COVID-19 and Its Repercussions on Oral Health: A Review
Abstract
:1. The Essentials about CoVs
2. Main Characteristics of COVID-19
2.1. General Characteristics
2.2. Severity by Age
2.3. Skin Involvement
3. Oral Lesions Related to COVID-19
4. The Association between Periodontal Disease and COVID-19
5. Temporomandibular Disorders Associated with COVID-19 Pandemic
6. Dental Medicine during the COVID-19 Pandemic
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Weiss, S.R.; Leibowitz, J.L. Coronavirus Pathogenesis. Adv. Appl. Microbiol. 2011, 81, 85–164. [Google Scholar] [CrossRef]
- Hui, D.S.; Azhar, E.I.; Memish, Z.A.; Zumla, A. Human Coronavirus Infections—Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS), and SARS-CoV-2. In Encyclopedia of Respiratory Medicine; Elsevier BV: Amsterdam, The Netherlands, 2021; pp. 146–161. [Google Scholar]
- Ramadan, N.; Shaib, H. Middle East respiratory syndrome coronavirus (MERS-CoV): A review. Germs 2019, 9, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Zhang, C.; Huang, F.; Wang, F.; Yuan, J.; Wang, Z.; Li, J.; Li, J.; Feng, C.; et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020, 63, 364–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Ge, H.; Wang, X.; Yuan, X.; Xiao, G.; Wang, C.; Deng, T.; Yuan, Q.; Xiao, X. The epidemiology and clinical information about COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J. SARS-CoV-2: An Emerging Coronavirus that Causes a Global Threat. Int. J. Biol. Sci. 2020, 16, 1678–1685. [Google Scholar] [CrossRef] [Green Version]
- Guarner, J. Three Emerging Coronaviruses in Two Decades. Am. J. Clin. Pathol. 2020, 153, 420–421. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- World Health Organization. Novel Coronavirus (2019-nCoV) Situation Report—22—Data as Reported by 11 February 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf?sfvrsn=fb6d49b1_2 (accessed on 15 June 2021).
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2020, 1866, 165878. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; Van De Veen, W.; Brüggen, M.-C.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.K.; Firmal, P.; Alam, A.; Ganguly, D.; Chattopadhyay, S. Overview of Immune Response During SARS-CoV-2 Infection: Lessons From the Past. Front. Immunol. 2020, 11, 1949. [Google Scholar] [CrossRef]
- Wei, C.; Liu, Y.; Liu, Y.; Zhang, K.; Su, D.; Zhong, M.; Meng, X. Clinical characteristics and manifestations in older patients with COVID-19. BMC Geriatr. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sciacqua, A.; Pujia, R.; Arturi, F.; Hribal, M.L.; Montalcini, T. COVID-19 and elderly: Beyond the respiratory drama. Intern. Emerg. Med. 2020, 15, 907–909. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, Z.; Zhang, T.; Guo, W.; Guo, W.; Zheng, J.; Zhang, J.; Dong, C.; Na, R.; Zheng, L.; et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J. Med. Virol. 2021, 93, 1057–1069. [Google Scholar] [CrossRef]
- Panahi, L.; Amiri, M.; Pouy, S. Clinical Characteristics of COVID-19 Infection in Newborns and Pediatrics: A Systematic Review. Arch. Acad. Emerg. Med. 2020, 18, e50. [Google Scholar]
- Zare-Zardini, H.; Soltaninejad, H.; Ferdosian, F.; Hamidieh, A.A.; Memarpoor-Yazdi, M. Coronavirus Disease 2019 (COVID-19) in Children: Prevalence, Diagnosis, Clinical Symptoms, and Treatment. Int. J. Gen. Med. 2020, 13, 477–482. [Google Scholar] [CrossRef]
- De Souza, T.H.; Nadal, J.A.; Nogueira, R.J.N.; Pereira, R.M.; Brandão, M.B. Clinical manifestations of children with COVID-19: A systematic review. Pediatr. Pulmonol. 2020, 55, 1892–1899. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Oto Rhino Laryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yan, H.; Guo, W. Clinical Characteristics of Children With COVID-19: A Meta-Analysis. Front. Pediatr. 2020, 8, 431. [Google Scholar] [CrossRef]
- Pal, M.; Berhanu, G.; Desalegn, C.; Kandi, V. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): An Update. Cureus 2020, 12, e7423. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.; Ryu, H.-S.; Kim, H.A.; Hyun, M.; Lee, J.Y.; Yi, H.-A. Prognostic Factors of COVID-19 Infection in Elderly Patients: A Multicenter Study. J. Clin. Med. 2020, 9, 3932. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, Y.; Lin, R.; Han, K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J. Infect. 2020, 80, e14–e18. [Google Scholar] [CrossRef] [Green Version]
- Rui, L.; Sirui, L.; Xuebei, D.; Xujun, Y.; Yanggan, W. Clinical observations in very elderly patients with COVID-19 in Wuhan. Geriatr. Gerontol. Int. 2020, 20, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Mehdizadeh, M.; Badv, R.S.; Navaeian, A.; Pourakbari, B.; Rostamyan, M.; Ekbatani, M.S.; Eshaghi, H.; Abdolsalehi, M.R.; Alimadadi, H.; et al. The Coronavirus Disease 2019 (COVID-19) in Children: A Study in an Iranian Children’s Referral Hospital. Infect. Drug Resist. 2020, 13, 2649–2655. [Google Scholar] [CrossRef]
- Mori, H.; Obinata, H.; Murakami, W.; Tatsuya, K.; Sasaki, H.; Miyake, Y.; Taniguchi, Y.; Ota, S.; Yamaga, M.; Suyama, Y.; et al. Comparison of COVID-19 disease between young and elderly patients: Hidden viral shedding of COVID-19. J. Infect. Chemother. 2021, 27, 70–75. [Google Scholar] [CrossRef]
- Unicef. Available online: https://www.unicef.org/coronavirus/what-you-need-know-about-delta-variant. (accessed on 21 October 2021).
