New Horizons in Myeloproliferative Neoplasms Treatment: A Review of Current and Future Therapeutic Options
Abstract
1. Introduction
2. Molecular Biology
3. The Clinical Course of the Disease
4. Ruxolitinib Revolution
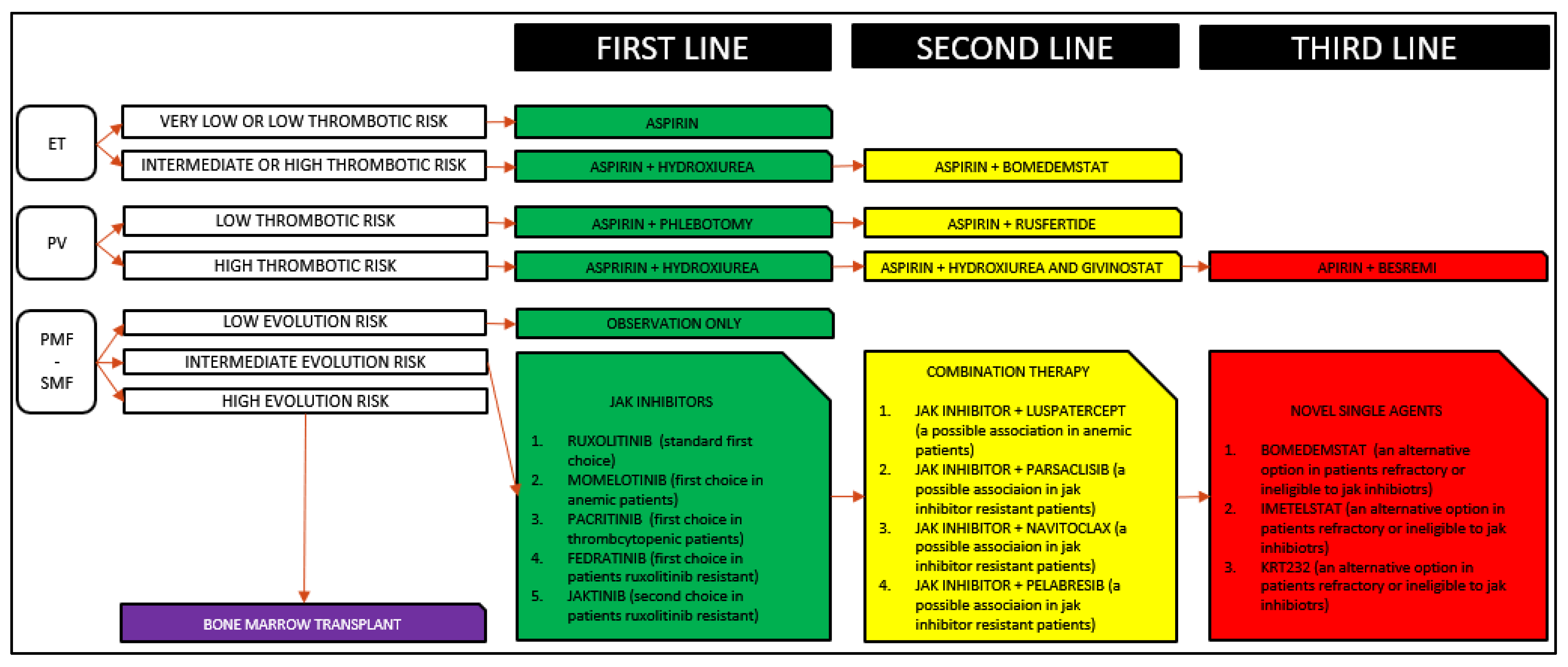
5. New Therapeutic Options
- new JAK inhibitors;
- combination therapies;
- non-JAK inhibitors new monotherapies.
