A Meta-Analysis and Meta-Regression of Frequency and Risk Factors for Poststroke Complex Regional Pain Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Study Selection and Data Extraction
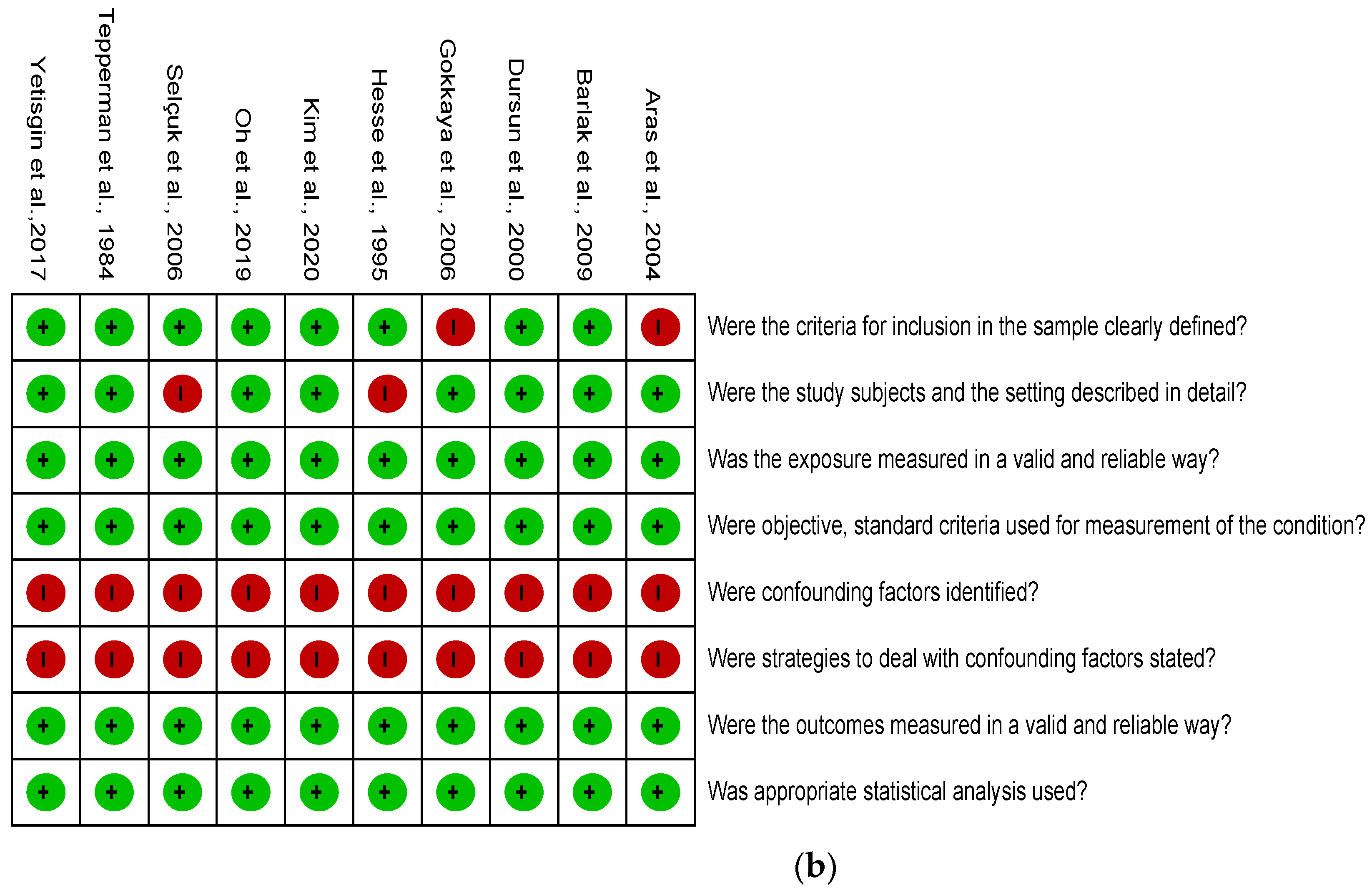
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Selection and Description
3.2. Risk of Bias Assessment
3.3. Outcomes
3.3.1. Shoulder Subluxation
3.3.2. Spasticity
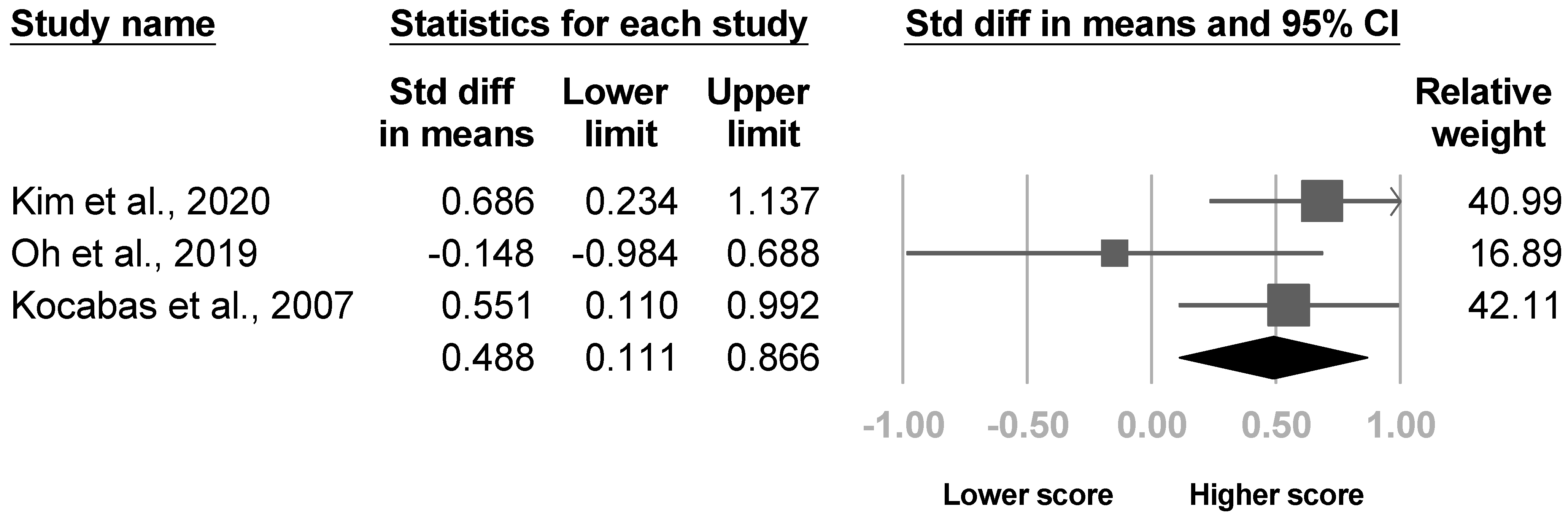
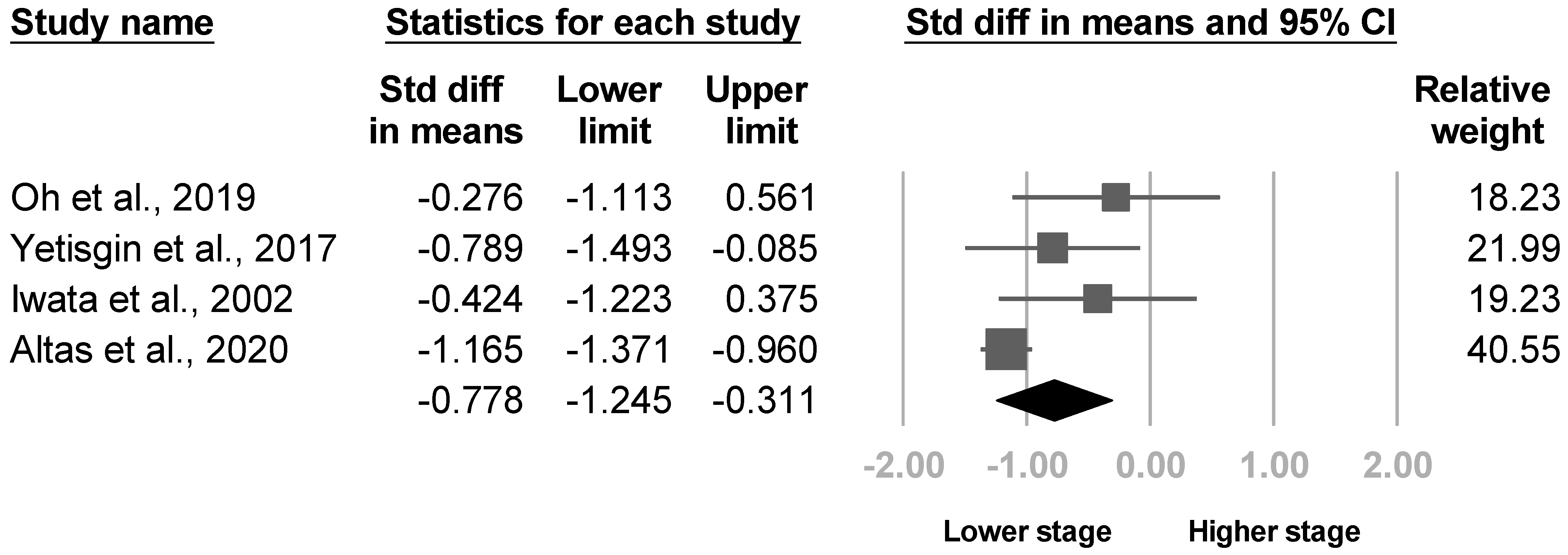
3.3.3. Brunnstrom Stage
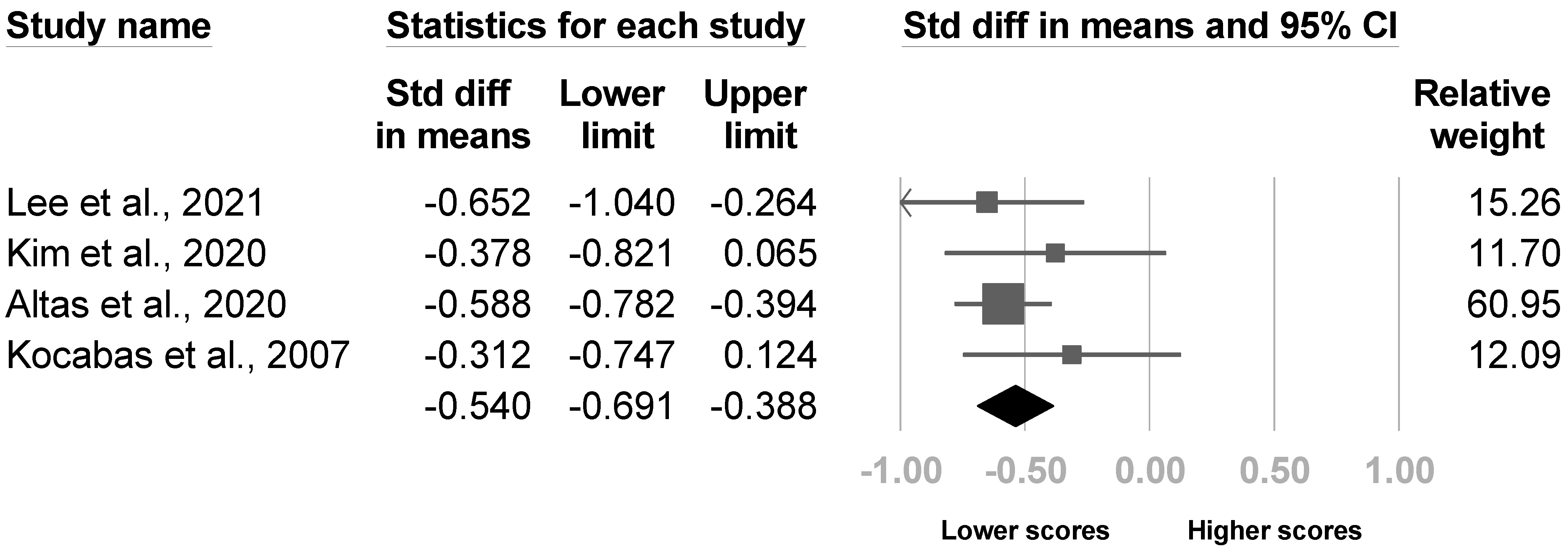
3.3.4. The Barthel Index
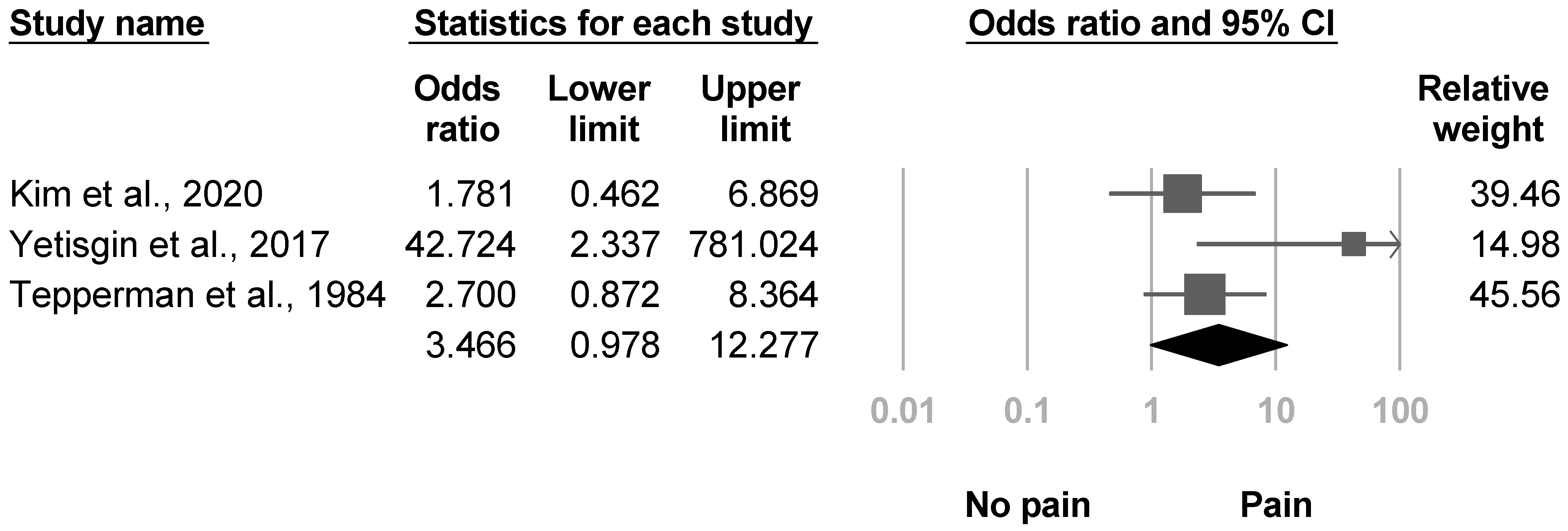
3.3.5. Shoulder Pain
3.3.6. Age
3.3.7. Sex
3.3.8. Etiology of Stroke
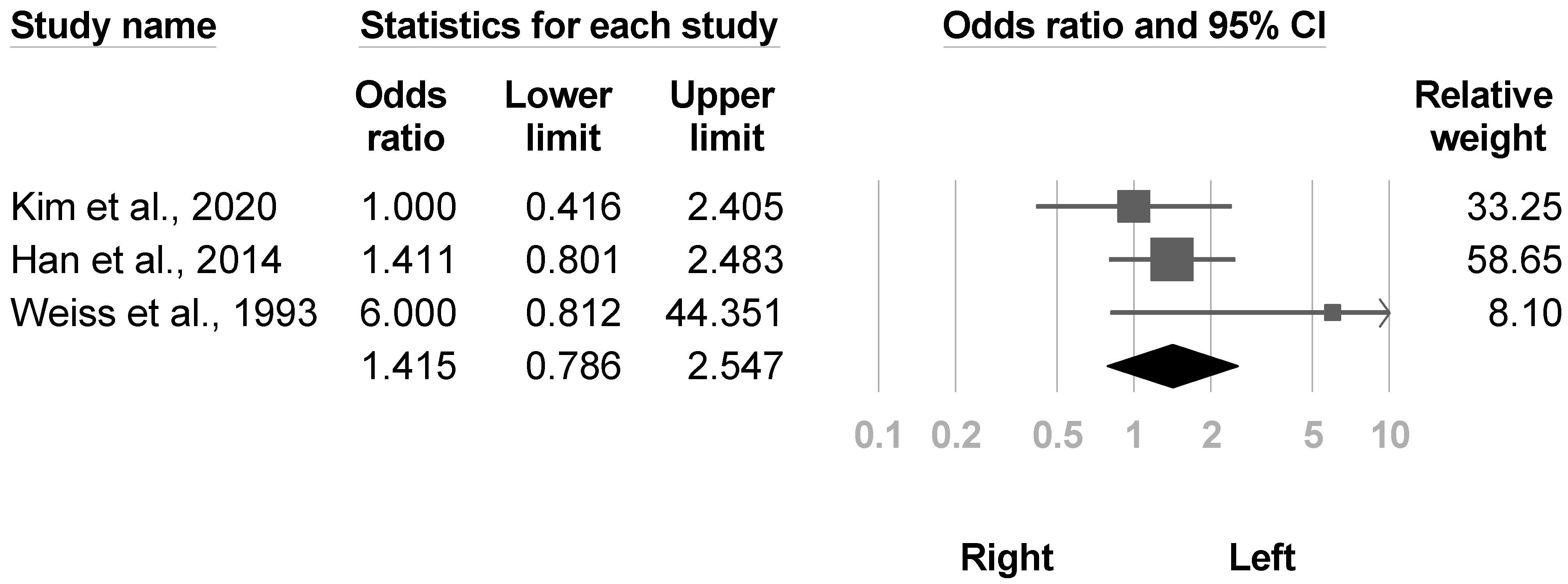
3.3.9. Side
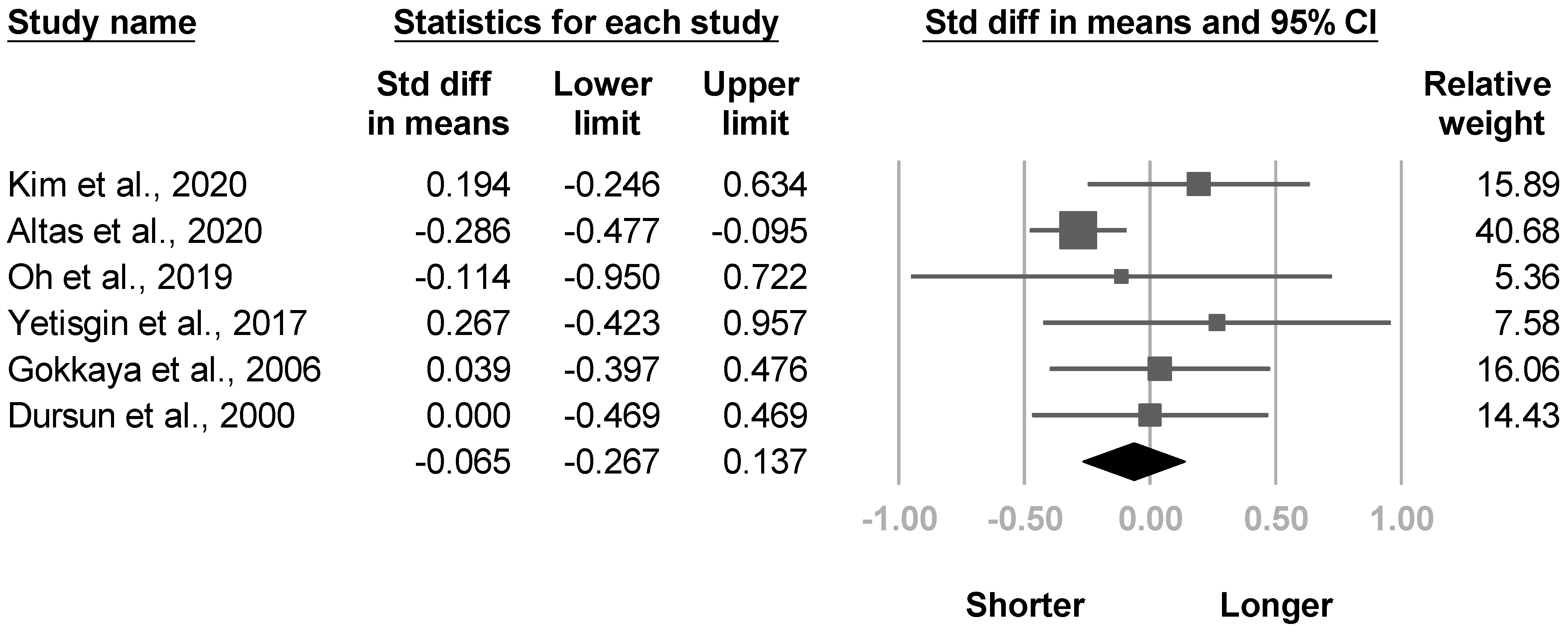
3.3.10. Duration of Stroke
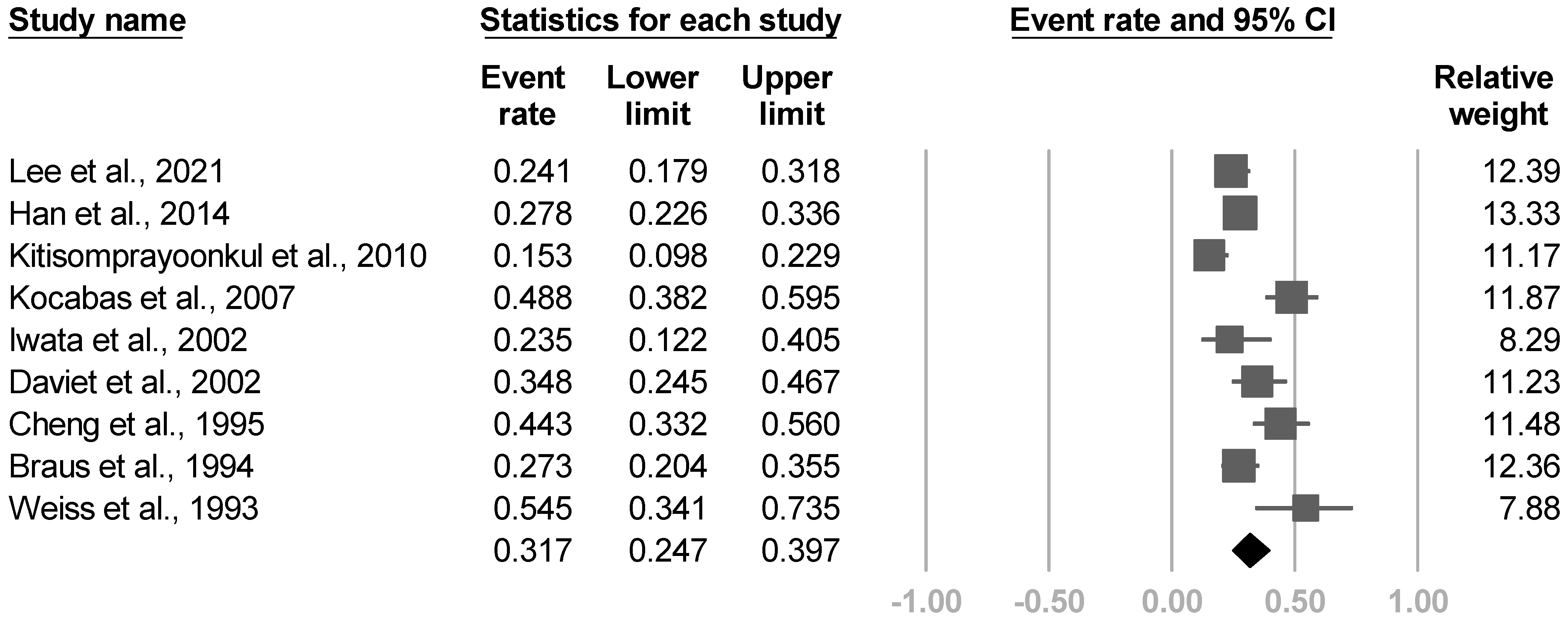
3.3.11. Frequency
3.3.12. Other Risk Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harden, R.N.; Bruehl, S.; Stanton-Hicks, M.; Wilson, P.R. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. 2007, 8, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Harden, R.N.; Oaklander, A.L.; Burton, A.W.; Perez, R.S.; Richardson, K.; Swan, M.; Barthel, J.; Costa, B.; Graciosa, J.R.; Bruehl, S. Complex regional pain syndrome: Practical diagnostic and treatment guidelines, 4th ed. Pain Med. 2013, 14, 180–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bussa, M.; Guttilla, D.; Lucia, M.; Mascaro, A.; Rinaldi, S. Complex regional pain syndrome type I: A comprehensive review. Acta Anaesthesiol. Scand. 2015, 59, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Yoon, S.Y.; Kim, J.; Jeong, Y.H.; Kim, Y.W. Neural substrates for poststroke complex regional pain syndrome type I: A retrospective case-control study using voxel-based lesion symptom mapping analysis. Pain 2020, 161, 1311–1320. [Google Scholar] [CrossRef]
- Kumar, B.; Kalita, J.; Kumar, G.; Misra, U.K. Central Poststroke Pain: A Review of Pathophysiology and Treatment. Anesth. Analg. 2009, 108, 1645–1657. [Google Scholar] [CrossRef]
- Kondo, I.; Hosokawa, K.; Soma, M.; Iwata, M.; Maltais, D. Protocol to prevent shoulder-hand syndrome after stroke. Arch. Phys. Med. Rehabil. 2001, 82, 1619–1623. [Google Scholar] [CrossRef]
- Meng, L.P.; Chen, C.Y.; Lu, Z.Q. Effect of early rehabilitation nursing on motor function, activities of daily living and incidence of shoulder-hand syndrome in stroke patients with hemiplegia. Acta Med. Mediterr. 2020, 36, 3053–3058. [Google Scholar] [CrossRef]
- Lee, J.I.; Kwon, S.W.; Lee, A.; Tae, W.S.; Pyun, S.B. Neuroanatomical correlates of poststroke complex regional pain syndrome: A voxel-based lesion symptom-mapping study. Sci. Rep. 2021, 11, 13093. [Google Scholar] [CrossRef]
- Geurts, A.C.H.; Visschers, B.; van Limbeek, J.; Ribbers, G.M. Systematic review of aetiology and treatment of post-stroke hand oedema and shoulder-hand syndrome. Scand. J. Rehabil. Med. 2000, 32, 4–10. [Google Scholar]
- Brooke, B.S.; Schwartz, T.A.; Pawlik, T.M. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021, 156, 787–788. [Google Scholar] [CrossRef]
