Effectiveness of Postoperative or Preoperative Radiotherapy on Prognosis in Patients with Stage II Resectable Non-Small Cell Lung Cancer: A Retrospective Study Based on the SEER Database
Abstract
:1. Introduction
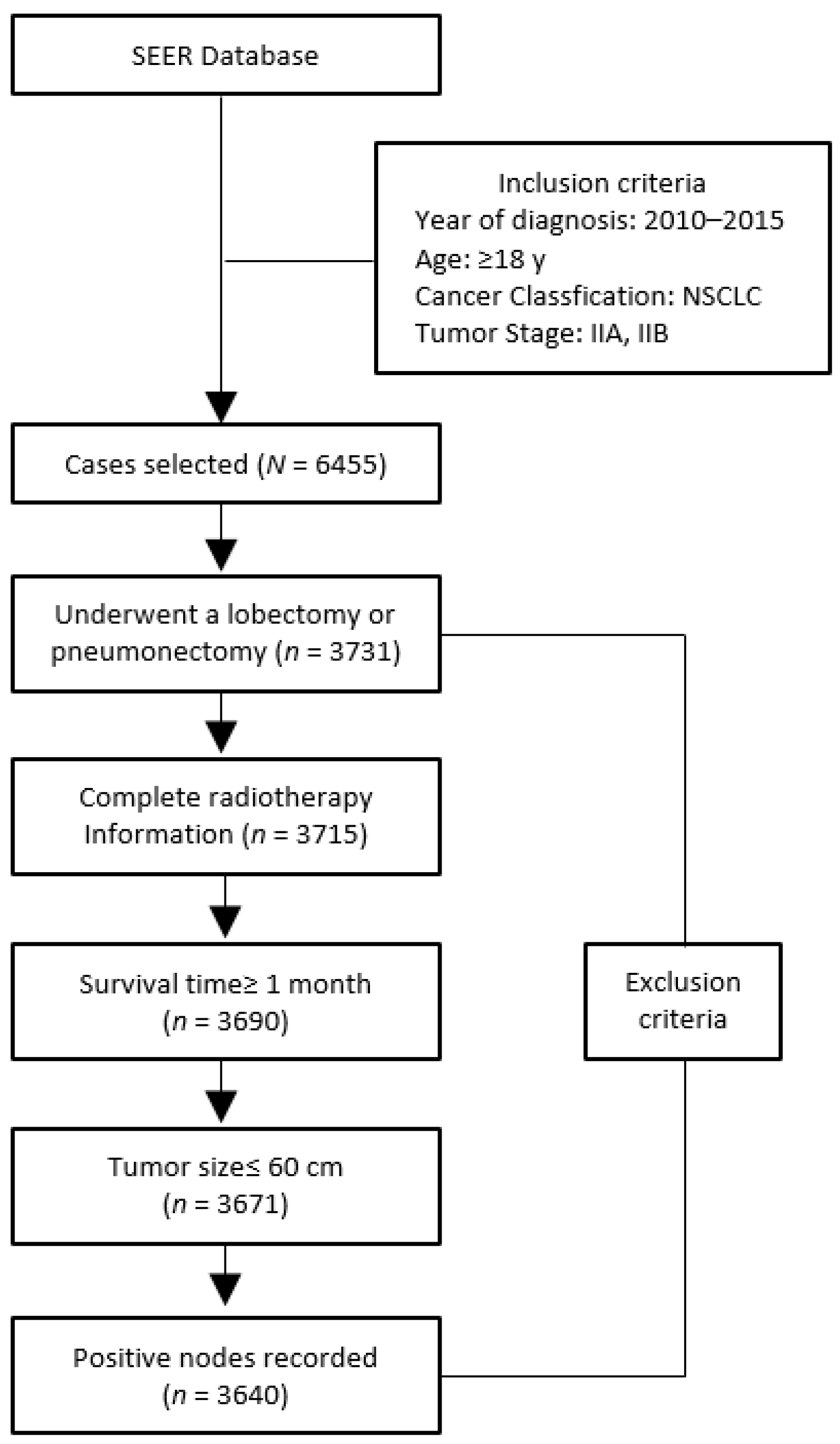
2. Methods
3. Results
3.1. Distribution Characteristic of Factors Related to Use of PORT or PRRT
3.2. Univariate and Multivariate Analysis of Variables Influencing Prognosis of Patients
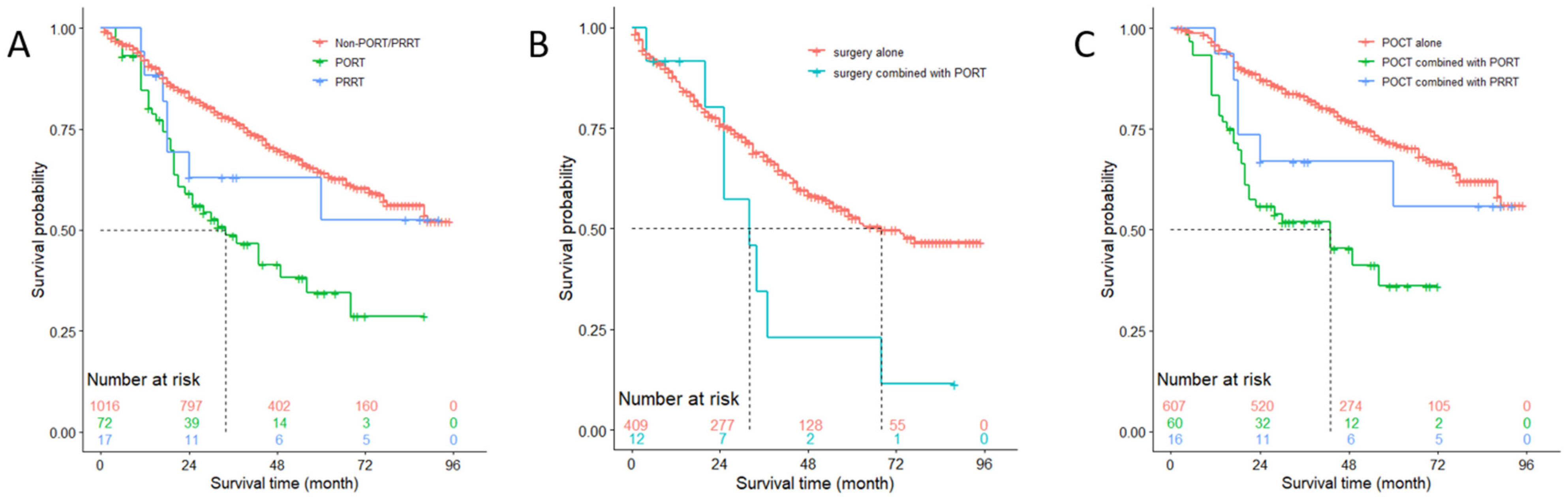
3.3. Multivariate Analysis of Important Variables Affecting Prognosis of Patients with N1 Stage
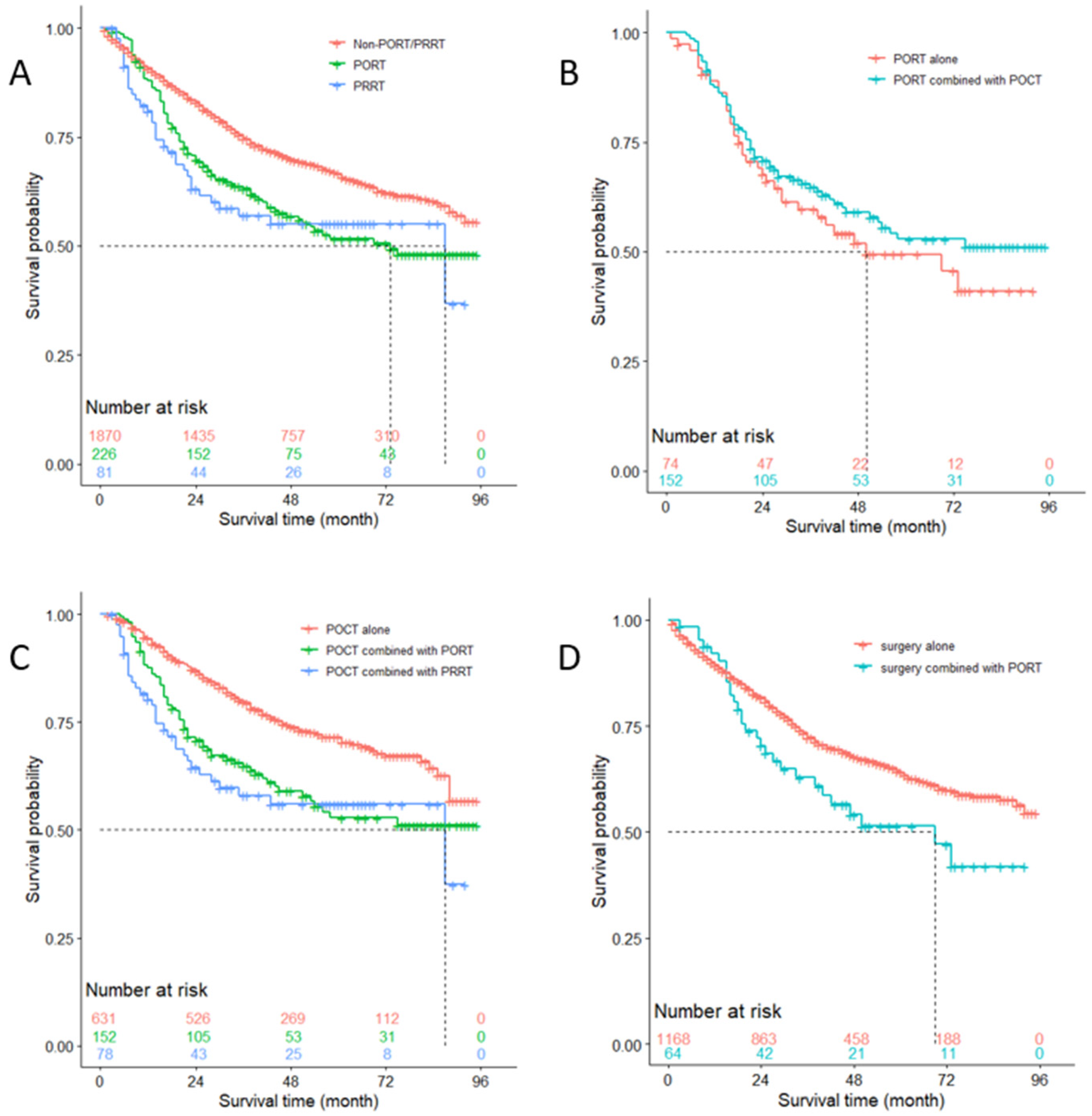
3.4. Subgroup Analysis of the Risk of Lung Cancer-Specific Death Caused by Using PORT
3.5. OS Analysis of Patients with N0 Stage
3.6. OS Analysis of Patients with N1 Stage
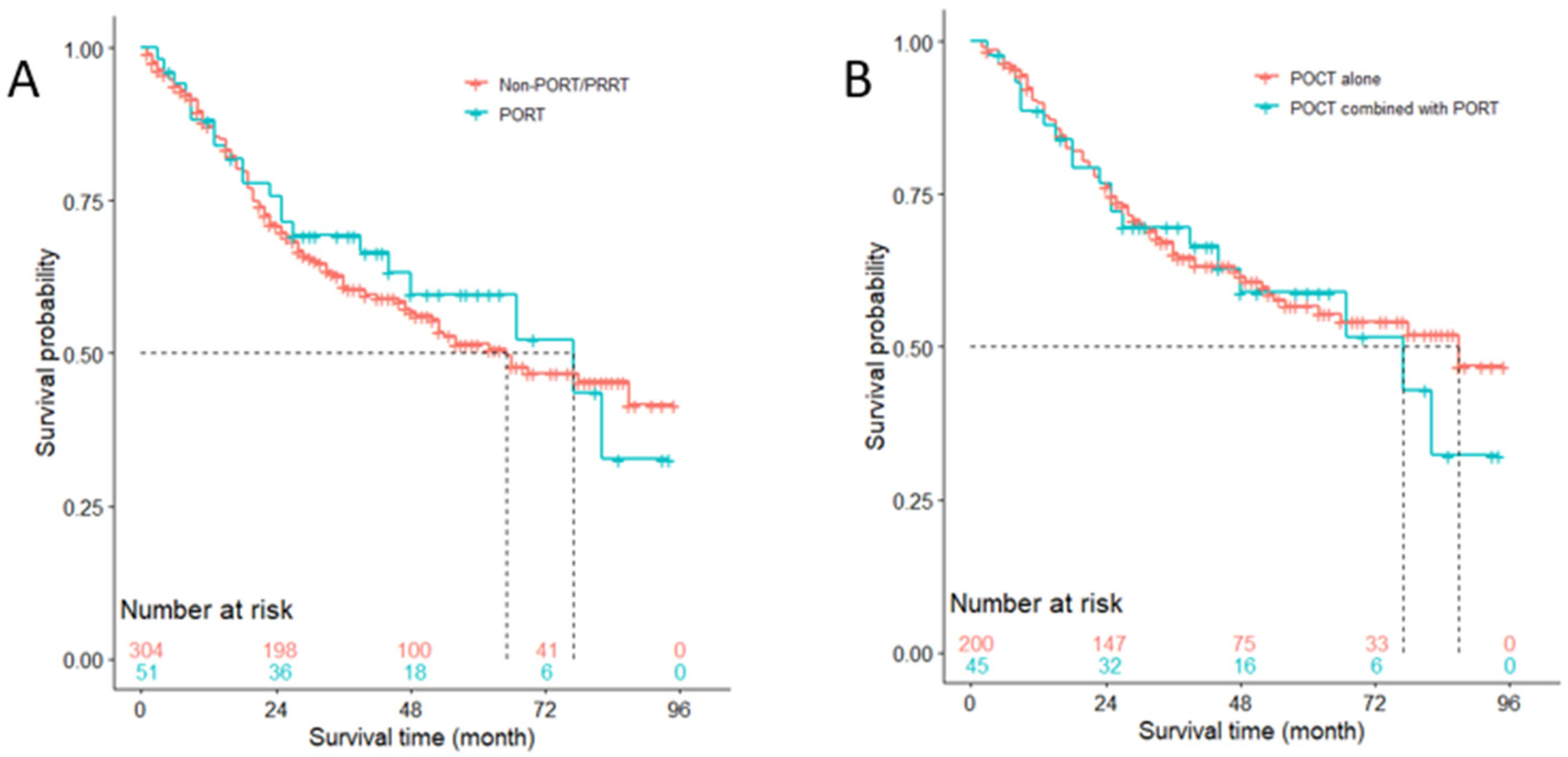
3.7. Competitive Risk Analysis of NSCLC-Specific Death
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.; Zheng, R.; Baade, P.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am. J. Respir. Crit. Care Med. 2002, 165, 277–304. [Google Scholar]
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.F.; Wong, K.-K. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat. Rev. Cancer 2014, 14, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Groome, P.A.; Bolejack, V.; Crowley, J.J.; Kennedy, C.; Krasnik, M.; Sobin, L.H.; Goldstraw, P.; IASLC International Staging Committee; Cancer Research and Biostatistics; Observers to the Committee; et al. The IASLC Lung Cancer Staging Project: Validation of the Proposals for Revision of the T, N, and M Descriptors and Consequent Stage Groupings in the Forthcoming (Seventh) Edition of the TNM Classification of Malignant Tumours. J. Thorac. Oncol. 2007, 2, 694–705. [Google Scholar] [CrossRef] [Green Version]