- Criado, P.; Abdalla, B.M.Z.; De Assis, I.C.; Mello, C.V.B.D.G.; Caputo, G.C.; Vieira, I.C. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? Revision of possible pathophysiologic mechanisms. Inflamm. Res. 2020, 69, 745–756. [Google Scholar] [CrossRef]
- Criado, P.R.; Pagliari, C.; Carneiro, F.R.O.; Quaresma, J.A.S. Lessons from dermatology about inflammatory responses in Covid-19. Rev. Med. Virol. 2020, 30, e2130. [Google Scholar] [CrossRef]
- Torres, T.; Puig, L. Managing Cutaneous Immune-Mediated Diseases During the COVID-19 Pandemic. Am. J. Clin. Dermatol. 2020, 21, 307–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miodońska, M.; Bogacz, A.; Mróz, M.; Mućka, S.; Bożek, A. The Effect of SARS-CoV-2 Virus Infection on the Course of Atopic Dermatitis in Patients. Medicina 2021, 57, 521. [Google Scholar] [CrossRef]
- Docampo-Simón, A.; Sánchez-Pujol, M.; Juan-Carpena, G.; Palazón-Cabanes, J.; Caso, E.V.; Berbegal, L.; Poveda-Montoyo, I.; Pastor-Tomás, N.; Mataix-Díaz, J.; Terencio-Alemany, C.; et al. Are chilblain-like acral skin lesions really indicative of COVID-19? A prospective study and literature review. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Daneshgaran, G.; Dubin, D.P.; Gould, D.J. Cutaneous Manifestations of COVID-19: An Evidence-Based Review. Am. J. Clin. Dermatol. 2020, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Recalcati, S. Cutaneous manifestations in COVID-19: A first perspective. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 212–213. [Google Scholar] [CrossRef]
- Freeman, E.E.; McMahon, D.E.; Lipoff, J.B.; Rosenbach, M.; Kovarik, C.; Desai, S.R.; Harp, J.; Takeshita, J.; French, L.E.; Lim, H.W.; et al. The spectrum of COVID-19–associated dermatologic manifestations: An international registry of 716 patients from 31 countries. J. Am. Acad. Dermatol. 2020, 83, 1118–1129. [Google Scholar] [CrossRef]
- Estébanez, A.; Pérez-Santiago, L.; Silva, E.; Guillen-Climent, S.; García-Vázquez, A.; Ramón, M.D. Cutaneous manifestations in COVID-19: A new contribution. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 250–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galván Casas, C.; Catala, A.; Carretero Hernández, G.; Rodríguez-Jiménez, P.; Fernández-Nieto, D.; Rodríguez-Villa Lario, A.; Navarro Fernández, I.; Ruiz-Villaverde, R.; Falkenhain-López, D.; Llamas Velasco, M. Classification of the Cutaneous Manifestations of COVID-19: A Rapid Prospective Nationwide Consensus Study in Spain with 375 Cases. Br. J. Dermatol. 2020, 183, 71–77. [Google Scholar] [CrossRef]
- Piccolo, V.; Neri, I.; Filippeschi, C.; Oranges, T.; Argenziano, G.; Battarra, V.C.; Berti, S.; Manunza, F.; Fortina, A.B.; Di Lernia, V.; et al. Chilblain-like lesions during COVID-19 epidemic: A preliminary study on 63 patients. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 291–293. [Google Scholar] [CrossRef]
- De Masson, A.; Bouaziz, J.D.; Sulimovic, L.; Cassius, C.; Jachiet, M.; Ionescu, M.A.; Rybojad, M.; Bagot, M.; Duong, T.A.; SNDV (French National Union of Dermatologists-Venereologists). Chilblains is a common cutaneous finding during the COVID-19 pandemic: A retrospective nationwide study from France. J. Am. Acad. Dermatol. 2020, 83, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Andina, D.; Noguera-Morel, L.; Bascuas-Arribas, M.; Gaitero-Tristán, J.; Alonso-Cadenas, J.A.; Escalada-Pellitero, S.; Hernández-Martín, Á.; De La Torre-Espi, M.; Colmenero, I.; Torrelo, A. Chilblains in children in the setting of COVID-19 pandemic. Pediatr. Dermatol. 2020, 37, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Tay, J.; Shoqirat, N. Skin and Mucosal Damage in Patients Diagnosed with COVID-19: A Case Report. J. Wound Ostomy Cont. Nurs. 2020, 47, 435–438. [Google Scholar] [CrossRef]
- Chaux-Bodard, A.-G.; Deneuve, S.; Desoutter, A. Oral manifestation of Covid-19 as an inaugural symptom? J. Oral Med. Oral Surg. 2020, 26, 18. [Google Scholar] [CrossRef]