- Ringer, R.; Wertli, M.; Bachmann, L.M.; Buck, F.M.; Brunner, F. Concordance of qualitative bone scintigraphy results with presence of clinical complex regional pain syndrome 1: Meta-analysis of test accuracy studies. Eur. J. Pain 2012, 16, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Wertli, M.M.; Brunner, F.; Steurer, J.; Held, U. Usefulness of bone scintigraphy for the diagnosis of Complex Regional Pain Syndrome 1: A systematic review and Bayesian meta-analysis. PLoS ONE 2017, 12, e0173688. [Google Scholar] [CrossRef]
- Held, U.; Brunner, F.; Steurer, J.; Wertli, M.M. Bayesian meta-analysis of test accuracy in the absence of a perfect reference test applied to bone scintigraphy for the diagnosis of complex regional pain syndrome. Biom. J. 2015, 57, 1020–1037. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Deeks, J.J. Selecting studies and collecting data. In Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 151–185. [Google Scholar]
- McGrath, S.; Zhao, X.; Steele, R.; Thombs, B.D.; Benedetti, A. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat. Methods Med. Res. 2020, 29, 2520–2537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sistrom, C.; Mergo, P. A Simple Method for Obtaining Original Data from Published Graphs and Plots. AJR Am. J. Roentgenol. 2000, 174, 1241–1244. [Google Scholar] [CrossRef]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Catalá-López, F.; Tobías, A. Meta-analysis of randomized trials, heterogeneity and prediction intervals. Med. Clin. 2014, 142, 270–274. [Google Scholar] [CrossRef]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Comparison of Two Methods to Detect Publication Bias in Meta-analysis. JAMA 2006, 295, 676–680. [Google Scholar] [CrossRef] [Green Version]
- Altas, E.U.; Onat, Ş.; Konak, H.E.; Polat, C.S. Post-stroke complex regional pain syndrome and related factors: Experiences from a tertiary rehabilitation center. J. Stroke Cerebrovasc. Dis. 2020, 29, 104995. [Google Scholar] [CrossRef]
- Oh, S.W.; Choi, S.U.; Park, M.; Shin, J.H. Validity of the Budapest Criteria For Poststroke Complex Regional Pain Syndrome. Clin. J. Pain 2019, 35, 831–835. [Google Scholar] [CrossRef]
- Yetisgin, A. Clinical characteristics affecting motor recovery and ambulation in stroke patients. J. Phys. Ther. Sci. 2017, 29, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Han, E.Y.; Jung, H.Y.; Kim, M.O. Absent median somatosensory evoked potential is a predictor of type I complex regional pain syndrome after stroke. Disabil. Rehabil. 2014, 36, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Kitisomprayoonkul, W.; Sungkapo, P.; Taveemanoon, S.; Chaiwanichsiri, D. Medical complications during inpatient stroke rehabilitation in Thailand: A prospective study. J. Med. Assoc. Thai. 2010, 93, 594–600. [Google Scholar]
