The Influence of Selected Meteorological Factors on the Prevalence and Course of Stroke
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- Air humidity and air temperature on the day of stroke onset, as well as air temperature, on the day preceding stroke are important for the functional status of patients in the acute disease period.
- A combination of the following meteorological parameters: lowered mean temperature and low sunshine, high humidity and high wind speed all increase the risk of stroke during the winter period.
- High humidity combined with high precipitation, low wind speed and low sunshine in the autumn period are associated with the lowest stroke incidence risk.
- A possible relationship between phases of the moon and the incidence of requires further investigation.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sipilä, J.O.; Ruuskanen, J.O.; Kauko, T.; Rautava, P.; Kytö, V. Seasonality of stroke in Finland. Ann. Med. 2016, 49, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Matsumaru, N.; Okada, H.; Suzuki, K.; Nachi, S.; Yoshida, T.; Tsukamoto, K.; Ogura, S. Weather Fluctuations May Have an Impact on Stroke Occurrence in a Society: A Population-Based Cohort Study. Cerebrovasc. Dis. Extra 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Royé, D.; Zarrabeitia, M.T.; Riancho, J.; Santurtún, A. A time series analysis of the relationship between apparent temperature, air pollutants and ischaemic stroke in Madrid, Spain. Environ. Res. 2019, 173, 349–358. [Google Scholar] [CrossRef]
- Telman, G.; Sviri, G.E.; Sprecher, E.; Amsalem, Y.; Avizov, R. Seasonal variation in spontaneous intracerebral hemorrhage in northern Israel. Chronobiol. Int. 2017, 34, 563–570. [Google Scholar] [CrossRef]
- The Lancet Planetary Health. More than a diet. Lancet Planet Health 2019, 3, e48. [Google Scholar] [CrossRef]
- Watts, N.; Adger, W.N.; Ayeb-Karlsson, S.; Bai, Y.; Byass, P.; Campbell-Lendrum, D.; Colbourn, T.; Cox, P.; Davies, M.; Depledge, M.; et al. The Lancet Countdown: Tracking progress on health and climate change. Lancet 2017, 389, 1151–1164. [Google Scholar] [CrossRef]
- Cameron, A.C.; Trivedi, P.K. Microeconometrics Using Stata; Stata Press: College Station, TX, USA, 2009. [Google Scholar]