- La Rosa, G.R.M.; Libra, M.; De Pasquale, R.; Ferlito, S.; Pedullà, E. Association of Viral Infections with Oral Cavity Lesions: Role of SARS-CoV-2 Infection. Front. Med. 2021, 7, 571214. [Google Scholar] [CrossRef]
- Imai, K.; Tanaka, H. SARS-CoV-2 Infection and Significance of Oral Health Management in the Era of “the New Normal with COVID-19. Int. J. Mol. Sci. 2021, 22, 6527. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.A.; Normando, A.G.; Da Silva, R.L.C.; De Paula, R.M.; Cembranel, A.C.; Santos-Silva, A.R.; Guerra, E.N.S. Oral mucosal lesions in a COVID-19 patient: New signs or secondary manifestations? Int. J. Infect. Dis. 2020, 97, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Picciani, B.L.S.; Santos, L.R.; Teixeira-Souza, T.; Dick, T.N.A.; Carneiro, S.; Pinto, J.M.N.; Avelleira, J.C.R.; Azulay, D.R.; Luiz, R.R.; Gonzaga, H.F.D.S. Geographic tongue severity index: A new and clinical scoring system. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 330–338. [Google Scholar] [CrossRef]
- Ansari, R.; Gheitani, M.; Heidari, F.; Heidari, F. Oral cavity lesions as a manifestation of the novel virus (COVID-19). Oral Dis. 2021, 27, 771–772. [Google Scholar] [CrossRef]
- Bezerra, T.M.M.; Feitosa, S.G.; Carneiro, D.T.O.; Costa, F.W.G.; Pires, F.R.; Pereira, K.M.A. Oral lesions in COVID-19 infection: Is long-term follow-up important in the affected patients? Oral Dis. 2020, 1, 1–2. [Google Scholar] [CrossRef]
- Brandão, T.B.; Gueiros, L.A.; Melo, T.S.; Prado-Ribeiro, A.C.; Nesrallah, A.C.F.A.; Prado, G.V.B.; Santos-Silva, A.R.; Migliorati, C.A. Oral lesions in patients with SARS-CoV-2 infection: Could the oral cavity be a target organ? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, e45–e51. [Google Scholar] [CrossRef]
- Kahraman, F.C.; Çaşkurlu, H. Mucosal involvement in a COVID-19-positive patient: A case report. Dermatol. Ther. 2020, 33, e13797. [Google Scholar] [CrossRef]
- Ciccarese, G.; Drago, F.; Boatti, M.; Porro, A.; Muzic, S.I.; Parodi, A. Oral erosions and petechiae during SARS-CoV-2 infection. J. Med. Virol. 2021, 93, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Corchuelo, J.; Ulloa, F.C. Oral manifestations in a patient with a history of asymptomatic COVID-19: Case report. Int. J. Infect. Dis. 2020, 100, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Tapia, R.O.C.; Labrador, A.J.P.; Guimaraes, D.M.; Valdez, L.H.M. Oral mucosal lesions in patients with SARS-CoV-2 infection. Report of four cases. Are they a true sign of COVID-19 disease? Spéc. Care Dent. 2020, 40, 555–560. [Google Scholar] [CrossRef]
- Rodríguez, M.D.; Romera, A.J.; Villarroel, M. Oral manifestations associated with COVID-19. Oral Dis. 2020. Epub ahead of print. [Google Scholar] [CrossRef]
- Domínguez-Santás, M.; Haya-Martínez, L.; Fernández-Nieto, D.; Jiménez-Cauhé, J.; Suárez-Valle, A.; Díaz-Guimaraens, B. Acute telogen effluvium associated with SARS-CoV-2 infection. Aust. J. Gen. Pract. 2020, 49, 1022–1023. [Google Scholar] [CrossRef]
- Glavina, A.; Biočina-Lukenda, D.; Mravak-Stipetić, M.; Markeljević, J. Oral symptoms and lesions in SARS-CoV-2-positive patient. Oral Dis. 2020. Epub ahead of print. [Google Scholar] [CrossRef]
- Jimenez-Cauhe, J.; Ortega-Quijano, D.; Carretero-Barrio, I.; Suarez-Valle, A.; Saceda-Corralo, D.; Del Real, C.M.; Fernandez-Nieto, D. Erythema multiforme-like eruption in patients with COVID-19 infection: Clinical and histological findings. Clin. Exp. Dermatol. 2020, 45, 892–895. [Google Scholar] [CrossRef]
- Kitakawa, D.; Oliveira, F.E.; Neves de Castro, P.; Carvalho, L.F.C.S. Short report—Herpes simplex lesion in the lip semimucosa in a COVID-19 patient. Eur. Rev. Med. Pharm. Sci. 2020, 24, 9151–9153. [Google Scholar] [CrossRef]
- Labé, P.; Ly, A.; Sin, C.; Nasser, M.; Chapelon-Fromont, E.; Ben Saïd, P.; Mahé, E. Erythema multiforme and Kawasaki disease associated with COVID-19 infection in children. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Shirato, K.; Imada, Y.; Kawase, M.; Nakagaki, K.; Matsuyama, S.; Taguchi, F. Possible involvement of infection with human coronavirus 229E, but not NL63, in Kawasaki disease. J. Med. Virol. 2014, 86, 2146–2153. [Google Scholar] [CrossRef]