5.1. New JAK Inhibitors
5.1.1. Momelotinib—An Option for Anemic Patients with MF
5.1.2. Pacritinib—An Option for Thrombocytopenic Patients with MF
5.1.3. Fedratinib—An Option for Patients with MF Resistant or with Sub-Optimal Response to JAK Inhibtors
5.1.4. Jaktinib—An Option for Patients with MF Resistant or with Sub-Optimal Response to JAK Inhibtors
5.2. Combination Therapies
5.2.1. Luspatercept—An Option for Anemic Patients with MF
5.2.2. Parsaclisib—An Option for Patients with MF Resistant or with Sub-Optimal Response to JAK Inhibitors
5.2.3. Navitoclax—An Option for Patients with MF Resistant or with Sub-Optimal Response to JAK Inhibitors
5.2.4. Pelabresib—An Option for Patients with MF Resistant or with Sub-Optimal Response to JAK Inhibitors
5.3. Non-JAK Inhibitors New Monotherapies
5.3.1. Imetelstat—An Option for Patients with MF Refractory or Ineligible for JAK Inhibitors
5.3.2. KRT232—An Option for Patients with MF Refractory or Ineligible for JAK Inhibitors
5.3.3. Bomedemstat—An Option for Patients with MF or ET with Thrombocytosis
5.3.4. Rusfertide—An Option for Patients with PV Needing Phlebotomy
5.3.5. Besremi—An Option for Patients with PV Needing Cytoreduction
5.3.6. Givinostat—An Option for Patients with PV Needing Cytoreduction
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Spivak, J.L. The chronic myeloproliferative disorders: Clonality and clinical heterogeneity. Semin. Hematol. 2004, 41, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Lasho, T.L.; Finke, C.M.; Elala, Y.; Hanson, C.A.; Ketterling, R.P.; Gangat, N.; Pardanani, A. Targeted deep sequencing in primary myelofibrosis. Blood 2016, 1, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Guglielmelli, P.; Larson, D.R.; Finke, C.; Wassie, E.A.; Pieri, L.; Gangat, N.; Fjerza, R.; Belachew, A.A.; Lasho, T.L.; et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 2014, 124, 2507–2513. [Google Scholar] [CrossRef]
- Tefferi, A. Primary myelofibrosis: 2019 update on diagnosis, risk stratification and management. Am. J. Hematol. 2018, 93, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Devlin, R.; Gupta, V. Myelofibrosis: To transplant or not to transplant? Hematol. Am. Soc. Hematol. Educ. Program 2016, 2016, 543–551. [Google Scholar] [CrossRef][Green Version]
- Baxter, E.J.; Scott, L.M.; Campbell, P.J.; East, C.; Fourouclas, N.; Swanton, S.; Vassiliou, G.S.; Bench, A.J.; Boyd, E.M.; Curtin, N.; et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005, 365, 1054–1061. [Google Scholar] [CrossRef]
- Levine, R.L.; Wadleigh, M.; Cools, J.; Ebert, B.L.; Wernig, G.; Huntly, B.J.; Boggon, T.J.; Wlodarska, I.; Clark, J.J.; Moore, S.; et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005, 7, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.V.; Kreil, S.; Zoi, K.; Waghorn, K.; Curtis, C.; Zhang, L.; Score, J.; Seear, R.; Chase, A.J.; Grand, F.H.; et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood 2005, 106, 2162–2168. [Google Scholar] [CrossRef] [PubMed]
- Vainchenker, W.; Kralovics, R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood 2017, 129, 667–679. [Google Scholar] [CrossRef]