- Barlak, A.; Unsal, S.; Kaya, K.; Sahin-Onat, S.; Ozel, S. Poststroke shoulder pain in Turkish stroke patients: Relationship with clinical factors and functional outcomes. Int. J. Rehabil. Res. 2009, 32, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Kocabas, H.; Levendoglu, F.; Ozerbil, O.M.; Yuruten, B. Complex regional pain syndrome in stroke patients. Int. J. Rehabil. Res. 2007, 30, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Selçuk, B.; Ersoz, M.; Inanir, M.; Kurtaran, A.; Akyuz, M. Sympathetic skin responses in hemiplegic patients with and without complex regional pain syndrome. Neurol. India 2006, 54, 279–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gokkaya, N.K.; Aras, M.; Yesiltepe, E.; Koseoglu, F. Reflex sympathetic dystrophy in hemiplegia. Int. J. Rehabil. Res. 2006, 29, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Aras, M.D.; Gokkaya, N.K.; Comert, D.; Kaya, A.; Cakci, A. Shoulder pain in hemiplegia: Results from a national rehabilitation hospital in Turkey. Am. J. Phys. Med. Rehabil. 2004, 83, 713–719. [Google Scholar] [CrossRef]
- Iwata, M.; Kondo, I.; Sato, Y.; Satoh, K.; Soma, M.; Bar-Or, O. Prediction of reflex sympathetic dystrophy in hemiplegia by evaluation of hand edema. Arch. Phys. Med. Rehabil. 2002, 83, 1428–1431. [Google Scholar] [CrossRef]
- Daviet, J.C.; Preux, P.M.; Salle, J.Y.; Lebreton, F.; Munoz, M.; Dudognon, P.; Pelissier, J.; Perrigot, M. Clinical factors in the prognosis of complex regional pain syndrome type I after stroke: A prospective study. Am. J. Phys. Med. Rehabil. 2002, 81, 34–39. [Google Scholar] [CrossRef]
- Dachy, B.; Denis, L.; Deltenre, P. Usefulness of transcranial magnetic stimulation to predict the development of reflex sympathetic dystrophy poststroke: A pilot study. Arch. Phys. Med. Rehabil. 2002, 83, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Dursun, E.; Dursun, N.; Ural, C.E.; Cakci, A. Glenohumeral joint subluxation and reflex sympathetic dystrophy in hemiplegic patients. Arch. Phys. Med. Rehabil. 2000, 81, 944–946. [Google Scholar] [CrossRef]
- Hesse, S.; Jahnke, M.T.; Ehret, R.; Mauritz, K.H. Shoulder-hand syndrome in hemiplegic patients: Temperature, sympathetic skin responses, and nerve latencies of the affected and nonaffected upper extremity. J. Neurol. Rehabil. 1995, 9, 229–233. [Google Scholar] [CrossRef]
- Cheng, P.T.; Hong, C.Z. Prediction of reflex sympathetic dystrophy in hemiplegic patients by electromyographic study. Stroke 1995, 26, 2277–2280. [Google Scholar] [CrossRef] [PubMed]
- Braus, D.F.; Krauss, J.K.; Strobel, J. The shoulder-hand syndrome after stroke: A prospective clinical trial. Ann. Neurol. 1994, 36, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.; Alfano, A.; Bardfeld, P.; Weiss, J.; Friedmann, L.W. Prognostic value of triple phase bone scanning for reflex sympathetic dystrophy in hemiplegia. Arch. Phys. Med. Rehabil. 1993, 74, 716–719. [Google Scholar] [CrossRef]
- Tepperman, P.S.; Greyson, N.D.; Hilbert, L.; Jimenez, J.; Williams, J.I. Reflex sympathetic dystrophy in hemiplegia. Arch. Phys. Med. Rehabil. 1984, 65, 442–447. [Google Scholar]
- Zyluk, A.; Zyluk, B. Shoulder-hand syndrome in patients after stroke. Neurol. Neurochir. Pol. 1999, 33, 131–142. [Google Scholar]