- Burns, J.C.; Glodé, M.P. Kawasaki syndrome. Lancet 2004, 364, 533–544. [Google Scholar] [CrossRef]
- Martín Carreras-Presas, C.; Amaro Sánchez, J.; López-Sánchez, A.F.; Jané-Salas, E.; Somacarrera Pérez, M.L. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis. 2020, 27, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Nuno-Gonzalez, A.; Martin-Carrillo, P.; Magaletsky, K.; Rios, M.M.; Mañas, C.H.; Almazan, J.A.; Casasola, G.G.; Castro, E.P.; Arenas, A.G.; Ibarguren, A.M.; et al. Prevalence of mucocutaneous manifestations in 666 patients with COVID-19 in a field hospital in Spain: Oral and palmoplantar findings. Br. J. Dermatol. 2021, 184, 184–185. [Google Scholar] [CrossRef]
- Patel, J.; Woolley, J. Necrotizing periodontal disease: Oral manifestation of COVID-19. Oral Dis. 2021, 27, 768–769. [Google Scholar] [CrossRef] [PubMed]
- Putra, B.E.; Adiarto, S.; Dewayanti, S.R.; Juzar, D.A. Viral exanthem with “Spins and needles sensation” on extremities of a COVID-19 patient: A self-reported case from an Indonesian medical frontliner. Int. J. Infect. Dis. 2020, 96, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Kassem, I.; Issa, J.; Badrah, M.; Klugar, M. Angular cheilitis of COVID-19 patients: A case-series and literature review. Oral Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Kassem, I.; Hockova, B.; Badrah, M.; Klugar, M. Tongue Ulcers Associated with SARS-COV-2 Infection: A case-series. Oral Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sakaida, T.; Tanimoto, I.; Matsubara, A.; Nakamura, M.; Morita, A. Unique skin manifestations of COVID-19: Is drug eruption specific to COVID-19? J. Dermatol. Sci. 2020, 99, 62–64. [Google Scholar] [CrossRef]
- Soares, C.; Carvalho, R.; Carvalho, M.; Almeida, O. Letter to Editor: Oral lesions in a patient with Covid-19. Med. Oral Patol. Oral Y Cir. Bucal 2020, 25, e563–e564. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Ho, Y.-C. SARS-CoV-2: A storm is raging. J. Clin. Investig. 2020, 130, 2202–2205. [Google Scholar] [CrossRef]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol. 2000 2014, 64, 57–80. [Google Scholar] [CrossRef] [Green Version]
- Coke, C.; Davison, B.; Fields, N.; Fletcher, J.; Rollings, J.; Roberson, L.; Challagundla, K.; Sampath, C.; Cade, J.; Farmer-Dixon, C.; et al. SARS-CoV-2 Infection and Oral Health: Therapeutic Opportunities and Challenges. J. Clin. Med. 2021, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Molayem, S.; Pontes, C.C. The Mouth-COVID Connection: Il-6 Levels in Periodontal Disease-Potential Role in COVID-19-Related Respiratory Complications. J. Calif. Dent. Assoc. 2020, 40, 68–80. [Google Scholar]
- Daniel, R.; Gokulanathan, S.; Shanmugasundaram, N.; Lakshmigandhan, M.; Kavin, T. Diabetes and periodontal disease. J. Pharm. Bioallied Sci. 2012, 4, S280. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2011, 55, 21–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, O.; Arora, P.; Mayer, M.; Chatterjee, S. Inflammation in Periodontal Disease: Possible Link to Vascular Disease. Front. Physiol. 2021, 11, 609614. [Google Scholar] [CrossRef]
- Pitones-Rubio, V.; Chávez-Cortez, E.; Hurtado-Camarena, A.; González-Rascón, A.; Serafín-Higuera, N. Is periodontal disease a risk factor for severe COVID-19 illness? Med. Hypotheses 2020, 144, 109969. [Google Scholar] [CrossRef] [PubMed]
- Marouf, N.; Cai, W.; Said, K.N.; Daas, H.; Diab, H.; Chinta, V.R.; Hssain, A.A.; Nicolau, B.; Sanz, M.; Tamimi, F. Association between periodontitis and severity of COVID-19 infection: A case–control study. J. Clin. Periodontol. 2021, 48, 483–491. [Google Scholar] [CrossRef] [PubMed]
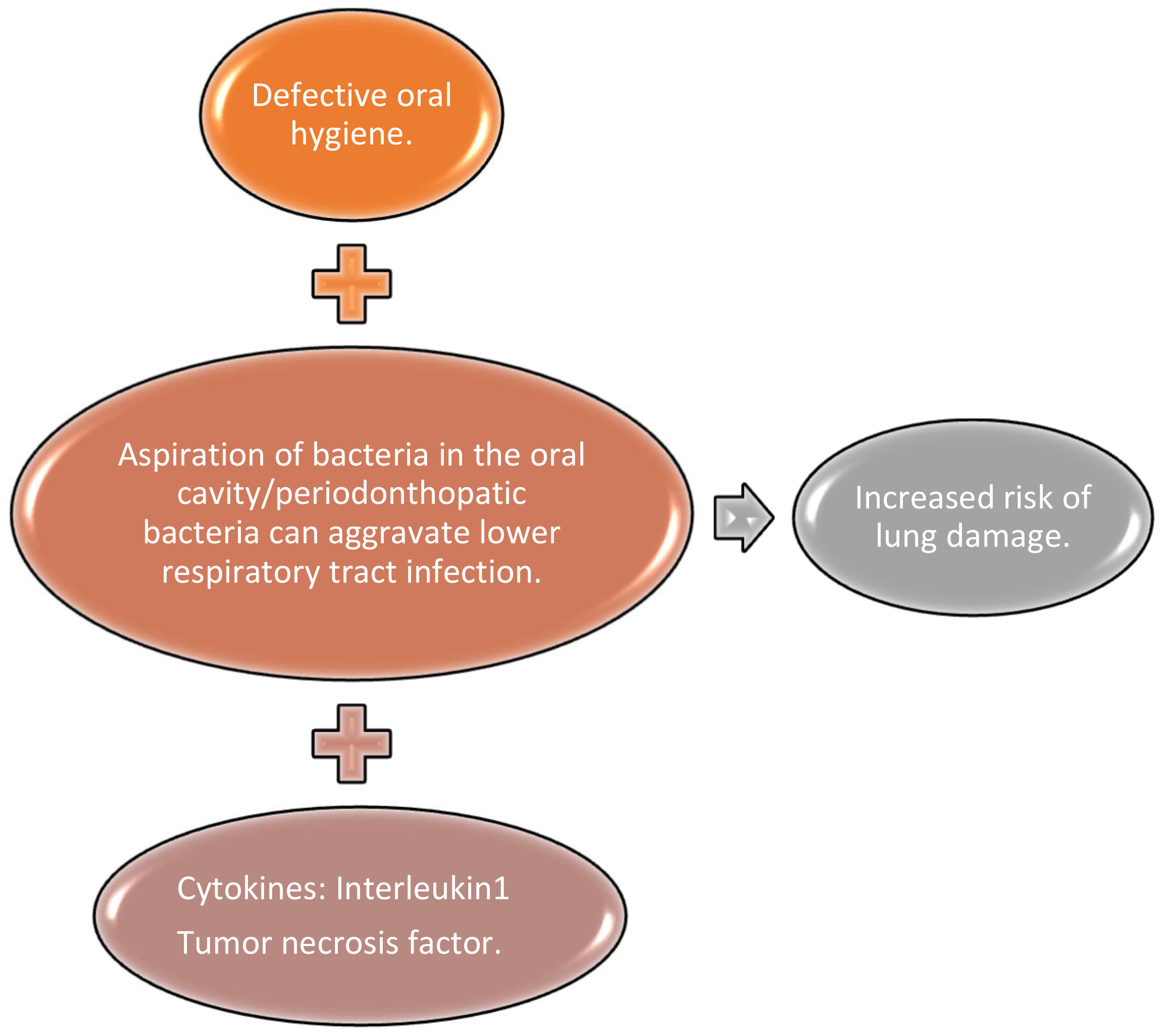