- Grinfeld, J.; Nangalia, J.; Green, A.R. Molecular determinants of pathogenesis and clinical phenotype in myeloproliferative neoplasms. Haematologica 2017, 102, 7–17. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Ugo, V.; Le Couédic, J.P.; Staerk, J.; Delhommeau, F.; Lacout, C.; Garçon, L.; Raslova, H.; Berger, R.; Bennaceur-Griscelli, A.; et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005, 434, 1144–1148. [Google Scholar] [CrossRef]
- Mejía-Ochoa, M.; Acevedo Toro, P.A.; Cardona-Arias, J.A. Systematization of analytical studies of polycythemia vera, essential thrombocythemia and primary myelofibrosis, and a meta-analysis of the frequency of JAK2, CALR and MPL mutations: 2000–2018. BMC Cancer 2019, 19, 590. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.M.; Tong, W.; Levine, R.L.; Scott, M.A.; Beer, P.A.; Stratton, M.R.; Futreal, P.A.; Erber, W.N.; McMullin, M.F.; Harrison, C.N.; et al. JAK2Exon 12 Mutations in Polycythemia Vera and Idiopathic Erythrocytosis. N. Engl. J. Med. 2007, 356, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Nangalia, J.; Massie, C.E.; Baxter, E.J.; Nice, F.L.; Gundem, G.; Wedge, D.C.; Avezov, E.; Li, J.; Kollmann, K.; Kent, D.G.; et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N. Engl. J. Med. 2013, 369, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Pikman, Y.; Lee, B.H.; Mercher, T.; McDowell, E.; Ebert, B.L.; Gozo, M.; Cuker, A.; Wernig, G.; Moore, S.; Galinsky, I.; et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006, 3, e270. [Google Scholar] [CrossRef] [PubMed]
- How, J.; Hobbs, G.S.; Mullally, A. Mutant calreticulin in myeloproliferative neoplasms. Blood 2019, 134, 2242–2248. [Google Scholar] [CrossRef] [PubMed]
- Benlabiod, C.; Cacemiro, M.D.C.; Nédélec, A.; Edmond, V.; Muller, D.; Rameau, P.; Touchard, L.; Gonin, P.; Constantinescu, S.N.; Raslova, H.; et al. Calreticulin del52 and ins5 knock-in mice recapitulate different myeloproliferative phenotypes observed in patients with MPN. Nat. Commun. 2020, 11, 4886. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Lasho, T.L.; Guglielmelli, P.; Finke, C.M.; Rotunno, G.; Elala, Y.; Pacilli, A.; Hanson, C.A.; Pancrazzi, A.; Ketterling, R.P.; et al. Targeted deep sequencing in polycythemia vera and essential thrombocythemia. Blood Adv. 2016, 1, 21–30. [Google Scholar] [CrossRef]
- Tefferi, A.; Guglielmelli, P.; Lasho, T.L.; Coltro, G.; Finke, C.M.; Loscocco, G.G.; Sordi, B.; Szuber, N.; Rotunno, G.; Pacilli, A.; et al. Mutation-enhanced international prognostic systems for essential thrombocythaemia and polycythaemia vera. Br. J. Haematol. 2020, 189, 291–302. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Lasho, T.L.; Rotunno, G.; Score, J.; Mannarelli, C.; Pancrazzi, A.; Biamonte, F.; Pardanani, A.; Zoi, K.; Reiter, A.; et al. The number of prognostically detrimental mutations and prognosis in primary myelofibrosis: An international study of 797 patients. Leukemia 2014, 28, 1804–1810. [Google Scholar] [CrossRef]
- Ernst, T.; Chase, A.J.; Score, J.; Hidalgo-Curtis, C.E.; Bryant, C.; Jones, A.V.; Waghorn, K.; Zoi, K.; Ross, F.M.; Reiter, A.; et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat. Genet. 2010, 42, 722–726. [Google Scholar] [CrossRef]
- Stegelmann, F.; Bullinger, L.; Schlenk, R.F.; Paschka, P.; Griesshammer, M.; Blersch, C.; Kuhn, S.; Schauer, S.; Döhner, H.; Döhner, K. DNMT3A mutations in myeloproliferative neoplasms. Leukemia 2011, 25, 1217–1219. [Google Scholar] [CrossRef] [PubMed]