- Vansteenkiste, J.; Crinò, L.; Dooms, C.; Douillard, J.Y.; Faivre-Finn, C.; Lim, E.; Rocco, G.; Senan, S.; Van Schil, P.; Veronesi, G.; et al. 2nd ESMO Consensus Conference on Lung Cancer: Early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, 1462–1474. [Google Scholar] [CrossRef]
- Van Schil, P.E.; Asamura, H.; Rusch, V.W.; Mitsudomi, T.; Tsuboi, M.; Brambilla, E.; Travis, W.D. Surgical implications of the new IASLC/ATS/ERS adenocarcinoma classification. Eur. Respir. J. 2012, 39, 478–486. [Google Scholar] [CrossRef] [Green Version]
- McDonald, F.; De Waele, M.; Hendriks, L.; Faivre-Finn, C.; Dingemans, A.-M.C.; Van Schil, P.E. Management of stage I and II nonsmall cell lung cancer. Eur. Respir. J. 2017, 49, 1600764. [Google Scholar] [CrossRef] [Green Version]
- Green, N.; Kurohara, S.S.; George, F.W., 3rd; Crews, Q.E., Jr. Postresection Irradiation for Primary Lung Cancer. Radiology 1975, 116, 405–407. [Google Scholar] [CrossRef]
- Choi, N.C.H.; Grillo, H.C.; Gardiello, M.; Scannell, J.G.; Wilkins, E.W. Basis for new strategies in postoperative radiotherapy of bronchogenic carcinoma. Int. J. Radiat. Oncol. 1980, 6, 31–35. [Google Scholar] [CrossRef]
- Martini, N.; Flehinger, B.J.; Zaman, M.B.; Beattie, E.J. Prospective study of 445 lung carcinomas with mediastinal lymph node metastases. J. Thorac. Cardiovasc. Surg. 1980, 80, 390–399. [Google Scholar] [CrossRef]
- Chung, C.K.; Stryker, J.A.; O’Neill, M., Jr.; DeMuth, W.E., Jr. Evaluation of adjuvant postoperative radiotherapy lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 1982, 8, 1877–1880. [Google Scholar] [CrossRef]
