolic free calcium in confluent mouse osteoblasts. J. Biol. Chem. 1987, 262, 13168–13173. [Google Scholar] [CrossRef]
- Oshima, J.; Watanabe, M.; Hirosumi, J.; Orimo, H. 1,25(OH)2D3 increases cytosolic Ca++ concentration of osteoblastic cells, clone MC3T3-E1. Biochem. Biophys. Res. Commun. 1987, 145, 956–960. [Google Scholar] [CrossRef]
- Aoshima, Y.; Mizobuchi, M.; Ogata, H.; Kumata, C.; Nakazawa, A.; Kondo, F.; Ono, N.; Koiwa, F.; Kinugasa, E.; Akizawa, T. Vitamin D receptor activators inhibit vascular smooth muscle cell mineralization induced by phosphate and TNF-α. Nephrol. Dial. Transplant. 2012, 27, 1800–1806. [Google Scholar] [CrossRef]
- Metzger, W.; Schimmelpfennig, L.; Schwab, B.; Sossong, D.; Dorst, N.; Bubel, M.; Görg, A.; Pütz, N.; Wennemuth, G.; Pohlemann, T.; et al. Expansion and differentiation of human primary osteoblasts in two- and three-dimensional culture. Biotech. Histochem. 2013, 88, 86–102. [Google Scholar] [CrossRef]
- Schröder, M.; Riksen, E.A.; He, J.; Skallerud, B.H.; Møller, M.E.; Lian, A.M.; Syversen, U.; Reseland, J.E. Vitamin K2 Modulates Vitamin D-Induced Mechanical Properties of Human 3D Bone Spheroids In Vitro. JBMR Plus 2020, 4, e10394. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Hyzy, S.L.; Boyan, B.D.; Schwartz, Z. Regulation of Osteoblast Differentiation by Acid-Etched and/or Grit-Blasted Titanium Substrate Topography Is Enhanced by 1,25(OH)2D3 in a Sex-Dependent Manner. Biomed. Res. Int. 2015, 2015, 365014. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Navarrete, R.; Sutha, K.; Hyzy, S.L.; Hutton, D.L.; Schwartz, Z.; McDevitt, T.; Boyan, B.D. Osteogenic differentiation of stem cells alters vitamin D receptor expression. Stem Cells Dev. 2012, 21, 1726–1735. [Google Scholar] [CrossRef]
- Shao, B.Y.; Wang, L.; Yu, Y.; Chen, L.; Gan, N.; Huang, W.M. Effects of CD4(+) T lymphocytes from ovariectomized mice on bone marrow mesenchymal stem cell proliferation and osteogenic differentiation. Exp. Ther. Med. 2020, 20, 84. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.; Long, L.; Xie, K.; Liu, J.; Zhou, L.; Pan, S.; Liang, J.; Tu, Z.; Gao, Z.; Tang, Y. miRNA-429 suppresses osteogenic differentiation of human adipose-derived mesenchymal stem cells under oxidative stress via targeting SCD-1. Exp. Ther. Med. 2020, 19, 696–702. [Google Scholar] [CrossRef]
- Petrenko, Y.; Syková, E.; Kubinová, Š. The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res. Ther. 2017, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Ko, Y.; Park, J.B. Evaluation of the maintenance of stemness, viability, and differentiation potential of gingiva-derived stem-cell spheroids. Exp. Ther. Med. 2017, 13, 1757–1764. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Min, S.K.; Kim, M.; Park, J.B. Insulin-like growth factor 2-enhanced osteogenic differentiation of stem cell spheroids by regulation of Runx2 and Col1 expression. Exp. Ther. Med. 2021, 21, 383. [Google Scholar] [CrossRef] [PubMed]
- Caputi, S.; Trubiani, O.; Sinjari, B.; Trofimova, S.; Diomede, F.; Linkova, N.; Diatlova, A.; Khavinson, V. Effect of short peptides on neuronal differentiation of stem cells. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419828613. [Google Scholar] [CrossRef]