- Delhommeau, F.; Dupont, S.; Della Valle, V.; James, C.; Trannoy, S.; Massé, A.; Kosmider, O.; Le Couedic, J.P.; Robert, F.; Alberdi, A.; et al. Mutation in TET2 in myeloid cancers. N. Engl. J. Med. 2009, 360, 2289–2301. [Google Scholar] [CrossRef]
- Figueroa, M.E.; Abdel-Wahab, O.; Lu, C.; Ward, P.S.; Patel, J.; Shih, A.; Li, Y.; Bhagwat, N.; Vasanthakumar, A.; Fernandez, H.F.; et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 2010, 18, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Sanada, M.; Shiraishi, Y.; Nowak, D.; Nagata, Y.; Yamamoto, R.; Sato, Y.; Sato-Otsubo, A.; Kon, A.; Nagasaki, M.; et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 2011, 478, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Brecqueville, M.; Rey, J.; Bertucci, F.; Coppin, E.; Finetti, P.; Carbuccia, N.; Cervera, N.; Gelsi-Boyer, V.; Arnoulet, C.; Gisserot, O.; et al. Mutation analysis of ASXL1, CBL, DNMT3A, IDH1, IDH2, JAK2, MPL, NF1, SF3B1, SUZ12, and TET2 in myeloproliferative neoplasms. Genes Chromosomes Cancer 2012, 51, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Harutyunyan, A.; Klampfl, T.; Cazzola, M.; Kralovics, R. p53 lesions in leukemic transformation. N. Engl. J. Med. 2011, 364, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Klampfl, T.; Harutyunyan, A.; Berg, T.; Gisslinger, B.; Schalling, M.; Bagienski, K.; Olcaydu, D.; Passamonti, F.; Rumi, E.; Pietra, D.; et al. Genome integrity of myeloproliferative neoplasms in chronic phase and during disease progression. Blood 2011, 118, 167–176. [Google Scholar] [CrossRef]
- Rampal, R.; Ahn, J.; Abdel-Wahab, O.; Nahas, M.; Wang, K.; Lipson, D.; Otto, G.A.; Yelensky, R.; Hricik, T.; McKenney, A.S.; et al. Genomic and functional analysis of leukemic transformation of myeloproliferative neoplasms. Proc. Natl. Acad. Sci. USA 2014, 111, 5401–5410. [Google Scholar] [CrossRef]
- Beer, P.A.; Delhommeau, F.; LeCouédic, J.P.; Dawson, M.A.; Chen, E.; Bareford, D.; Kusec, R.; McMullin, M.F.; Harrison, C.N.; Vannucchi, A.M.; et al. Two routes to leukemic transformation after a JAK2 mutation-positive myeloproliferative neoplasm. Blood 2010, 115, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Policitemia, G.I.S. Polycythemia vera: The natural history of 1213 patients followed for 20 years. Ann. Intern. Med. 1995, 123, 656–664. [Google Scholar]
- De Stefano, V.; Za, T.; Rossi, E.; Vannucchi, A.M.; Ruggeri, M.; Elli, E.; Micò, C.; Tieghi, A.; Cacciola, R.R.; Santoro, C.; et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: Incidence, risk factors, and effect of treatments. Haematologica 2008, 93, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Hultcrantz, M.; Björkholm, M.; Dickman, P.W.; Landgren, O.; Derolf, Å.R.; Kristinsson, S.Y.; Andersson, T.M.L. Risk of Arterial and Venous Thrombosis in 11,155 Patients with Myeloproliferative Neoplasms and 44,620 Matched Controls; a Population-Based Study; American Society of Hematology: Washington, DC, USA, 2014. [Google Scholar]
- Kennedy, J.A.; Atenafu, E.G.; Messner, H.A.; Craddock, K.J.; Brandwein, J.M.; Lipton, J.H.; Minden, M.D.; Schimmer, A.D.; Schuh, A.C.; Yee, K.W.; et al. Treatment outcomes following leukemic transformation in Philadelphia-negative myeloproliferative neoplasms. Blood J. Am. Soc. Hematol. 2013, 121, 2725–2733. [Google Scholar]