- Magalhães, R.; Silva, M.C.; Correia, M.; Bailey, T. Are Stroke Occurrence and Outcome Related to Weather Parameters Results from a Population-Based Study in Northern Portugal. Cerebrovasc. Dis. 2011, 32, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.; Weir, C.; Wright, F.; Bryden, C.; Aslanyan, S.; Lees, K.; Bird, W.; Walters, M. Associations between meteorological variables and acute stroke hospital admissions in the west of Scotland. Acta Neurol. Scand. 2007, 117, 85–89. [Google Scholar] [CrossRef]
- Salam, A.; Kamran, S.; Bibi, R.; Korashy, H.M.; Parray, A.; Al Mannai, A.; Al Ansari, A.; Kanikicharla, K.K.; Gashi, A.Z.; Shuaib, A. Meteorological Factors and Seasonal Stroke Rates: A Four-year Comprehensive Study. J. Stroke Cerebrovasc. Dis. 2019, 28, 2324–2331. [Google Scholar] [CrossRef]
- Han, M.-H.; Yi, H.-J.; Ko, Y.; Kim, Y.-S.; Lee, Y.-J. Association between hemorrhagic stroke occurrence and meteorological factors and pollutants. BMC Neurol. 2016, 16, 59. [Google Scholar] [CrossRef]
- Han, M.-H.; Yi, H.-J.; Kim, Y.-S. Effect of Seasonal and Monthly Variation in Weather and Air Pollution Factors on Stroke Incidence in Seoul, Korea. Stroke 2015, 46, 927–935. [Google Scholar] [CrossRef]
- Morabito, M.; Crisci, A.; Vallorani, R.; Modesti, P.A.; Gensini, G.F.; Orlandini, S. Innovative Approaches Helpful to Enhance Knowledge on Weather-Related Stroke Events Over a Wide Geographical Area and a Large Population. Stroke 2011, 42, 593–600. [Google Scholar] [CrossRef]
- Basu, R.; Pearson, D.; Malig, B.; Broadwin, R.; Green, R. The Effect of High Ambient Temperature on Emergency Room Visits. Epidemiology 2012, 23, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Zhou, J.; Wang, S.; Li, T.; Fan, X.; Fan, J.; Xie, J. Differences of hemorrhagic and ischaemic strokes in age spectra and responses to climatic thermal conditions. Sci. Total Environ. 2018, 644, 1573–1579. [Google Scholar] [CrossRef]