- Kirsh, M.-M.; Herbert, S. Mediastinal Metastases in Bronchogenic Carcinoma: Influence of Postoperative Irradiation, Cell Type, and Location. Ann. Thorac. Surg. 1982, 33, 459–463. [Google Scholar] [CrossRef]
- Wang, E.H.; Corso, C.D.; Rutter, C.E.; Park, H.S.; Chen, A.B.; Kim, A.W.; Wilson, L.D.; Decker, R.H.; Yu, J.B. Postoperative Radiation Therapy Is Associated with Improved Overall Survival in Incompletely Resected Stage II and III Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2015, 33, 2727–2734. [Google Scholar] [CrossRef]
- Faber, L.P.; Kittle, C.F.; Warren, W.H.; Bonomi, P.D.; Taylor, S.G., 4th; Reddy, S.; Lee, M.S. Preoperative chemotherapy and irradiation for stage III non-small cell lung cancer. Ann. Thorac. Surg. 1989, 47, 669–677. [Google Scholar] [CrossRef]
- Choi, N.C.; Carey, R.W.; Daly, W.; Mathisen, D.; Wain, J.; Wright, C.; Lynch, T.; Grossbard, M.; Grillo, H. Potential impact on survival of improved tumor downstaging and resection rate by preoperative twice-daily radiation and concurrent chemotherapy in stage IIIA non-small-cell lung cancer. J. Clin. Oncol. 1997, 15, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, H.; Song, X.; Yue, J.; Yu, J. Preoperative radiation may improve the outcomes of resectable IIIA/N2 non-small-cell lung cancer patients: A propensity score matching-based analysis from surveillance, epidemiology, and end results database. Cancer Med. 2018, 7, 4354–4360. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.F.; Wang, M.; Wang, L.J.; Yang, Z.Y.; Zhang, Y.G.; Zhang, D.W.; Yin, W.B. A study of postoperative radiotherapy in patients with non–small-cell lung cancer: A randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 925–929. [Google Scholar] [CrossRef]