- Tefferi, A.; Rumi, E.; Finazzi, G.; Gisslinger, H.; Vannucchi, A.M.; Rodeghiero, F.; Randi, M.L.; Vaidya, R.; Cazzola, M.; Rambaldi, A.; et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: An international study. Leukemia 2013, 27, 1874–1881. [Google Scholar] [CrossRef]
- Barbui, T.; Finazzi, G.; Carobbio, A.; Thiele, J.; Passamonti, F.; Rumi, E.; Ruggeri, M.; Rodeghiero, F.; Randi, M.L.; Bertozzi, I.; et al. Development and validation of an International Prognostic Score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood 2012, 120, 5128–5133. [Google Scholar] [CrossRef]
- Passamonti, F.; Cervantes, F.; Vannucchi, A.M.; Morra, E.; Rumi, E.; Pereira, A.; Guglielmelli, P.; Pungolino, E.; Caramella, M.; Maffioli, M.; et al. A dynamic prognostic model to predict survival in primary myelofibrosis: A study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 2010, 115, 1703–1708. [Google Scholar]
- Gangat, N.; Caramazza, D.; Vaidya, R.; George, G.; Begna, K.; Schwager, S.; Van Dyke, D.; Hanson, C.; Wu, W.; Pardanani, A.; et al. DIPSS plus: A refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J. Clin. Oncol. 2011, 29, 392–397. [Google Scholar] [CrossRef]
- Tefferi, A.; Guglielmelli, P.; Lasho, T.L.; Gangat, N.; Ketterling, R.P.; Pardanani, A.; Vannucchi, A.M. MIPSS70+ Version 2.0: Mutation and Karyotype-Enhanced International Prognostic Scoring System for Primary Myelofibrosis. J. Clin. Oncol. 2018, 36, 1769–1770. [Google Scholar] [CrossRef]
- Tefferi, A.; Guglielmelli, P.; Nicolosi, M.; Mannelli, F.; Mudireddy, M.; Bartalucci, N.; Finke, C.M.; Lasho, T.L.; Hanson, C.A.; Ketterling, R.P.; et al. GIPSS: Genetically inspired prognostic scoring system for primary myelofibrosis. Leukemia 2018, 32, 1631–1642. [Google Scholar] [CrossRef]
- Landolfi, R.; Marchioli, R.; Kutti, J.; Gisslinger, H.; Tognoni, G.; Patrono, C.; Barbui, T. Efficacy and Safety of Low-Dose Aspirin in Polycythemia Vera. N. Engl. J. Med. 2004, 350, 114–124. [Google Scholar] [CrossRef]
- Marchioli, R.; Finazzi, G.; Specchia, G.; Masciulli, A.; Mennitto, M.R.; Barbui, T. The CYTO-PV: A large-scale trial testing the intensity of CYTOreductive therapy to prevent cardiovascular events in patients with polycythemia vera. Thrombosis 2011, 2011, 794240. [Google Scholar] [CrossRef] [PubMed]
- Gisslinger, H.; Klade, C.; Georgiev, P.; Krochmalczyk, D.; Gercheva-Kyuchukova, L.; Egyed, M.; Rossiev, V.; Dulicek, P.; Illes, A.; Pylypenko, H.; et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): A randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol. 2020, 7, e196–e208. [Google Scholar] [CrossRef]
- Jain, T.; Mesa, R.A.; Palmer, J.M. Allogeneic Stem Cell Transplantation in Myelofibrosis. Biol. Blood Marrow Transplant. 2017, 23, 1429–1436. [Google Scholar] [CrossRef]
- Gupta, V.; Kennedy, J.A.; Capo-Chichi, J.M.; Kim, S.; Hu, Z.H.; Alyea, E.P.; Popat, U.R.; Sobecks, R.M.; Scott, B.L.; Gerds, A.T.; et al. Genetic factors rather than blast reduction determine outcomes of allogeneic HCT in BCR-ABL-negative MPN in blast phase. Blood Adv. 2020, 4, 5562–5573. [Google Scholar] [CrossRef] [PubMed]