- Takahashi, Y.; Watanabe, N.; Kamio, N.; Kobayashi, R.; Iinuma, T.; Imai, K. Aspiration of periodontopathic bacteria due to poor oral hygiene potentially contributes to the aggravation of COVID-19. J. Oral Sci. 2021, 63, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Brian, Z.; Weintraub, J.A. Oral Health and COVID-19: Increasing the Need for Prevention and Access. Prev. Chronic Dis. 2020, 17, E82. [Google Scholar] [CrossRef] [PubMed]
- Sampson, V.; Kamona, N.; Sampson, A. Could there be a link between oral hygiene and the severity of SARS-CoV-2 infections? Br. Dent. J. 2020, 228, 971–975. [Google Scholar] [CrossRef]
- Botros, N.; Iyer, P.; Ojcius, D.M. Is there an association between oral health and severity of COVID-19 complications? Biomed. J. 2020, 43, 325–327. [Google Scholar] [CrossRef]
- Karayanni, H.; Dror, A.A.; Oren, D.; Sela, E.; Granot, I.; Srouji, S. Exacerbation of chronic myofascial pain during COVID-19. Adv. Oral Maxillofac. Surg. 2021, 1, 100019. [Google Scholar] [CrossRef]
- Consolo, U.; Bellini, P.; Bencivenni, D.; Iani, C.; Checchi, V. Epidemiological Aspects and Psychological Reactions to COVID-19 of Dental Practitioners in the Northern Italy Districts of Modena and Reggio Emilia. Int. J. Environ. Res. Public Health 2020, 17, 3459. [Google Scholar] [CrossRef]
- Saccomanno, S.; Bernabei, M.; Scoppa, F.; Pirino, A.; Mastrapasqua, R.; Visco, M.A. Coronavirus Lockdown as a Major Life Stressor: Does It Affect TMD Symptoms? Int. J. Environ. Res. Public Health 2020, 17, 8907. [Google Scholar] [CrossRef]
- Rokaya, D.; Koontongkaew, S. Can Coronavirus Disease-19 Lead to Temporomandibular Joint Disease? Open Access Maced. J. Med. Sci. 2020, 8, 142–143. [Google Scholar] [CrossRef]
- Almeida-Leite, C.M.; Stuginski-Barbosa, J.; Conti, P.C.R. How psychosocial and economic impacts of COVID-19 pandemic can interfere on bruxism and temporomandibular disorders? J. Appl. Oral Sci. 2020, 28, e20200263. [Google Scholar] [CrossRef]
- Di Giacomo, P.; Serritella, E.; Imondi, F.; Di Paolo, C. Psychological impact of COVID-19 pandemic on TMD subjects. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4616–4626. [Google Scholar] [CrossRef]
- Di Blasi, M.; Gullo, S.; Mancinelli, E.; Freda, M.F.; Esposito, G.; Gelo, O.C.G.; Lagetto, G.; Giordano, C.; Mazzeschi, C.; Pazzagli, C.; et al. Psychological distress associated with the COVID-19 lockdown: A two-wave network analysis. J. Affect. Disord. 2021, 284, 18–26. [Google Scholar] [CrossRef]
- Rocha, J.R.; Neves, M.J.; Pinheiro, M.R.R.; Feitosa, M.; Áurea, L.; Casanovas, R.C.; Lima, D.M. Alterações psicológicas durante a pandemia por COVID-19 e sua relação com bruxismo e DTM. Res. Soc. Dev. 2021, 10, 48710615887. [Google Scholar] [CrossRef]
- Emodi-Perlman, A.; Eli, I.; Smardz, J.; Uziel, N.; Wieckiewicz, G.; Gilon, E.; Grychowska, N.; Wieckiewicz, M. Temporomandibular Disorders and Bruxism Outbreak as a Possible Factor of Orofacial Pain Worsening during the COVID-19 Pandemic—Concomitant Research in Two Countries. J. Clin. Med. 2020, 9, 3250. [Google Scholar] [CrossRef]
- Asquini, G.; Bianchi, A.E.; Borromeo, G.; Locatelli, M.; Falla, D. The impact of Covid-19-related distress on general health, oral behaviour, psychosocial features, disability and pain intensity in a cohort of Italian patients with temporomandibular disorders. PLoS ONE 2021, 16, e0245999. [Google Scholar] [CrossRef]
- Alona, E.-P.; Ilana, E. One year into the COVID-19 pandemic—temporomandibular disorders and bruxism: What we have learned and what we can do to improve our manner of treatment. Dent. Med. Probl. 2021, 58, 215–218. [Google Scholar] [CrossRef] [PubMed]
- De Boni, R.B.; Balanzá-Martínez, V.; Mota, J.C.; Cardoso, T.D.A.; Ballester, P.; Atienza-Carbonell, B.; Bastos, I.F.; Kapczinski, F. Depression, Anxiety, and Lifestyle Among Essential Workers: A Web Survey from Brazil and Spain During the COVID-19 Pandemic. J. Med. Internet Res. 2020, 22, e22835. [Google Scholar] [CrossRef]