- Guan, W.; Clay, S.J.; Sloan, G.J.; Pretlow, L.G. Effects of Barometric Pressure and Temperature on Acute Ischaemic Stroke Hospitalization in Augusta, GA. Transl. Stroke Res. 2019, 10, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Cowperthwaite, M.C.; Burnett, M.G. An analysis of admissions from 155 United States hospitals to determine the influence of weather on stroke incidence. J. Clin. Neurosci. 2011, 18, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Hori, A.; Hashizume, M.; Tsuda, Y.; Tsukahara, T.; Nomiyama, T. Effects of weather variability and air pollutants on emergency admissions for cardiovascular and cerebrovascular diseases. Int. J. Environ. Heal. Res. 2012, 22, 416–430. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, D.; Salomaa, V.; Sivenius, J.; Tamminen, M.; Sarti, C.; Salmi, K.; Kaarsalo, E.; Narva, V.; Immonen-Räihä, P.; Torppa, J.; et al. Seasonal variation in the occurrence of stroke in a Finnish adult population: The FINMONICA Stroke Register. Stroke 1996, 27, 1774–1779. [Google Scholar] [CrossRef]
- Knezovic, M.; Pintaric, S.; Jelavic, M.M.; Kes, V.B.; Nesek, V.; Bogovic, S.; Cvetkovic, B.; Pintaric, H. The role of weather conditions and normal level of air pollution in appearance of stroke in the region of Southeast Europe. Acta Neurol. Belg. 2018, 118, 267–275. [Google Scholar] [CrossRef]
- Lim, J.-S.; Kwon, H.-M.; Kim, S.-E.; Lee, J.; Lee, Y.-S.; Yoon, B.-W. Effects of Temperature and Pressure on Acute Stroke Incidence Assessed Using a Korean Nationwide Insurance Database. J. Stroke 2017, 19, 295–303. [Google Scholar] [CrossRef][Green Version]
- Cornelissen Guillaume, G.; Gubin, D.; Beaty, L.A.; Otsuka, K. Some Near- and Far-Environmental Effects on Human Health and Disease with a Focus on the Cardiovascular System. Int. J. Environ. Res. Public Health 2020, 17, 3083. [Google Scholar] [CrossRef]
- Çevik, Y.; Doğan, N.Ö.; Daş, M.; Ahmedali, A.; Kul, S.; Bayram, H. The association between weather conditions and stroke admissions in Turkey. Int. J. Biometeorol. 2015, 59, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Tamasauskiene, L.; Rastenyte, D.; Radisauskas, R.; Tamosiunas, A.; Tamasauskas, D.; Vaiciulis, V.; Kranciukaite-Butylkiniene, D.; Milinaviciene, E. Relationship of meteorological factors and acute stroke events in Kaunas (Lithuania) in 2000–2010. Environ. Sci. Pollut. Res. 2017, 24, 9286–9293. [Google Scholar] [CrossRef]