- Kralovics, R.; Passamonti, F.; Buser, A.S.; Teo, S.S.; Tiedt, R.; Passweg, J.R.; Tichelli, A.; Cazzola, M.; Skoda, R.C. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 2005, 352, 1779–1790. [Google Scholar] [CrossRef]
- Deisseroth, A.; Kaminskas, E.; Grillo, J.; Chen, W.; Saber, H.; Lu, H.L.; Rothmann, M.D.; Brar, S.; Wang, J.; Garnett, C.; et al. U.S. food and drug administration approval: Ruxolitinib for the treatment of patients with intermediate and high-risk myelofibrosis. Clin. Cancer Res. 2012, 18, 3212–3217. [Google Scholar] [CrossRef]
- Verstovsek, S.; Gotlib, J.; Mesa, R.A.; Vannucchi, A.M.; Kiladjian, J.J.; Cervantes, F.; Harrison, C.N.; Paquette, R.; Sun, W.; Naim, A.; et al. Long-term survival in patients treated with ruxolitinib for myelofibrosis: COMFORT-I and -II pooled analyses. J. Hematol. Oncol. 2017, 10, 156. [Google Scholar] [CrossRef]
- Verstovsek, S.; Mesa, R.A.; Gotlib, J.; Gupta, V.; DiPersio, J.F.; Catalano, J.V.; Deininger, M.W.; Miller, C.B.; Silver, R.T.; Talpaz, M.; et al. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORTI trial. J. Hematol. Oncol. 2017, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.N.; Vannucchi, A.M.; Kiladjian, J.J.; Al-Ali, H.K.; Gisslinger, H.; Knoops, L.; Cervantes, F.; Jones, M.M.; Sun, K.; McQuitty, M.; et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia 2016, 30, 1701–1707. [Google Scholar] [CrossRef]
- Galli, S.; McLornan, D.; Harrison, C. Safety evaluation of ruxolitinib for treating myelofibrosis. Expert Opin. Drug Saf. 2014, 13, 967–976. [Google Scholar] [CrossRef]
- McLornan, D.P.; Khan, A.A.; Harrison, C.N. Immunological consequences of JAK inhibition: Friend or foe? Curr. Hematol. Malig. Rep. 2015, 10, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Barbui, T.; Ghirardi, A.; Masciulli, A.; Carobbio, A.; Palandri, F.; Vianelli, N.; De Stefano, V.; Betti, S.; Di Veroli, A.; Iurlo, A.; et al. Second cancer in philadelphia negative myeloproliferative neoplasms (MPN-K). A nested casecontrol study. Leukemia 2019, 33, 1996–2005. [Google Scholar] [CrossRef]
- Masarova, L.; Cherry, M.; Newberry, K.J.; Estrov, Z.; Cortes, J.E.; Kantarjian, H.M.; Verstovsek, S. Secondary solid tumors and lymphoma in patients with essential thrombocythemia and polycythemia vera —Single center experience. Leuk. Lymphoma 2016, 57, 237–239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rumi, E.; Passamonti, F.; Elena, C.; Pietra, D.; Arcaini, L.; Astori, C.; Zibellini, S.; Boveri, E.; Pascutto, C.; Lazzarino, M. Increased risk of lymphoid neoplasm in patients with myeloproliferative neoplasm: A study of 1,915 patients. Haematologica 2011, 96, 454–458. [Google Scholar] [CrossRef][Green Version]
- Vannucchi, A.M.; Masala, G.; Antonioli, E.; Chiara Susini, M.; Guglielmelli, P.; Pieri, L.; Maggi, L.; Caini, S.; Palli, D.; Bogani, C.; et al. Increased risk of lymphoid neoplasms in patients with philadelphia chromosomenegative myeloproliferative neoplasms. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2068–2073. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Asshoff, M.; Petzer, V.; Warr, M.R.; Haschka, D.; Tymoszuk, P.; Demetz, E.; Seifert, M.; Posch, W.; Nairz, M.; Maciejewski, P.; et al. Momelotinib inhibits ACVR1/ALK2, decreases hepcidin production, and ameliorates anemia of chronic disease in rodents. Blood 2017, 129, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Mesa, R.A.; Kiladjian, J.J.; Catalano, J.V.; Devos, T.; Egyed, M.; Hellmann, A.; McLornan, D.; Shimoda, K.; Winton, E.F.; Deng, W.; et al. SIMPLIFY-1: A phase III randomized trial of momelotinib versus ruxolitinib in Janus kinase inhibitor-Naive patients with myelofibrosis. J. Clin. Oncol. 2017, 35, 3844–3850. [Google Scholar] [CrossRef]