- Peixoto, K.O.; de Resende, C.M.B.M.; de Almeida, E.O.; Almeida-Leite, C.M.; Conti, P.C.R.; Barbosa, G.A.S.; Barbosa, J.S. Association of sleep quality and psychological aspects with reports of bruxism and TMD in Brazilian dentists during the COVID-19 pandemic. J. Appl. Oral Sci. 2021, 29, e20201089. [Google Scholar] [CrossRef] [PubMed]
- Gaş, S.; Özsoy, H.E.; Aydın, K.C. The association between sleep quality, depression, anxiety and stress levels, and temporomandibular joint disorders among Turkish dental students during the COVID-19 pandemic. CRANIO 2021, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, R.A.; Vieira, D.L.; Da Silva, E.V.F.; Rezende, L.V.M.D.L.; Dos Santos, R.W.; Tabata, L.F. Prevalence of symptoms of temporomandibular disorders, oral behaviors, anxiety, and depression in Dentistry students during the period of social isolation due to COVID-19. J. Appl. Oral Sci. 2020, 28, e20200445. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, S.; Ahmed, Z.; Allana, R.; Peretti, A.; Amenta, F.; Bijle, M.N.; Seow, L.; Daood, U. Pivoting Dental Practice Management during the COVID-19 Pandemic—A Systematic Review. Medicina 2020, 56, 644. [Google Scholar] [CrossRef] [PubMed]
- Ather, A.; Patel, B.; Ruparel, N.B.; Diogenes, A.; Hargreaves, K.M. Coronavirus Disease 19 (COVID-19): Implications for Clinical Dental Care. J. Endod. 2020, 46, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Jouhar, R. Dissemination of Aerosol and Splatter in Clinical Environment during Cavity Preparation: An In Vitro Study. Int. J. Environ. Res. Public Health 2021, 18, 3773. [Google Scholar] [CrossRef] [PubMed]
- Persoon, I.; Stankiewicz, N.; Smith, A.; de Soet, H.; Volgenant, C. A review of respiratory protection measures recommended in Europe for dental procedures during the COVID-19 pandemic. J. Hosp. Infect. 2020, 106, 330–331. [Google Scholar] [CrossRef] [PubMed]
- Soltani, P.; Baghaei, K.; Tafti, K.T.; Spagnuolo, G. Science Mapping Analysis of COVID-19 Articles Published in Dental Journals. Int. J. Environ. Res. Public Health 2021, 18, 2110. [Google Scholar] [CrossRef]
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 12, 9. [Google Scholar] [CrossRef]
- Shi, A.H.; Guo, W.; Chng, C.K.; Chan, B.H. Precautions When Providing Dental Care During Coronavirus Disease 2019 (COVID-19) Pandemic. Ann. Acad. Med. Singap. 2020, 49, 312–319. [Google Scholar] [CrossRef]
- Ghai, S. Facial Trauma Management During the COVID-19 Era: A Primer for Surgeons. Curr. Med. Res. Pract. 2020, 10, 169–173. [Google Scholar] [CrossRef]
- Sinjari, B.; Rexhepi, I.; Santilli, M.; D′addazio, G.; Chiacchiaretta, P.; Di Carlo, P.; Caputi, S. The Impact of COVID-19 Related Lockdown on Dental Practice in Central Italy—Outcomes of A Survey. Int. J. Environ. Res. Public Health 2020, 17, 5780. [Google Scholar] [CrossRef]
- Tysiąc-Miśta, M.; Dziedzic, A. The Attitudes and Professional Approaches of Dental Practitioners during the COVID-19 Outbreak in Poland: A Cross-Sectional Survey. Int. J. Environ. Res. Public Health 2020, 17, 4703. [Google Scholar] [CrossRef]
- Al-Omiri, M.K.; Lynch, E.; Patil, S.; Al-Shayyab, M.H.; Al Nazeh, A.A.; Alraheam, A.I.; Malkawi, A.Z.; Alomiri, A.K.; Alzoubi, A.I. COVID-19 and Dentistry: An Updated Overview of Dental Perspectives and a Recommended Protocol for Dental Care and Emergency Dental Treatment. J. Contemp. Dent. Pract. 2021, 22, 572–586. [Google Scholar] [CrossRef]
- Tonkaboni, A.; Amirzade-Iranaq, M.H.; Ziaei, H.; Ather, A. Impact of COVID-19 on Dentistry. Adv. Exp. Med. Biol. 2021, 1318, 623–636. [Google Scholar] [CrossRef]
- Alharbi, A.; Alharbi, S.; Alqaidi, S. Guidelines for dental care provision during the COVID-19 pandemic. Saudi Dent. J. 2020, 32, 181–186. [Google Scholar] [CrossRef]
- Izzetti, R.; Nisi, M.; Gabriele, M.; Graziani, F. COVID-19 Transmission in Dental Practice: Brief Review of Preventive Measures in Italy. J. Dent. Res. 2020, 99, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, R. The Severe Acute Respiratory Syndrome Coronavirus-2 (SARS CoV-2) in Dentistry. Management of Biological Risk in Dental Practice. Int. J. Environ. Res. Public Health 2020, 17, 3067. [Google Scholar] [CrossRef] [PubMed]