- Gunes, H.; Kandis, H.; Saritas, A.; Dikici, S.; Buyukkaya, R. The relationship between ischaemic stroke and weather conditions in Duzce, Turkey. World J. Emerg. Med. 2015, 6, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, Y.; Wang, B.; Yang, H.; Ban, J.; Liu, F.; Li, T. Acute effects of temperature exposure on blood pressure: An hourly level panel study. Environ. Int. 2019, 124, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-Y.; Li, H.-M.; Yan, Z.-X.; Li, M.-C.; Wei, J.-P.; Zheng, W.-X.; Liu, S.-Q.; Deng, Y.-T.; Xie, H.-F.; Li, C.-G. Renin-angiotensin system activation and imbalance of matrix metalloproteinase-9/tissue inhibitor of matrix metalloproteinase-1 in cold-induced stroke. Life Sci. 2019, 231, 116563. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Wang, Z.; Xia, X.; Xue, J.; Gu, Y.; Han, S.; Wang, L.; Li, X.; Leng, S.X. Potential Impacts of Meteorological Variables on Acute Ischaemic Stroke Onset. Risk Manag. Healthc. Policy 2020, 13, 615–621. [Google Scholar] [CrossRef]
- Lee, H.C.; Hu, C.J.; Chen, C.S.; Lin, H.C. Seasonal variation in ischaemic stroke incidence and association with climate: A six-year population-based study. Chronobiol. Int. 2008, 25, 938–949. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, H.; Si, Y.; Cao, Y.; Song, J.; Li, M.; Wu, Y.; Wang, X.; Xiang, X.; Juan, J.; et al. Association between temperature variability and daily hospital admissions for cause-specific cardiovascular disease in urban China: A national time-series study. PLoS Med. 2019, 16, e1002738. [Google Scholar] [CrossRef]
- Lei, L.; Bao, J.; Guo, Y.; Wang, Q.; Peng, J.; Huang, C. Effects of diurnal temperature range on first-ever strokes in different seasons: A time-series study in Shenzhen, China. BMJ Open 2020, 10, e033571. [Google Scholar] [CrossRef]
- Lim, Y.-H.; Kim, H.; Kim, J.H.; Bae, S.; Hong, Y.-C. Effect of diurnal temperature range on cardiovascular markers in the elderly in Seoul, Korea. Int. J. Biometeorol. 2013, 57, 597–603. [Google Scholar] [CrossRef]
- Houck, P.D.; Lethen, J.E.; Riggs, M.W.; Gantt, D.S.; Dehmer, G.J. Relation of Atmospheric Pressure Changes and the Occurrences of Acute Myocardial Infarction and Stroke. Am. J. Cardiol. 2005, 96, 45–51. [Google Scholar] [CrossRef]