- Harrison, C.N.; Vannucchi, A.M.; Platzbecker, U.; Cervantes, F.; Gupta, V.; Lavie, D.; Passamonti, F.; Winton, E.F.; Dong, H.; Kawashima, J.; et al. Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): A randomised, open-label, phase 3 trial. Lancet Haematol. 2018, 5, e73–e81. [Google Scholar] [CrossRef]
- Mesa, R.A.; Vannucchi, A.M.; Mead, A.; Egyed, M.; Szoke, A.; Suvorov, A.; Jakucs, J.; Perkins, A.; Prasad, R.; Mayer, J.; et al. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): An international, randomised, phase 3 trial. Lancet Haematol. 2017, 4, e225–e236. [Google Scholar] [CrossRef]
- Gerds, A.T.; Savona, M.R.; Scott, B.L.; Talpaz, M.; Egyed, M.; Harrison, C.N.; Yacoub, A.; Vannucchi, A.; Mead, A.J.; Kiladjian, J.J.; et al. Results of PAC203: A randomized phase 2 dose-finding study and determination of the recommended dose of pacritinib in patients with myelofibrosis. Blood 2019, 136, 667–1667. [Google Scholar] [CrossRef]
- Harrison, C.N.; Gerds, A.T.; Kiladjian, J.J.; Döhner, K.; Buckley, S.A.; Smith, J.A.; Craig, A.R.; Mascarenhas, J.; Verstovsek, S. Pacifica: A randomized, controlled phase 3 study of pacritinib vs. Physician’s choice in patients with primary myelofibrosis, post polycythemia vera myelofibrosis, or post essential thrombocytopenia myelofibrosis with severe thrombocytopenia (platelet count <50,000/mL). Blood 2019, 134, 4175. [Google Scholar]
- Harrison, C.N.; Schaap, N.; Vannucchi, A.M.; Kiladjian, J.J.; Tiu, R.V.; Zachee, P.; Jourdan, E.; Winton, E.; Silver, R.T.; Schouten, H.C.; et al. Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA-2): A single-arm, open-label, non-randomised, phase 2, multicentre study. Lancet Haematol. 2017, 4, 317–324. [Google Scholar] [CrossRef]
- Pardanani, A.; Tefferi, A.; Jamieson, C.; Gabrail, N.Y.; Lebedinsky, C.; Gao, G.; Liu, F.; Xu, C.; Cao, H.; Talpaz, M. A phase 2 randomized dose-ranging study of the JAK2-selective inhibitor fedratinib (SAR302503) in patients with myelofibrosis. Blood Cancer J. 2015, 5, e335. [Google Scholar] [CrossRef]
- Pardanani, A.; Harrison, C.; Cortes, J.E.; Cervantes, F.; Mesa, R.A.; Milligan, D.; Masszi, T.; Mishchenko, E.; Jourdan, E.; Vannucchi, A.M.; et al. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: A randomized clinical trial. JAMA Oncol. 2015, 1, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, A.; Gotlib, J.R.; Jamieson, C.; Cortes, J.E.; Talpaz, M.; Stone, R.M.; Silverman, M.H.; Gilliland, D.G.; Shorr, J.; Tefferi, A. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J. Clin. Oncol. 2011, 29, 789–796. [Google Scholar] [CrossRef]
- Harrison, C.N.; Mesa, R.A.; Jamieson, C.; Hood, J.; Bykowski, J.; Zuccoli, G.; Brewer, J. Case series of potential wernicke’s encephalopathy in patients treated with fedratinib. Blood 2017, 130, 14197. [Google Scholar]