- Lally, B.E.; Zelterman, D.; Colasanto, J.M.; Haffty, B.G.; Detterbeck, F.C.; Wilson, L.D. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J. Clin. Oncol. 2006, 24, 2998–3006. [Google Scholar] [CrossRef] [PubMed]
- Burdett, S.; Stewart, L. Postoperative radiotherapy in non-small-cell lung cancer: Update of an individual patient data meta-analysis. Lung Cancer 2005, 47, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Burdett, S.; Rydzewska, L.; Tierney, J.; Fisher, D.; Parmar, M.K.; Arriagada, R.; Pignon, J.P.; Le Pechoux, C.; PORT Meta-Analysis Trialists Group. Postoperative radiotherapy for non-small cell lung cancer. Cochrane. Database Syst. Rev. 2016, 10, D2142. [Google Scholar]
- Scott, W.-J.; John, H.; Benjamin, M. Treatment of Stage II Non-small Cell Lung Cancer. Chest 2003, 123 (Suppl. 1), 188S–201S. [Google Scholar] [CrossRef] [PubMed]
- PORT Meta-Analysis Trialists Group. Postoperative radiotherapy in non-small-cell lung cancer: Systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Lancet 1998, 352, 257–263. [Google Scholar] [CrossRef]
- Videtic, G.M.; Chang, J.Y.; Chetty, I.J.; Ginsburg, M.E.; Kestin, L.L.; Kong, F.M.; Lally, B.E.; Loo, B.W., Jr.; Movsas, B.; Stinchcombe, T.E.; et al. ACR Appropriateness Criteria® Early-Stage Non–Small–Cell Lung Cancer. Am. J. Clin. Oncol. 2014, 37, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Okawara, G.; Ung, Y.C.; Markman, B.R.; Mackay, J.A.; Evans, W.K.; Lung Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based Care. Postoperative radiotherapy in stage II or IIIA completely resected non-small cell lung cancer: A systematic review and practice guideline. Lung Cancer 2004, 44, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gudbjartsson, T.; Gyllstedt, E.; Pikwer, A.; Jönsson, P. Early Surgical Results After Pneumonectomy for Non-Small Cell Lung Cancer are not Affected by Preoperative Radiotherapy and Chemotherapy. Ann. Thorac. Surg. 2008, 86, 376–382. [Google Scholar] [CrossRef] [PubMed]