- Cuadrado-Godia, E.; Subirana, I.; Roquer, J. Weather as a trigger of stroke. Daily meteorological factors and incidence of stroke subtypes. Cerebrovasc. Dis. 2008, 26, 348–354. [Google Scholar] [CrossRef]
- Hirel, C.; Berton, L.; Preda, C.; Richard, O.; Lambert, Y.; Pico, F. Air pollution and humidity as triggering factors for stroke. Results of a 12-year analysis in the West Paris area. Rev. Neurol. 2019, 175, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yoon, K.; Choi, J.C.; Kim, H.; Song, J.-K. The association between wind-related variables and stroke symptom onset: A case-crossover study on Jeju Island. Environ. Res. 2016, 150, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Rakers, F.; Schiffner, R.; Rupprecht, S.; Brandstädt, A.; Witte, O.W.; Walther, M.; Schlattmann, P.; Schwab, M. Rapid weather changes are associated with increased ischaemic stroke risk: A case-crossover study. Eur. J. Epidemiol. 2016, 31, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Ruuskanen, J.O.; Sipilä, J.O.T.; Rautava, P.; Kytö, V. No association of moon phase with stroke occurrence. Chronobiol. Int. 2018, 35, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Busija, L.; Davis, S.; Yan, B.; Mao, Y.; Schnytzer, Y.; Churilov, L. “MOONSTROKE”: Lunar patterns of stroke occurrence combined with circadian and seasonal rhythmicity—A hospital based study. Chronobiol. Int. 2015, 32, 881–888. [Google Scholar] [CrossRef]
- Wang, R.R.; Hao, Y.; Chen, J.; Wang, M.Q.; Zheng, R.Y.; Shi, L.S.; He, J. Sex differences in the effects of the moon on ischaemic stroke incidence: New findings from Beijing, China. Chronobiol. Int. 2020, 37, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Engardt, M.; Bergström, R.; Andersson, C. Climate and emission changes contributing to changes in near-surface ozone in Europe over the coming decades: Results from model studies. Ambio 2009, 38, 452–458. [Google Scholar] [CrossRef] [PubMed]
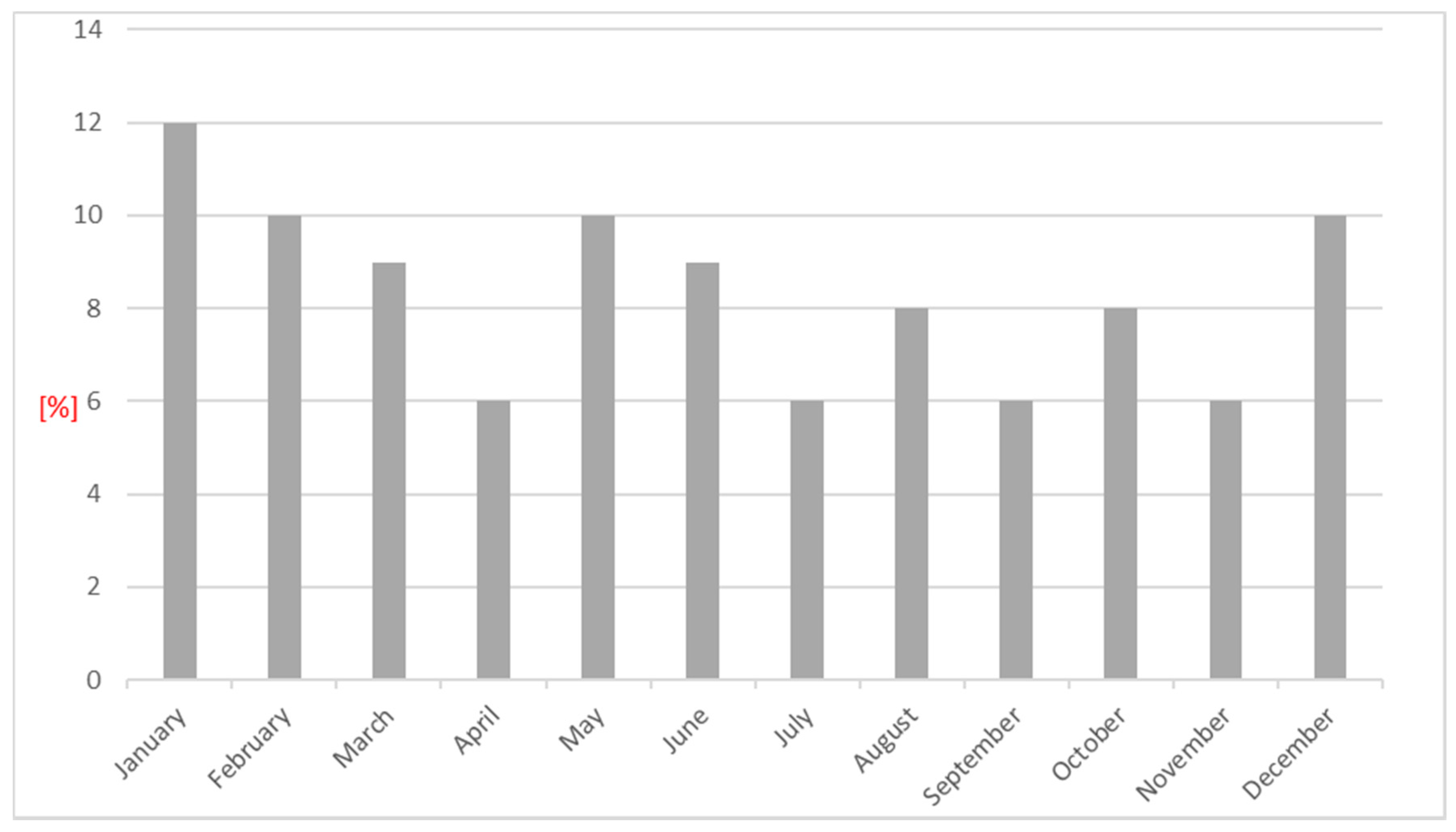
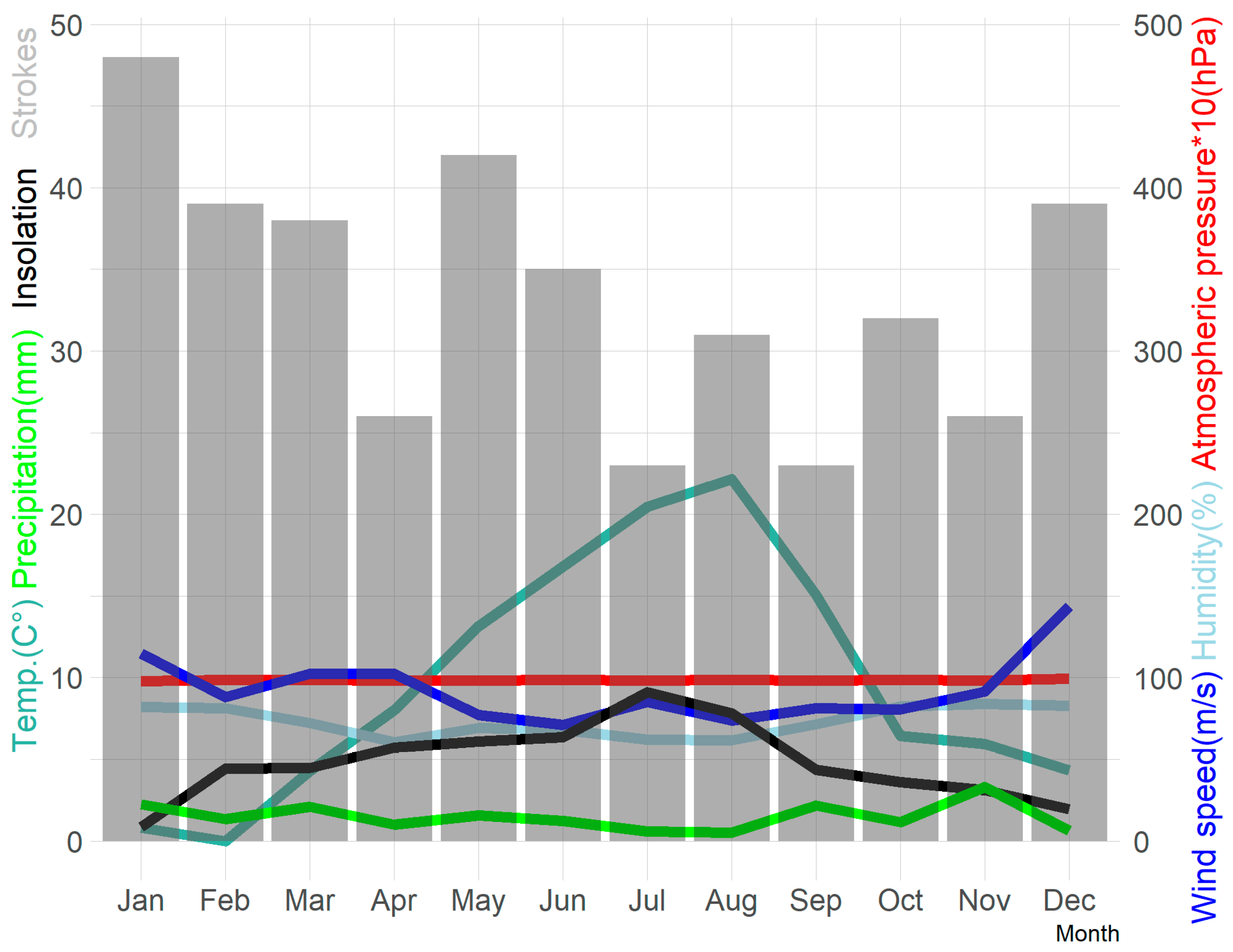
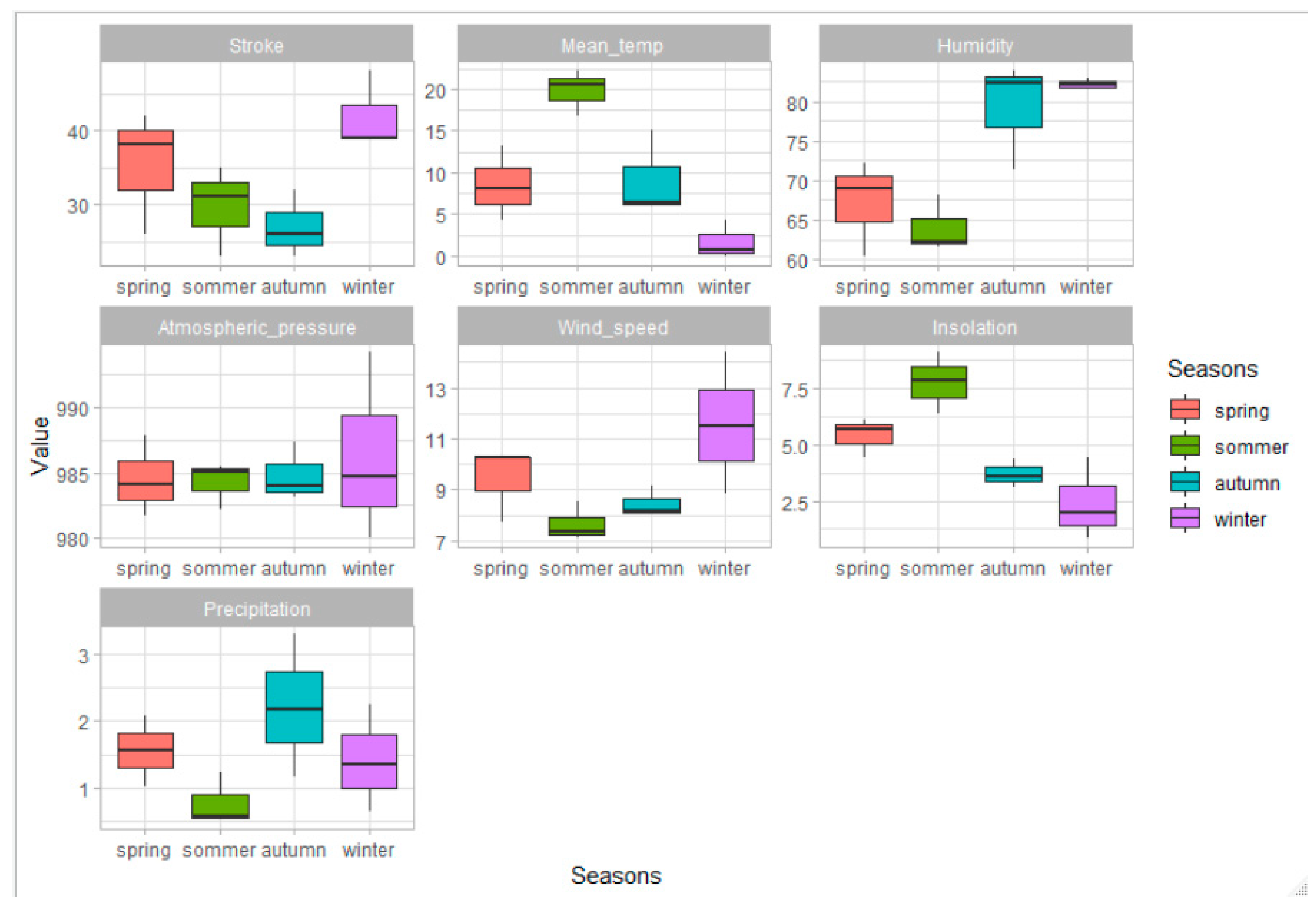
| Parameter | N = 402 | Women N = 200 | Men N = 202 | p | Patients ≤65 Years of Age N = 124 | Patients >65 Years of Age N = 278 | p |
|---|---|---|---|---|---|---|---|