- Zorowitz, R.D.; Hughes, M.B.; Idank, D.; Ikai, T.; Johnston, M.V. Shoulder pain and subluxation after stroke: Correlation or coincidence? Am. J. Occup. Ther. 1996, 50, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Suethanapornkul, S.; Kuptniratsaikul, P.S.; Kuptniratsaikul, V.; Uthensut, P.; Dajpratha, P.; Wongwisethkarn, J. Post stroke shoulder subluxation and shoulder pain: A cohort multicenter study. J. Med. Assoc. Thai. 2008, 91, 1885–1892. [Google Scholar] [PubMed]
- Jush, S.-D.; Wang, C.-H.; Hsieh, C.-L.; Chen, M.-H.; Chen, C.-L. The Brunnstrom recovery scale: Its reliability and concurrent validity. J. Occup. Ther. Assoc. ROC 1996, 14, 1–12. [Google Scholar] [CrossRef]
- Sandroni, P.; Benrud-Larson, L.M.; McClelland, R.L.; Low, P.A. Complex regional pain syndrome type I: Incidence and prevalence in Olmsted county, a population-based study. Pain 2003, 103, 199–207. [Google Scholar] [CrossRef]
- Ringman, J.M.; Saver, J.L.; Woolson, R.F.; Clarke, W.R.; Adams, H.P. Frequency, risk factors, anatomy, and course of unilateral neglect in an acute stroke cohort. Neurology 2004, 63, 468. [Google Scholar] [CrossRef]
- Elsamadicy, A.A.; Yang, S.; Sergesketter, A.R.; Ashraf, B.; Charalambous, L.; Kemeny, H.; Ejikeme, T.; Ren, X.; Pagadala, P.; Parente, B.; et al. Prevalence and Cost Analysis of Complex Regional Pain Syndrome (CRPS): A Role for Neuromodulation. Neuromodul. J. Int. Neuromodul. Soc. 2018, 21, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Oray, M.; Abu Samra, K.; Ebrahimiadib, N.; Meese, H.; Foster, C.S. Long-term side effects of glucocorticoids. Expert Opin. Drug Saf. 2016, 15, 457–465. [Google Scholar] [CrossRef]
- Long, A.N.; Dagogo-Jack, S. Comorbidities of diabetes and hypertension: Mechanisms and approach to target organ protection. J. Clin. Hypertens 2011, 13, 244–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matayoshi, S.; Shimodozono, M.; Hirata, Y.; Ueda, T.; Horio, S.; Kawahira, K. Use of calcitonin to prevent complex regional pain syndrome type I in severe hemiplegic patients after stroke. Disabil. Rehabil. 2009, 31, 1773–1779. [Google Scholar] [CrossRef]
- Woon, C. Nursing at the centre of stroke recovery in the acute setting: Prioritising early rehabilitation. Br. J. Neurosci. Nurs. 2016, 12, 23–28. [Google Scholar] [CrossRef]










| Research | Country/Area, Year of Study | Study Design | Study Population | Study Size | Age (Years) | Sex (% of Females) |
|---|---|---|---|---|---|---|
| Lee et al., 2021 [8] | November 2012 to August 2019, Korea | Retrospective nested case-control study | Hospitalized, first-ever episode of ischemic stroke, unilateral hemispheric, supratentorial | CRPS group: 35, control group: 110 | CRPS group: median 72 (IQR 65–78); control group: median 72 (IQR 58.75–80)) | 42.1% |
| Kim et al., 2020 [4] | January 2009 and May 2019, Korea | Cross-sectional study | Hospitalized, first-ever episode of stroke within 3 months, supratentorial | CRPS group: 38, control group: 42 | CRPS group: 67.9 (10.3); control group: 62.7 (10.9) | 43.8% |