- Sarapultseva, M.; Hu, D.; Sarapultsev, A. SARS-CoV-2 Seropositivity among Dental Staff and the Role of Aspirating Systems. JDR Clin. Transl. Res. 2021, 6, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Caggiano, M.; Amato, M.; Moccia, G.; Capunzo, M.; De Caro, F. Infection Control in Dental Practice During the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2020, 17, 4769. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Little, R.; Howell, J.; Nixon, P. COVID-19 and beyond: Implications for dental radiography. Br. Dent. J. 2020, 229, 105–109. [Google Scholar] [CrossRef]
- Dziedzic, A.; Wojtyczka, R. The impact of coronavirus infectious disease 19 (COVID-19) on oral health. Oral Dis. 2021, 27, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L.; Fakhruddin, K.S.; Bandara, N. Oral Manifestations of Coronavirus Disease 2019 (COVID-19): An Overview. Dent. Updat. 2021, 48, 418–422. [Google Scholar] [CrossRef]
- Sinjari, B.; D’Ardes, D.; Santilli, M.; Rexhepi, I.; D’Addazio, G.; Di Carlo, P.; Chiacchiaretta, P.; Caputi, S.; Cipollone, F. SARS-CoV-2 and Oral Manifestation: An Observational, Human Study. J. Clin. Med. 2020, 9, 3218. [Google Scholar] [CrossRef]
- Godinho, G.V.; Paz, A.L.L.M.; Gomes, E.P.A.D.A.; Garcia, C.L.; Volpato, L.E.R. Extensive hard palate hyperpigmentation associated with chloroquine use. Br. J. Clin. Pharmacol. 2020, 86, 2325–2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study | Number of Cases | Patient Data | Oral Lesion | Localization |
|---|---|---|---|---|
| Amorim dos Santos et al. [51] | 1 | M, 67, confirmed | White plaque Yellowish pinpoint ulcers Geographical tongue | Tongue dorsum |
| Ansari et al. [53] | 2 | 1. M, 75, confirmed 2. F, 56, confirmed | Several painful ulcers, with irregular margins and varying sizes against red and nonhemorrhagic backgrounds | 1. Hard palate 2. Anterior region of the tongue |
| Bezerra et al. [54] | 1 | M, 33, suspicion | Painful mouth ulceration Two crateriform ulcers with a necrotic background and no erythematous halo | The floor of the mouth Left retromolar region and lip mucosa |
| Brandao et al. [55] | 8 | 1. M, 81, confirmed 2. F, 71, confirmed 3. F, 83, confirmed 4. M, 72, confirmed 5. F, 32, confirmed 6. M, 35, confirmed 7. M, 29, confirmed 8. M, 28, confirmed | 1. Multiple painful, shallow aphthous-like ulcers covered with mucopurulent membrane HSV-1 identified 2. Small hemorrhagic ulcerations Areas of superficial necrosis HSV-1 identified 3. Ulcer of 1.5 × 1.5 cm2 Discrete area affected by petechiae and a superficial necrotic area 4. Small hemorrhagic ulcerations Painful necrotic ulceration HSV-1 identified 5. Multiple ulcers, superficial and circular lesions with a whitish center and surrounded by an erythematous halo 6. Superficial, circular ulcer, covered by a fibrinopurulent membrane and surrounded by an erythematous halo of 0.5 cm Mild odynophagia. 7. Superficial, painful ulcer, with a diameter of 1 cm and a whitish pseudomembrane surrounded by an erythematous halo 8. Aphthous-like ulcers | 1. The mucosa of the upper and lower lips Anterior tongue dorsum 2. Upper and lower lip Anterior tongue dorsum 3. Right lateral edge of the tongue Anterior hard palate 4. Upper and lower lip Lower right lip 5. Tip and lateral edges of the tongue 6. Tonsillar pillar 7. Ventral portion of the tongue 8. Upper and lower labial mucosae, right side of the tongue |
| Cebeci Kahraman and Caskurlu [56] | 1 | M, 51, confirmed | Large erythematous surface A few petechiae Numerous pustular enanthemata | Oropharynx Hard palate midline Left side of soft palate border |
| Chaux-Bodard et al. [48] | 1 | F, 45, confirmed | Painful inflammation of tongue papilla, followed by an erythematous macula and an asymptomatic irregular ulcer | Tongue dorsum |