| Age | 71.1 ± 13.1 Median 72; range (20–102) | 74.7 ± 13.4 Median 76 range (24–102) | 67.5 ± 11.9 Median 68 range (20–96) | 0.000 | - | - | - |
| Diabetes | 119 (29.6%) | 63 (31.5%) | 56 (27.7%) | 0.407 | 25 (20.2%) | 94 (33.8%) | 0.006 |
| Ischaemic heart disease | 180 (44.8%) | 102 (51.0%) | 78 (38.6%) | 0.013 | 31 (25%) | 149 (53.6%) | 0.000 |
| History of myocardial infarction | 72 (17.9%) | 38 (19.0%) | 34 (16.8%) | 0.571 | 20 (16.1%) | 52 (18.7%) | 0.534 |
| Carotid artery stenosis ≥50% * | 72 (17.9%) | 27 (13.5%) | 45 (22.3%) | 0.022 | 23 (18.5%) | 49 (17.6%) | 0.824 |
| Lipid disorders | 159 (39.6%) | 86 (43.0%) | 73 (36.1%) | 0.160 | 51 (41.1%) | 108 (38.8%) | 0.666 |
| Arterial hypertension | 353 (87.8%) | 177 (88.5%) | 176 (87.1%) | 0.674 | 96 (77.4%) | 257 (92.4%) | 0.000 |
| Atrial fibrillation | 123 (30.6%) | 65 (32.5%) | 58 (28.7%) | 0.410 | 15 (12.1%) | 108 (38.8%) | 0.000 |
| Previous stroke | 103 (25.6%) | 54 (27.0%) | 49 (24.3%) | 0.529 | 27 (21.8%) | 76 (27.3%) | 0.238 |
| NIHSS ** mean ± SD; med. (range); IQR (range Q1 Q3) | 4.90 ± 5.13 3 (0–30) 3 (1–16) | 5.21 ± 5.24 3 (0–23) 3 (0–18) | 4.59 ± 5.0 3 (0–30) 3 (0–17) | 0.268 | 4.46 ± 5.10 3 (0–30) 3 (0–21) | 5.09 ± 5.14 3 (0–28) 3 (1–26) | 0.129 |
| mRS *** mean ± SD; med. (range); IQR (range Q1 Q3) | 2.81 ± 1.96 3 (0–6) 3 (0–6); 3(2–5) | 3.1 ± 1.96 3 (0–6); 3 (0–6); 3 (2–5) | 2.52 ± 1.93 2 (0–6); 2 (0–6); 2 (2–5) | 0.004 | 2.31 ± 2.02 2 (0–6); 2 (0–6); 2 (2–5) | 3.04 ± 1.90 3 (0–6); 3 (0–6); 3 (2–5) | 0.001 |
| Hemorrhagic stroke | 38 (9.5%) | 23 (11.5%) | 15 (7.4%) | 0.163 | 13 (10.5%) | 25 (9.0%) | 0.637 |
| Ischaemic stroke | 364 (90.5%) | 177 (88.5%) | 187 (92.6%) | 0.163 | 111 (89.5%) | 253 (91.0%) | 0.637 |
| LACI | 51 (12.7%) | 24 (12%) | 27 (13.4%) | 0.681 | 21 (16.9%) | 30 (10.8%) | 0.087 |
| PACI | 212 (52.7%) | 112 (56%) | 100 (49.5%) | 0.192 | 64 (51.6%) | 148 (53.2%) | 0.763 |
| POCI | 91 (22.6%) | 37 (18.5%) | 54 (26.7%) | 0.049 | 21 (16.9%) | 70 (25.2%) | 0.068 |
| TACI | 10 (2.5%) | 4 (2%) | 6 (3.0%) | 0.532 | 5 (4.0%) | 5 (1.8%) | 0.184 |
| ASCOD—A | 60 (14.9%) | 22 (11%) | 38 (18.8%) | 0.028 | 21 (16.9%) | 39 (14%) | 0.450 |
| ASCOD—S | 59 (14.7%) | 28 (14%) | 31 (15.3%) | 0.703 | 23 (18.5%) | 36 (12.9%) | 0.143 |