| Variable | Non-PORT/PRRT (n = 3190) | PORT (n = 349) | PRRT (n = 101) | Overall (N = 3640) | p Value |
|---|---|---|---|---|---|
| Year of diagnosis, n (%) | |||||
| 2010 | 534 (16.7) | 70 (20.1) | 25 (24.8) | 629 (17.3) | 0.263 |
| 2011 | 579 (18.2) | 60 (17.2) | 18 (17.8) | 657 (18.0) | |
| 2012 | 520 (16.3) | 54 (15.5) | 22 (21.8) | 596 (16.4) | |
| 2013 | 552 (17.3) | 55 (15.8) | 14 (13.9) | 621 (17.1) | |
| 2014 | 513 (16.1) | 61 (17.5) | 11 (10.9) | 585 (16.1) | |
| 2015 | 492 (15.4) | 49 (14.0) | 11 (10.9) | 552 (15.2) | |
| Age at diagnosis | |||||
| Mean (SD) | 67.9 (9.4) | 66.9 (9.7) | 64.8 (9.3) | 67.8 (9.5) | <0.001 |
| Median (Min, Max) | 68.0 (30.0, 92.0) | 67.0 (35.0, 88.0) | 66.0 (36.0, 85.0) | 68.0 (30.0, 92.0) | |
| Sex, n (%) | |||||
| Female | 1521 (47.7) | 149 (42.7) | 39 (38.6) | 1709 (47.0) | 0.049 |
| Male | 1669 (52.3) | 200 (57.3) | 62 (61.4) | 1931 (53.0) | |
| Race, n (%) | |||||
| Black | 290 (9.1) | 32 (9.2) | 15 (14.9) | 337 (9.3) | 0.052 |
| White | 2654 (83.2) | 278 (79.7) | 80 (79.2) | 3012 (82.7) | |
| Others | 246 (7.7) | 39 (11.2) | 6 (5.9) | 291 (8.0) | |
| Pathologic Grade, n (%) | |||||
| Grade I | 369 (11.6) | 16 (4.6) | 4 (4.0) | 389 (10.7) | <0.001 |
| Grade II | 1343 (42.1) | 137 (39.3) | 22 (21.8) | 1502 (41.3) | |
| Grade III | 1221 (38.3) | 168 (48.1) | 46 (45.5) | 1435 (39.4) | |
| Grade IV | 69 (2.2) | 7 (2.0) | 2 (2.0) | 78 (2.1) | |
| Unknown | 188 (5.9) | 21 (6.0) | 27 (26.7) | 236 (6.5) | |
| Tumor size | |||||
| Mean (SD) | 45.3 (33.1) | 46.1 (26.8) | 57.2 (22.5) | 45.7 (32.4) | 0.001 |