| Ciccarese et al. [57] | 1 | F, 19, confirmed | Erosions, ulcerations, and blood crust Petechiae | The inner surface of the lips Palate and gingiva |
| Corchuelo and Ulloa [58] | 1 | F, 40, confirmed | Reddish plaques Dark brown pigmentation Aphtous-like ulcer White area, probably candida | Lower lip Gingiva Attached lower left gingiva Tongue dorsum |
| Cruz Tapia et al. [59] | 4 | 1. F, 41, confirmed 2. F, 42, confirmed 3. F, 55, confirmed 4. M, 41, confirmed | 1. Bulla 2. Macule 3. Bulla 4. Small macule | 1. Hard palate 2. Hard palate (left side) 3. Tongue 4. Hard palate |
| Diaz Rodriguez et al. [60] | 3 | 1. F, 43, confirmed 2. M, 53, confirmed 3. F, 78, confirmed | 1. Aphthous-like lesions, burning sensation, and tongue depapillation 2. Burning-mouth sensation and unilateral angular cheilitis 3. Pseudomembranous candidiasis and angular cheilitis | 1. N/A 2. Lips 3. Tongue, palate |
| Dominguez-Santos et al. [61] | 4 | 1. F, 43, confirmed 2. M, 33, confirmed 3. M, 37, confirmed 4. M, 19, confirmed | 1. Single ulcer, with peripheral erythematous rim 2. Single aphtous ulcer 3. Seven aphtae 4. Four clustered aphtae | 1. Right buccal mucosa 2. Superior mucogingival junction 3. Ventral right side of the tongue 4. Right side of the inferior labial mucosa |
| Glavina et al. [62] | 1 | F, 40, confirmed | Pain and burning in the oral cavity Recurent HSV White, hairy tongue | Hard palate Tongue |
| Jimenez Cauhe et al. [63] | 3 | F, 77, confirmed F, 58, confirmed F, 69, confirmed | Macules and petechiae | Palate |
| Kitakawa et al. [64] | 1 | F, 20, confirmed | Herpetic lesions, pruritus | Median lower lip semimucosa |
| Labe et al. [65] | 2 | 1. M, 6, confirmed 2. M, 3, suspicion | 1. Painful cheilitis 2. Cheilitis, stomatitis, glossitis | N/A N/A |
| Martin Carreras-Presas et al. [68] | 3 | 1. M, 56, suspicion 2. M, 58, suspicion 3. F, 65, confimed | 1. Painful lesions resembling a herpetic recurrent stomatitis 2. Multiple small, painful yellowish ulcers with erythematous halo 3. Pain Blisters Desquamative gingivitis | 1. Palate 2. Palate 3. Tongue Internal lip mucosa Gingiva |
| Nuno-Gonzalez et al. [69] | 78 | Adults, average age of 55.7 years, confirmed/suspicion | Lingual papillitis, glossitis, aphthous stomatitis, mucositis, and burning sensation | N/A |
| Patel et al. [70] | 1 | F, 35, suspicion | Erithematous and edematous gingiva and necrotic interdental papillae | Gingiva and interdental papillae |
| Putra et al. [71] | 1 | M, 29, confirmed | Aphthous stomatitis | N/A |
| Riad et al. [72] | 17 | 12 F, 5 M; average age of 39.94, confirmed | Angular cheilitis | Lips |
| Riad et al. [73] | 26 | 9 M, 17 F; average age of 36.81, confirmed | Tongue ulcers | Tongue |
| Sakaida et al. [74] | 1 | F, 52, confirmed | Erythematous and erosive lesions | Lips and oral mucosa |
| Soares et al. [75] | 1 | M, 42, confirmed | Painful ulceration Multiple reddish macules of different sizes | Buccal mucosa Hard palate, tongue, and lips |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu, L.-C.; Ardelean, L.C.; Tigmeanu, C.V.; Matichescu, A.; Sauciur, I.; Bratu, E.A. COVID-19 and Its Repercussions on Oral Health: A Review. Medicina 2021, 57, 1189. https://doi.org/10.3390/medicina57111189
Rusu L-C, Ardelean LC, Tigmeanu CV, Matichescu A, Sauciur I, Bratu EA. COVID-19 and Its Repercussions on Oral Health: A Review. Medicina. 2021; 57(11):1189. https://doi.org/10.3390/medicina57111189
Chicago/Turabian StyleRusu, Laura-Cristina, Lavinia Cosmina Ardelean, Codruta Victoria Tigmeanu, Anamaria Matichescu, Iulia Sauciur, and Emanuel Adrian Bratu. 2021. "COVID-19 and Its Repercussions on Oral Health: A Review" Medicina 57, no. 11: 1189. https://doi.org/10.3390/medicina57111189
APA StyleRusu, L.-C., Ardelean, L. C., Tigmeanu, C. V., Matichescu, A., Sauciur, I., & Bratu, E. A. (2021). COVID-19 and Its Repercussions on Oral Health: A Review. Medicina, 57(11), 1189. https://doi.org/10.3390/medicina57111189