| ASCOD—C | 145 (36.1%) | 74 (37%) | 71 (35.1%) | 0.699 | 23 (18.5%) | 122 (43.9%) | 0.000 |
| ASCOD—O | 100 (24.9%) | 53 (26.5%) | 47 (23.3%) | 0.454 | 44 (35.5%) | 56 (20.1%) | 0.001 |
| ASCOD—D | 0 (0%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Parameter | mRS 0–2 N = 177 | mRS 3–6 N = 225 | p |
|---|---|---|---|
| Mean temperature on the day of stroke [°C] | 7.98 ± 8.01 Median 6.1 (−7.2–27) | 9.63 ± 7.78 Median 8.8 (−7.2–26) | 0.041 |
| Mean temperature on the day preceding stroke [°C] | 8.13 ± 7.72 Median 6.9 (−7.2–27) | 9.70 ± 7.50 Median 9.3 (−7.2–26) | 0.048 |
| Mean atmospheric pressure on the day of stroke [hPa] | 984.3 ± 9.2 Median 984.8 (946.9–1004.7) | 985.6 ± 7.8 Median 986.9 (946.9–1001.6) | 0.129 |
| Mean atmospheric pressure on the day preceding stroke [hPa] | 984.5 ± 8.0 Median 984.5 (958.4–1004.7) | 985.7 ± 7.5 Median 985.6 (963.5–1004.4) | 0.122 |
| Mean relative humidity [%] | 75.1 ± 12.9 Median 76.2 (43.2–96.4) | 73.2 ± 13.4 Median 72.5 (43.6–95.8) | 0.146 |
| Mean wind speed [km/h] | 9.8 ± 5.1 Median 8.3 (1.8–24.1) | 9.1 ± 4.6 Median 7.8 (1.8–22.2) | 0.257 |
| Parameter | p | OR |
|---|---|---|
| Sex (men) | 0.001 | 2.00 |
| Age ≤ 65 years | 0.000 | 2.28 |
| No atrial fibrillation | 0.014 | 1.72 |
| Humidity > 75% | 0.016 | 1.61 |
| Parameter | Wind ≤ 8 m/s N = 202 | Wind > 8 m/s N = 200 | p |
|---|---|---|---|
| Ht [%] | 39.1 ± 4.8 Median 39.55 Range (23.2–48.4) | 39.8 ± 4.6 Median 40.45 Range (25–52.6) | 0.550 |
| RBC [M/µL] | 4.44 ± 0.57 Median 4.455 Range (2.54–5.88) | 4.51 ± 0.57 Median 4.585 Range (2.66–6.11) | 0.197 |
| PLTs [K/µL] | 235.3 ± 80.7 Median 219 Range (100–578) | 230.4 ± 79.3 Median 216.5 Range (73–606) | 0.537 |
| Systolic blood pressure [mmHg] | 160.2 ± 29.8 Median 160 Range (90–240) | 153.7 ± 26.0 Median 150 Range (110–240) | 0.021 |
| Diastolic blood pressure [mmHg] | 85.7 ± 16.3 Median 80 Range (50–160) | 85.6 ± 14.3 Median 80 Range (40–130) | 0.973 |
| NIHSS * mean ± SD; med. [range]; IQR [range Q1 Q3] | 4.86 ± 4.62 3 (0–22) 3 (0–16) | 4.94 ± 5.60 3 (0–30) 3 (0–19) | 0.335 |
| mRS ** mean ± SD; med. [range]; IQR [range Q1 Q3] | 2.91 ± 1.93 3 (0–6); 3 (2–5) | 2.72 ± 2.00 3 (0–6); 3 (2–5) | 0.323 |