| Altas et al., 2020 [20] | June 1, 2014 and June 1, 2019, Turkey | Retrospective nested case-control study | Hospitalized, stroke | CRPS group: 213, control: 213 | CRPS group: 67.9 (10.3); control group: 66.1 (9.9) | 60.1% |
| Oh et al., 2019 [21] | May 2016 and June 2017, Korea | Cross-sectional study | Hospitalized, first-ever stroke with hemiplegia | CRPS group: 66, control group: 6 | CRPS group: 50.8 (SEM 5.7); control group: 48.9 (SEM 10.9) | 22.2% |
| Yetisgin et al., 2017 [22] | January 2015 to May 2016, Turkey | Cross-sectional study | Outpatient clinic, first ever stroke with hemiplegia | CRPS group: 10, control group: 43 | CRPS group: 60.6 (16.2); control group: 59.8 (12.7) | 41.5% |
| Han et al., 2014 [23] | January, 2003, to December, 2007, Korea | Retrospective nested case-control study | Hospitalized, first ever stroke, and performed somatosensory evoked tests of potentials in the hemiparetic limb 2–4 weeks post stroke | CRPS group: 70, control group: 182 | CRPS group: 61.5 (12.8); control group: 63.1 (13) | 43.7% |
| Kitisomprayoonkul et al., 2010 [24] | August 2006 to January 2007, Thailand | Prospective nested case-control study | Hospitalized for stroke | 118 | 63.4 (11.2) | 39.0% |
| Barlak et al., 2009 [25] | January 2005 and May 2007, Turkey | Cross-sectional study | Hospitalized, first ever unilateral stroke | CRPS group: 114, control group: 73 | 61.3 (11.3) | 50.8% |
| Kocabas et al., 2007 [26] | September 2002 to May 2003, Turkey | Prospective nested case-control study | Hospitalized, stroke | CRPS group: 40, control group: 42 | 64.0 (9.9) | 53.7% |
| Selçuk et al., 2006 [27] | 2000 to 2005, Turkey | Cross-sectional study | Stroke with hemiplegia or hemiparesis | CRPS group: 41, control (stroke) group: 28, control (healthy): 20 | Stroke patients: 60.0 (12.9); healthy individuals: 57.9 (15.6) | 37.7% |
| Gokkaya et al., 2006 [28] | NR, Turkey | Cross-sectional study | Hospitalized for stroke | CRPS group: 29, control group: 66 | CRPS group: 60.7 (11.4); control group: 58.7 (12) | 33.7% |
| Aras et al., 2004 [29] | NR, Turkey | Cross-sectional study | Hospitalized for stroke | 85 | 59.5 (11.7) | 30.6% |
| Iwata et al., 2002 [30] | NR, Japan | Prospective nested case-control study | Hospitalized, first ever unilateral stroke within 3 weeks | CRPS group: 8, control group: 26 | CRPS group: 58.9 (8.7); control group: 62.5 (9.7) | 41.2% |
| Daviet et al., 2002 [31] | November 1997 to August 1998, France | Prospective cohort study | Hospitalized for stroke with hemiplegia within 1 month | 69 | NR | 42.0% |
| Dachy et al., 2002 [32] | NR, Belgium | Prospective cohort study | Hospitalized for first ever hemispheric stroke | 20 | 67.9 (range 41–85) | 45% |
| Dursun et al., 2000 [33] | NR, Turkey | Cross-sectional study | Hospitalized for first ever unilateral stroke with Ashworth scale 2 and below | CRPS group: 35, control group: 35 | CRPS group: 59.1 (SEM 1.6); control group: 59.8 (SEM 1.7) | 45.7% |
| Hesse et al., 1995 [34] | NR, Germany | Cross-sectional study | Stroke with hemiparesis | CRPS group: 21, control group: 18 | 60.6 (range 43–79) | 33.3% |