- Rexhepi, I.; Paolantonio, M.; Romano, L.; Serroni, M.; Santamaria, P.; Secondi, L.; Paolantonio, G.; Sinjari, B.; De Ninis, P.; Femminella, B. Efficacy of inorganic bovine bone combined with leukocyte and platelet-rich fibrin or collagen membranes for treating unfavorable periodontal infrabony defects: Randomized non-inferiority trial. J. Periodontol. 2021. [Google Scholar] [CrossRef]
- Rastegar, A.; Mahmoodi, M.; Mirjalili, M.; Nasirizadeh, N. Platelet-rich fibrin-loaded PCL/chitosan core-shell fibers scaffold for enhanced osteogenic differentiation of mesenchymal stem cells. Carbohydr. Polym. 2021, 269, 118351. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, H.; Na, C.B.; Song, I.S.; Ryu, J.J.; Park, J.B. Evaluation of the Age- and Sex-Related Changes of the Osteogenic Differentiation Potentials of Healthy Bone Marrow-Derived Mesenchymal Stem Cells. Medicina 2021, 57, 520. [Google Scholar] [CrossRef]
- Lee, H.; Park, J.B. Dimethyl sulfoxide leads to decreased osteogenic differentiation of stem cells derived from gingiva via Runx2 and Collagen I expression. Eur. J. Dent. 2019, 13, 131–136. [Google Scholar] [CrossRef]
- Kang, S.H.; Park, J.B.; Kim, I.; Lee, W.; Kim, H. Assessment of stem cell viability in the initial healing period in rabbits with a cranial bone defect according to the type and form of scaffold. J. Periodontal Implant Sci. 2019, 49, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.B.; Tae, J.Y.; Ko, Y.; Park, J.B. Lovastatin increases the proliferation and osteoblastic differentiation of human gingiva-derived stem cells in three-dimensional cultures. Exp. Ther. Med. 2019, 18, 3425–3430. [Google Scholar] [CrossRef]
- Lee, H.; Son, J.; Min, S.K.; Na, C.B.; Yi, G.; Koo, H.; Park, J.B. A study of the effects of doxorubicin-containing liposomes on osteogenesis of 3D stem cell spheroids derived from gingiva. Materials 2019, 12, 2693. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.; Na, C.B.; Park, J.B. The effects of simvastatin on cellular viability, stemness and osteogenic differentiation using 3-dimensional cultures of stem cells and osteoblast-like cells. Adv. Clin. Exp. Med. 2019, 28, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Min, S.K.; Kim, M.; Park, J.B. Bone morphogenetic protein 2-enhanced osteogenic differentiation of stem cell spheres by regulation of Runx2 expression. Exp. Ther. Med. 2020, 20, 79. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Tae, J.Y.; Min, S.K.; Ko, Y.; Park, J.B. Fibroblast growth factor-4 maintains cellular viability while enhancing osteogenic differentiation of stem cell spheroids in part by regulating RUNX2 and BGLAP expression. Exp. Ther. Med. 2020, 20, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, Y.; Li, C.; Jiang, Q.; Zhu, L. Effect of TNF-α on the proliferation and osteogenesis of human periodontal mesenchymal stem cells. Exp. Ther. Med. 2021, 21, 434. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Bolland, M.J.; Grey, A. Effects of vitamin D supplements on bone mineral density: A systematic review and meta-analysis. Lancet 2014, 383, 146–155. [Google Scholar] [CrossRef]
- DeLuca, H.F. The metabolism and functions of vitamin D. Adv. Exp. Med. Biol. 1986, 196, 361–375. [Google Scholar]