| Median (Min, Max) | 40.0 (1.00, 540) | 40.0 (1.00, 225) | 54.0 (15.0, 140) | 40.0 (1.00, 540) | |
| Location, n (%) | |||||
| Upper lobe | 1769 (55.5) | 226 (64.8) | 80 (79.2) | 2075 (57.0) | <0.001 |
| Middle lobe | 157 (4.9) | 14 (4.0) | 0 (0) | 171 (4.7) | |
| Lower lobe | 1146 (35.9) | 92 (26.4) | 16 (15.8) | 1254 (34.5) | |
| Main bronchus | 26 (0.8) | 6 (1.7) | 1 (1.0) | 33 (0.9) | |
| Multi-lobe | 92 (2.9) | 11 (3.2) | 4 (4.0) | 107 (2.9) | |
| Histology, n (%) | |||||
| Ad | 2064 (64.7) | 180 (51.6) | 43 (42.6) | 2287 (62.8) | <0.001 |
| Sq | 958 (30.0) | 143 (41.0) | 47 (46.5) | 1148 (31.5) | |
| Others | 168 (5.3) | 26 (7.4) | 11 (10.9) | 205 (5.6) | |
| Stage, n (%) | |||||
| IIA | 1706 (53.5) | 129 (37.0) | 25 (24.8) | 1860 (51.1) | <0.001 |
| IIB | 1484 (46.5) | 220 (63.0) | 76 (75.2) | 1780 (48.9) | |
| T, n (%) | |||||
| T1 | 460 (14.4) | 43 (12.3) | 3 (3.0) | 506 (13.9) | <0.001 |
| T2 | 1451 (45.5) | 109 (31.2) | 27 (26.7) | 1587 (43.6) | |
| T3 | 1279 (40.1) | 197 (56.4) | 71 (70.3) | 1547 (42.5) | |
| N, n (%) | |||||
| N0 | 1870 (58.6) | 226 (64.8) | 81 (80.2) | 2177 (59.8) | <0.001 |
| N1 | 1320 (41.4) | 123 (35.2) | 20 (19.8) | 1463 (40.2) | |
| Positive Lymph Nodes, n (%) | |||||
| <3 | 2787 (87.4) | 273 (78.2) | 89 (88.1) | 3149 (86.5) | <0.001 |
| ≥3 | 403 (12.6) | 76 (21.8) | 12 (11.9) | 491 (13.5) | |
| POCT, n (%) | |||||
| No | 1752 (54.9) | 92 (26.4) | 5 (5.0) | 1849 (50.8) | <0.001 |
| Yes | 1438 (45.1) | 257 (73.6) | 96 (95.0) | 1791 (49.2) | |
| <3 Positive Lymph Nodes (n = 1105) | ≥3 Positive Lymph Nodes (n = 358) | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95 CI | p Value | HR | 95 CI | p Value |
| Treatment | ||||||
| Non-PORT/ PRRT | Ref | Ref | ||||