| Cheng et al., 1995 [35] | NR, Taiwan | Prospective nested case-control study | Hospitalized for first ever stroke within 3 weeks | CRPS group: 31, control group: 39 | 62.3 | 42.9% |
| Braus et al., 1994 [36] | NR, Germany | Prospective nested case-control study | Hospitalized for stroke with hemiplegia | CRPS group: 36, control group: 96 | 61.6 (11.9) | 31.8% |
| Weiss et al., 1993 [37] | NR, United States | Prospective cohort study | Hospitalized for stroke with hemiparesis | 22 | NR | 50.0% |
| Tepperman et al., 1984 [38] | NR, Canada | Cross-sectional study | Hospitalized for stroke with hemiplegia | 85 | CRPS group: 66.6 (8.3); control group: 66.7 (10.4) | 43.5% |
| Research | Time of Outcome Assessment | % Developing CRPS (CRPS/Total) | Criteria | Risk Factors Assessed with Statistical Significance | Risk Factors Assessed without Statistical Significance |
|---|---|---|---|---|---|
| Lee et al., 2021 [8] | NR | 24.1% (35/145) | Budapest Criteria | Manual Function Test, Modified Barthel Index, Fugl‒Meyer assessment (total and upper extremity), strength of shoulder flexion and wrist extension of the hemiplegic side (MRC), Berg Balance Scale, MMSE, somatosensory evoked potentials in median nerve, damage to the white matter of the CST, caudate nucleus, and putamen | Age and sex, presence of shoulder subluxation and spasticity, stroke lesion location (middle cerebral arterial region or others), and lesion volume |
| Kim et al., 2020 [4] | NR | 47.5% (38/80) | Budapest criteria | pain intensity of affected wrist, spasticity (Modified Ashworth Scale), strength of shoulder (MRC), medication during admission (Medication Quantification Scale) scores, shoulder subluxation; damage to the white matter of the CST | Age, sex, etiology of stroke, side of lesion, duration of stroke, lesion volume, MMSE, Geriatric Depression Scale, Modified Barthel Index, Prevalence of shoulder pain at rest |
| Altas et al., 2020 [20] | NR | NR | Budapest criteria | Duration of stroke, time to hospitalization, admission duration, coronary artery disease, upper extremity Brunnstrom stage, Brunnstrom hand stage, spasticity (Modified Ashworth scale), Barthel index, shoulder subluxation, shoulder soft tissue lesion, adhesive capsulitis, previous orthopedic surgeries, fracture in the upper extremities, neglect, visual field defect, heterotopic ossification, entrapment neuropathy, pressure wound, lower respiratory tract infection, urinary infection, epilepsy, depression | Age, sex, affected side, etiology of stroke, smoking, hypertension, diabetes, atrial fibrillation, dyslipidemia, deep vein thrombosis, brachial plexus injury, protein-energy malnutrition |
| Oh et al., 2019 [21] | NR | 8.3% (6/72) | Budapest criteria | Etiology of stroke | Age, sex, affected side, disease duration, strength of elbow, wrist, shoulder summarized (MRC), Brunnstrom stage, spasticity (Modified Ashworth scale), shoulder subluxation (acromion-greater tuberosity distance) |
| Yetisgin et al., 2017 [22] | NR | 18.9% (10/53) | NR | Sex, Brunnstrom hand stage, shoulder pain | Age, etiology of stroke, affected side, duration after stroke, rehabilitated, Brunnstrom stage of arm and lower extremity, functional ambulation scale |