- Fischer, V.; Haffner-Luntzer, M.; Prystaz, K.; Vom Scheidt, A.; Busse, B.; Schinke, T.; Amling, M.; Ignatius, A. Calcium and vitamin-D deficiency marginally impairs fracture healing but aggravates posttraumatic bone loss in osteoporotic mice. Sci. Rep. 2017, 7, 7223. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barral, A.; Costales-Carrera, A.; Buira, S.P.; Jung, P.; Ferrer-Mayorga, G.; Larriba, M.J.; Bustamante-Madrid, P.; Domínguez, O.; Real, F.X.; Guerra-Pastrián, L.; et al. Vitamin D differentially regulates colon stem cells in patient-derived normal and tumor organoids. FEBS J. 2020, 287, 53–72. [Google Scholar] [CrossRef]
- Hansen, A.K.; Figenschau, Y.; Zubiaurre-Martinez, I. Co-expression of 1α-hydroxylase and vitamin D receptor in human articular chondrocytes. BMC Musculoskelet. Disord. 2017, 18, 432. [Google Scholar] [CrossRef] [PubMed]
- Kwiatek, J.; Jaroń, A.; Trybek, G. Impact of the 25-Hydroxycholecalciferol Concentration and Vitamin D Deficiency Treatment on Changes in the Bone Level at the Implant Site during the Process of Osseointegration: A Prospective, Randomized, Controlled Clinical Trial. J. Clin. Med. 2021, 10, 526. [Google Scholar] [CrossRef]
- Grønborg, I.M.; Tetens, I.; Andersen, E.W.; Kristensen, M.; Larsen, R.E.K.; Tran, T.L.L.; Andersen, R. Effect of vitamin D fortified foods on bone markers and muscle strength in women of Pakistani and Danish origin living in Denmark: A randomised controlled trial. Nutr. J. 2019, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Zheng, M.; Kim, D.K.; Lee, W.P.; Yu, S.J.; Kim, B.O. Effects of 1,25-dihydroxyvitamin D(3) on the differentiation of MC3T3-E1 osteoblast-like cells. J. Periodontal Implant Sci. 2018, 48, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, N.B.; Jurj, A.; Sorițău, O.; Lucaciu, O.P.; Dirzu, N.; Raduly, L.; Berindan-Neagoe, I.; Cenariu, M.; Boșca, B.A.; Campian, R.S.; et al. Cannabidiol and Vitamin D3 Impact on Osteogenic Differentiation of Human Dental Mesenchymal Stem Cells. Medicina 2020, 56, 607. [Google Scholar] [CrossRef]
- Abdelgawad, L.M.; Abdelaziz, A.M.; Sabry, D.; Abdelgwad, M. Influence of photobiomodulation and vitamin D on osteoblastic differentiation of human periodontal ligament stem cells and bone-like tissue formation through enzymatic activity and gene expression. Biomol. Concepts 2020, 11, 172–181. [Google Scholar] [CrossRef]
- Romeu Montenegro, K.; Carlessi, R.; Cruzat, V.; Newsholme, P. Effects of vitamin D on primary human skeletal muscle cell proliferation, differentiation, protein synthesis and bioenergetics. J. Steroid Biochem. Mol. Biol. 2019, 193, 105423. [Google Scholar] [CrossRef]
- Gülden, M.; Jess, A.; Kammann, J.; Maser, E.; Seibert, H. Cytotoxic potency of H2O2 in cell cultures: Impact of cell concentration and exposure time. Free Radic. Biol. Med. 2010, 49, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Song, I.S.; Lee, J.E.; Park, J.B. The Effects of Various Mouthwashes on Osteoblast Precursor Cells. Open Life Sci. 2019, 14, 376–383. [Google Scholar] [CrossRef]
- Choi, Y.S.; Baek, K.; Choi, Y. Estrogen reinforces barrier formation and protects against tumor necrosis factor alpha-induced barrier dysfunction in oral epithelial cells. J. Periodontal Implant Sci. 2018, 48, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Song, Y.M.; Min, S.K.; Lee, H.J.; Lee, H.L.; Kim, M.J.; Park, Y.H.; Park, J.U.; Park, J.B. NELL-1 Increased the Osteogenic Differentiation and mRNA Expression of Spheroids Composed of Stem Cells. Medicina 2021, 57, 586. [Google Scholar] [CrossRef] [PubMed]