| Parameter | Risk Ratio | Estimate | Robust SE | Pr (>|z|) | CI 95% | |
|---|---|---|---|---|---|---|
| LL | UL | |||||
| Mean temperature [°C] | 1.019 | 0.019 | 0.003 | <0.0001 | 0.014 | 0.024 |
| Relative humidity [%] | 1.028 | 0.028 | 0.003 | <0.0001 | 0.023 | 0.033 |
| Atmospheric pressure [hPa] | 0.997 | −0.003 | 0.004 | 0.37 | −0.01 | 0.004 |
| Wind speed [km/h] | 0.923 | −0.08 | 0.008 | <0.0001 | −0.096 | −0.065 |
| Insolation [h] | 0.885 | −0.122 | 0.01 | <0.0001 | −0.141 | −0.103 |
| Precipitation [mm] | 0.914 | −0.089 | 0.017 | <0.0001 | −0.123 | −0.056 |
| Season—summer | 1.839 | 0.609 | 0.053 | <0.0001 | 0.506 | 0.713 |
| Season—spring | 2.309 | 0.837 | 0.032 | <0.0001 | 0.775 | 0.899 |
| Season—winter | 1.698 | 0.53 | 0.031 | <0.0001 | 0.468 | 0.591 |
| Moon Phase | Hemorrhagic Stroke N = 10 | Subarachnoid Hemorrhage N = 28 | LACI N = 51 | PACI N = 212 | POCI N = 91 | TACI N = 10 |
|---|---|---|---|---|---|---|
| New moon | 1 (10%) | 6 (21.43%) | 12 (23.5%) | 54 (25.5%) | 21 (23%) | 2 (20%) |
| 1st quarter moon | 4 (40%) | 6 (21.43%) | 21 (41.2%) | 50 (23.6%) | 31 (34%) | 1 (10%) |
| Full moon | 2 (20%) | 9 (32.14%) | 8 (15.7%) | 52 (24.5%) | 17 (19%) | 0 |
| 3rd quarter moon | 3 (30%) | 7 (25.0%) | 10 (19.6%) | 56 (26.4%) | 22 (24%) | 7 (70%) |
| p | 0.7011 | 0.5697 | 0.137 | 1.000 | 0.524 | 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaręba, K.; Lasek-Bal, A.; Student, S. The Influence of Selected Meteorological Factors on the Prevalence and Course of Stroke. Medicina 2021, 57, 1216. https://doi.org/10.3390/medicina57111216
Zaręba K, Lasek-Bal A, Student S. The Influence of Selected Meteorological Factors on the Prevalence and Course of Stroke. Medicina. 2021; 57(11):1216. https://doi.org/10.3390/medicina57111216
Chicago/Turabian StyleZaręba, Katarzyna, Anetta Lasek-Bal, and Sebastian Student. 2021. "The Influence of Selected Meteorological Factors on the Prevalence and Course of Stroke" Medicina 57, no. 11: 1216. https://doi.org/10.3390/medicina57111216
APA StyleZaręba, K., Lasek-Bal, A., & Student, S. (2021). The Influence of Selected Meteorological Factors on the Prevalence and Course of Stroke. Medicina, 57(11), 1216. https://doi.org/10.3390/medicina57111216