| Han et al., 2014 [23] | NR | 27% (70/252) | Orlando criteria | Etiology of stroke, somatosensory evoked potentials in median nerve, shoulder subluxation | Age, sex, stroke lesion location (cortical or subcortical), affected side |
| Kitisomprayoonkul et al., 2010 [24] | Until discharge from hospital (mean 122.5 days) | 15.3% (18/118) | Bonica’s management of pain. 3rd edition | Limit range of motion of weak shoulder | NR |
| Barlak et al., 2009 [25] | NR | 37.4% (70/187) | Bonica’s management of pain. 3rd edition | Brunnstrom stage | Shoulder subluxation |
| Kocabas et al., 2007 [26] | 28 weeks after stroke | 48.8% (40/82) | Bonica’s management of pain. 3rd edition | Spasticity (Ashworth scale), Brunnstrom score, strength of arm (Motricity Index), shoulder ROM, shoulder subluxation, depression (regression analysis of Beck score) | Sex, affected side, etiology of stroke, presence of hypoesthesia, leg strength (Motricity Index), Barthel index, depression (mean difference in Beck score) |
| Selçuk et al., 2006 [27] | NR | 59.4% (41/69) | Kozin’s clinical criteria | Sympathetic skin responses (absent or not, amplitude) | Sympathetic skin responses (latency) |
| Gokkaya et al., 2006 [28] | NR | 30.5% (29/95) | Clinical criteria of Tepperman et al., 1984 | Brunnstrom stage, shoulder subluxation | Age, sex, affected side, etiology of stroke, disease duration, duration of admission for rehabilitation |
| Aras et al., 2004 [29] | NR | 30.6% (26/85) | Clinical criteria of Tepperman et al., 1984 | Shoulder subluxation | NR |
| Iwata et al., 2002 [30] | 2–4 months after stroke | 23.5% (8/34) | Clinical diagnosis | Ratio circumference of the middle finger between hands | Age, sex, affected side, etiology of stroke, hemispatial neglect, dominant hand, Brunnstrom arm and hand stage |
| Daviet et al., 2002 [31] | 3 months after stroke | 34.8% (24/69) | Labrousse severity scale | Severity of symptoms (Labrousse scale), strength (Motricity Index), spasticity (Ashworth scale), shoulder subluxation, length of stay in acute ward, initial coma, Perrigot score | Age, sex, affected side, vibration sensitivity, depression (Montgomery-Asberg Depression Rating Scale), hemineglect, proprioception, diabetes, hypertension, dyslipidemia, thyroid disorder, barbituric treatment, anticoagulant treatment, edema of hand and forearm |
| Dachy et al., 2002 [32] | 70 days after stroke | NR | Enjalbert score | Transcranial magnetic stimulation induced motor evoked potential | NR |
| Dursun et al., 2000 [33] | NR | 50% (35/70) | Either clinical or bone scintigraphy | Shoulder subluxation | Age, sex, side, etiology of stroke, duration of disease, Brunnstrom stage |
| Hesse et al., 1995 [34] | NR | NR | Clinical diagnosis | Amplitude, area, F/M ratio of sympathetic skin response, temperature difference between limbs | NR |