- Alnahash, A.Z.; Song, Y.-M.; Min, S.-K.; Lee, H.-J.; Kim, M.-J.; Park, Y.-H.; Park, J.-U.; Park, J.-B. Effects of Connective Tissue Growth Factor on the Cell Viability, Proliferation, Osteogenic Capacity and mRNA Expression of Stem Cell Spheroids. Appl. Sci. 2021, 11, 6572. [Google Scholar] [CrossRef]
- Tae, J.Y.; Ko, Y.; Park, J.B. Evaluation of fibroblast growth factor-2 on the proliferation of osteogenic potential and protein expression of stem cell spheroids composed of stem cells derived from bone marrow. Exp. Ther. Med. 2019, 18, 326–331. [Google Scholar] [CrossRef]
- Lee, H.; Song, Y.; Park, Y.H.; Uddin, M.S.; Park, J.B. Evaluation of the Effects of Cuminum cyminum on Cellular Viability, Osteogenic Differentiation and Mineralization of Human Bone Marrow-Derived Stem Cells. Medicina 2021, 57, 38. [Google Scholar] [CrossRef]
- Yang, H.; Cao, Z.; Wang, Y.; Wang, J.; Gao, J.; Han, B.; Yu, F.; Qin, Y.; Guo, Y. Treadmill exercise influences the microRNA profiles in the bone tissues of mice. Exp. Ther. Med. 2021, 22, 1035. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Rim, Y.A.; Park, N.; Nam, Y.; Ju, J.H. Restoration of Osteogenesis by CRISPR/Cas9 Genome Editing of the Mutated COL1A1 Gene in Osteogenesis Imperfecta. J. Clin. Med. 2021, 10, 3141. [Google Scholar] [CrossRef]
- Bosetti, M.; Borrone, A.; Leigheb, M.; Shastri, V.P.; Cannas, M. Injectable Graft Substitute Active on Bone Tissue Regeneration. Tissue Eng. Part A 2017, 23, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Nah, H.; Lee, D.; Heo, M.; Lee, J.S.; Lee, S.J.; Heo, D.N.; Seong, J.; Lim, H.N.; Lee, Y.H.; Moon, H.J.; et al. Vitamin D-conjugated gold nanoparticles as functional carriers to enhancing osteogenic differentiation. Sci. Technol. Adv. Mater. 2019, 20, 826–836. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Kim, K. Bone Tissue Engineering Strategies in Co-Delivery of Bone Morphogenetic Protein-2 and Biochemical Signaling Factors. Adv. Exp. Med. Biol. 2018, 1078, 233–244. [Google Scholar]
- Xiong, Y.; Zhang, Y.; Guo, Y.; Yuan, Y.; Guo, Q.; Gong, P.; Wu, Y. 1α,25-Dihydroxyvitamin D(3) increases implant osseointegration in diabetic mice partly through FoxO1 inactivation in osteoblasts. Biochem. Biophys. Res. Commun. 2017, 494, 626–633. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-J.; Song, Y.-M.; Baek, S.; Park, Y.-H.; Park, J.-B. Vitamin D Enhanced the Osteogenic Differentiation of Cell Spheroids Composed of Bone Marrow Stem Cells. Medicina 2021, 57, 1271. https://doi.org/10.3390/medicina57111271
Lee H-J, Song Y-M, Baek S, Park Y-H, Park J-B. Vitamin D Enhanced the Osteogenic Differentiation of Cell Spheroids Composed of Bone Marrow Stem Cells. Medicina. 2021; 57(11):1271. https://doi.org/10.3390/medicina57111271
Chicago/Turabian StyleLee, Hyun-Jin, Young-Min Song, Seunghoon Baek, Yoon-Hee Park, and Jun-Beom Park. 2021. "Vitamin D Enhanced the Osteogenic Differentiation of Cell Spheroids Composed of Bone Marrow Stem Cells" Medicina 57, no. 11: 1271. https://doi.org/10.3390/medicina57111271
APA StyleLee, H.-J., Song, Y.-M., Baek, S., Park, Y.-H., & Park, J.-B. (2021). Vitamin D Enhanced the Osteogenic Differentiation of Cell Spheroids Composed of Bone Marrow Stem Cells. Medicina, 57(11), 1271. https://doi.org/10.3390/medicina57111271