| PORT | 2.698 | (1.910, 3.812) | <0.001 | 0.903 | (0.537, 1.517) | 0.700 |
| PRRT | 1.453 | (0.641, 3.294) | 0.370 | 0.659 | (0.076, 5.693) | 0.700 |
| Year of diagnosis | ||||||
| 2010 | Ref | Ref | ||||
| 2011 | 0.889 | (0.651, 1.213) | 0.460 | 0.955 | (0.585, 1.559) | 0.850 |
| 2012 | 0.951 | (0.687, 1.315) | .760 | 1.088 | (0.633, 1.871) | 0.760 |
| 2013 | 0.663 | (0.470, 0.937) | 0.020 | 0.934 | (0.517, 1.690) | 0.820 |
| 2014 | 0.707 | (0.487, 1.026) | 0.068 | 0.757 | (0.420, 1.362) | 0.350 |
| 2015 | 0.532 | (0.338, 0.837) | 0.006 | 0.716 | (0.364, 1.410) | 0.330 |
| Age at diagnosis | 1.009 | (0.997, 1.020) | 0.150 | 1.015 | (0.996, 1.034) | 0.130 |
| Sex | ||||||
| Female | Ref | Ref | ||||
| Male | 1.328 | (1.073, 1.644) | 0.009 | 1.137 | (0.786, 1.647) | 0.500 |
| Race | ||||||
| Black | Ref | Ref | ||||
| White | 0.683 | (0.499, 0.933) | 0.017 | 0.387 | (0.197, 0.762) | 0.006 |
| Others | 0.588 | (0.377, 0.916) | 0.019 | 1.145 | (0.595, 2.201) | 0.690 |
| Pathologic Grade, n () | ||||||
| I | Ref | Ref | ||||
| II | 1.302 | (0.801, 2.117) | 0.290 | 0.809 | (0.362, 1.808) | 0.610 |
| III | 1.748 | (1.075, 2.843) | 0.024 | 1.122 | (0.522, 2.412) | 0.770 |
| IV | 1.779 | (0.737, 4.297) | 0.200 | 1.414 | (0.386, 5.176) | 0.600 |
| Unknown | 0.904 | (0.401, 2.036) | 0.810 | 0.7 | (0.246, 1.994) | 0.500 |
| Tumor Size | 1.001 | (0.998, 1.003) | 0.670 | 1.019 | (1.001, 1.038) | 0.035 |
| Location | ||||||
| Upper lobe | Ref | Ref | ||||
| Middle lobe | 0.952 | (0.645, 1.404) | 0.800 | 1.031 | (0.515, 2.065) | 0.930 |
| Lower lobe | 0.986 | (0.780, 1.246) | 0.900 | 1.419 | (1.001, 2.011) | 0.049 |
| Main bronchus | 0.819 | (0.278, 2.411) | 0.720 | 0.721 | (0.125, 4.168) | 0.710 |
| Multi-lobe | 2.587 | (1.318, 5.075) | 0.006 | 1.428 | (0.624, 3.266) | 0.400 |