| Cheng et al., 1995 [35] | 6 months after admission | 44.2% (31/70, including 15 definite, 9 probable and 7 possible) | Clinical criteria of Tepperman et al., 1984 | Sensory impairment, shoulder subluxation, spontaneous electromyography activity of affected limb, | Age, sex, spasticity, etiology of stroke, affected side, distal latency and amplitude of thenar compound muscle action potential |
| Braus et al., 1994 [36] | 6 months after discharge | 27.2% (36/132, all definite) | Kozin et al., 1981 | Subluxation, motor deficit, spasticity (Ashworth scale), deficits in confrontation visual field testing | Age, sex, affected side, etiology of stroke |
| Weiss et al., 1993 [37] | 6 months after admission | 54.5% (12/22) | Clinical diagnosis | NR | Sex, sensory deficit, side |
| Tepperman et al., 1984 [38] | NR | 24.7% (21/85) | Bone scintigraphy | Peptic ulcer, vasomotor changes, wrist tenderness, metacarpal phalangeal joint tenderness, interphalangeal joint tenderness | Age, sex, affected side, etiology of stroke, hypertension, diabetes mellitus, atherosclerotic heart disease, congestive heart failure, atrial fibrillation, peripheral vascular disease, chronic obstructive lung disease, mitral stenosis, shoulder pain and tenderness, swelling of the wrist and hand |
| Newcastle-Ottawa Scale | Selection | Comparability | Outcome | Total Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| a Cohort | ||||||||||
| Weiss et al., 1993 [37] | * | * | * | - | - | - | * | * | * | 6 |
| Daviet et al., 2002 [31] | * | * | * | - | - | - | * | * | * | 6 |
| Dachy et al., 2002 [32] | * | * | * | * | - | - | * | * | * | 7 |
| b Case-control | ||||||||||
| Braus et al., 1994 [36] | * | * | * | * | - | - | * | * | * | 7 |
| Cheng et al., 1995 [35] | * | * | * | * | - | - | * | * | * | 7 |
| Iwata et al., 2002 [30] | * | - | * | * | - | - | * | * | * | 6 |
| Kocabas et al., 2007 [26] | * | * | * | * | - | - | * | * | * | 7 |
| Kitisomprayoonkul et al., 2010 [24] | * | * | * | * | - | - | * | * | * | 7 |
| Han et al., 2014 [23] | * | * | * | * | - | - | * | * | * | 7 |
| Altas et al., 2020 [20] | * | * | * | * | - | - | * | * | * | 7 |
| Lee et al., 2021 [8] | * | * | * | * | - | - | * | * | * | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.-C.; Guo, Y.-H.; Hsieh, P.-C.; Lin, Y.-C. A Meta-Analysis and Meta-Regression of Frequency and Risk Factors for Poststroke Complex Regional Pain Syndrome. Medicina 2021, 57, 1232. https://doi.org/10.3390/medicina57111232
Su Y-C, Guo Y-H, Hsieh P-C, Lin Y-C. A Meta-Analysis and Meta-Regression of Frequency and Risk Factors for Poststroke Complex Regional Pain Syndrome. Medicina. 2021; 57(11):1232. https://doi.org/10.3390/medicina57111232
Chicago/Turabian StyleSu, Yu-Chi, Yao-Hong Guo, Pei-Chun Hsieh, and Yu-Ching Lin. 2021. "A Meta-Analysis and Meta-Regression of Frequency and Risk Factors for Poststroke Complex Regional Pain Syndrome" Medicina 57, no. 11: 1232. https://doi.org/10.3390/medicina57111232
APA StyleSu, Y.-C., Guo, Y.-H., Hsieh, P.-C., & Lin, Y.-C. (2021). A Meta-Analysis and Meta-Regression of Frequency and Risk Factors for Poststroke Complex Regional Pain Syndrome. Medicina, 57(11), 1232. https://doi.org/10.3390/medicina57111232