| Histology | ||||||
| Ad | Ref | Ref | ||||
| Sq | 0.763 | (0.597, 0.975) | 0.030 | 0.689 | (0.458, 1.036) | 0.073 |
| Others | 0.858 | (0.520, 1.415) | 0.550 | 1.023 | (0.505, 2.072) | 0.950 |
| Stage | ||||||
| IIA | Ref | Ref | ||||
| IIB | 0.963 | (0.689, 1.346) | 0.820 | 0.642 | (0.344, 1.200) | 0.160 |
| T | ||||||
| T1 | Ref | Ref | ||||
| T2 | 1.334 | (1.051, 1.694) | 0.018 | 0.999 | (0.631, 1.583) | 1.000 |
| POCT | ||||||
| No | Ref | Ref | ||||
| Yes | 0.640 | (0.510, 0.803) | <.001 | 0.664 | (0.456, 0.967) | 0.033 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Yu, J. Effectiveness of Postoperative or Preoperative Radiotherapy on Prognosis in Patients with Stage II Resectable Non-Small Cell Lung Cancer: A Retrospective Study Based on the SEER Database. Medicina 2021, 57, 1202. https://doi.org/10.3390/medicina57111202
Chen D, Yu J. Effectiveness of Postoperative or Preoperative Radiotherapy on Prognosis in Patients with Stage II Resectable Non-Small Cell Lung Cancer: A Retrospective Study Based on the SEER Database. Medicina. 2021; 57(11):1202. https://doi.org/10.3390/medicina57111202
Chicago/Turabian StyleChen, Deng, and Jinming Yu. 2021. "Effectiveness of Postoperative or Preoperative Radiotherapy on Prognosis in Patients with Stage II Resectable Non-Small Cell Lung Cancer: A Retrospective Study Based on the SEER Database" Medicina 57, no. 11: 1202. https://doi.org/10.3390/medicina57111202
APA StyleChen, D., & Yu, J. (2021). Effectiveness of Postoperative or Preoperative Radiotherapy on Prognosis in Patients with Stage II Resectable Non-Small Cell Lung Cancer: A Retrospective Study Based on the SEER Database. Medicina, 57(11), 1202. https://doi.org/10.3390/medicina57111202