- Zhang, Y.; Zhou, H.; Jiang, Z.; Wu, D.; Zhuang, J.; Wei, L.; Jiang, Q.; Wang, X.; Huang, J.; Zhu, H.; et al. Jaktinib, a Noval Jak Inhibitor in Treatment of Patients with Advanced Myelofibrosis: Preliminary Results from a Phase 2 Study; EHA Library: The Hague, The Netherlands, 2021; A 324612; S204. [Google Scholar]
- Carrancio, S.; Markovics, J.; Wong, P.; Leisten, J.; Castiglioni, P.; Groza, M.C.; Raymon, H.K.; Heise, C.; Daniel, T.; Chopra, R.; et al. An activin receptor II A ligand trap promotes erythropoiesis resulting in a rapid induction of red blood cells and haemoglobin. Br. J. Haematol. 2014, 165, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Platzbecker, U.; Mufti, G.J.; Manero, G.G.; Buckstein, R.; Santini, V.; Campelo, M.D.; Finelli, C.; Cazzola, M.; Ilhan, O.; et al. Luspatercept in patients with lower-risk myelodysplastic syndromes. N. Engl. J. Med. 2020, 382, 140–151. [Google Scholar] [CrossRef]
- Gerds, A.T.; Vannucchi, A.M.; Passamonti, F.; Kremyanskaya, M.; Gotlib, J.; Palmer, J.M.; McCaul, K.; Ribrag, V.; Mead, A.J.; Harrison, C.; et al. A phase 2 study of luspatercept in patients with myelofibrosis-associated anemia. Blood 2019, 136, 557–1557. [Google Scholar] [CrossRef]
- Daver, N.G.; Kremyanskaya, M.; O’Connell, C.; Dao, K.; Oh, S.T.; Gerds, A.T.; Verstovsek, S.; Erickson-Viitanen, S.; Zhou, F.; Assad, A.; et al. A phase 2 study of the safety and efficacy of INCB050465, a selective PI3Kδ inhibitor, in combination with ruxolitinib in patients with myelofibrosis. Blood 2018, 132, 353. [Google Scholar] [CrossRef]
- Yacoub, A.; Wang, E.S.; Rampal, R.K.; Borate, U.; Kremyanskaya, M.; Ali, H.; Hobbs, G.; O’Connell, C.; Assad, A.; Erickson-Viitanen, S.; et al. Addition of Parsaclisib, a PI3KDELTA Inhibitor, in Patients (PTS) with Suboptimal Response to Ruxolitinib (RUX): A Phase 2 Study in PTS with Myelofibrosis (MF); EHA Library: The Hague, The Netherlands, 2020; A 295036; S216. [Google Scholar]
- Harrison, C.N.; Garcia, J.S.; Mesa, R.A.; Somervaille, T.; Komrokji, R.S.; Pemmaraju, N.; Jamieson, C.; Papadantonakis, N.; Foran, J.M.; O’Connell, C.L.; et al. Results from a phase 2 study of navitoclax in combination with ruxolitinib in patients with primary or secondary myelofibrosis. Blood 2019, 134, 671. [Google Scholar] [CrossRef]
- Saenz, D.T.; Fiskus, W.; Qian, Y.; Manshouri, T.; Rajapakshe, K.; Raina, K.; Coleman, K.G.; Crew, A.P.; Shen, A.; Mill, C.P.; et al. Novel BET protein proteolysis-targeting chimera exerts superior lethal activity than bromodomain inhibitor (BETi) against post-myeloproliferative neoplasm secondary (s) AML cells. Leukemia 2017, 31, 1951–1961. [Google Scholar] [CrossRef]
- Mascarenhas, J.; Kremyanskaya, M.; Hoffman, R.; Bose, P.; Talpaz, M.; Harrison, C.N.; Gupta, V.; Leber, B.; Sirhan, S.; Kabir, S.; et al. MANIFEST, a phase 2 study of CPI-0610, a Bromodomain and Extraterminal Domain Inhibitor (BETi), as monotherapy or “add-on” to ruxolitinib, in patients with refractory or intolerant advanced myelofibrosis. Blood 2019, 134, 670. [Google Scholar] [CrossRef]
- Tefferi, A.; Lasho, T.L.; Begna, K.H.; Patnaik, M.M.; Zblewski, D.L.; Finke, C.M.; Laborde, R.R.; Wassie, E.; Schimek, L.; Hanson, C.A.; et al. A pilot study of the telomerase inhibitor imetelstat for myelofibrosis. N. Engl. J. Med. 2015, 373, 908–919. [Google Scholar] [CrossRef]
- Mascarenhas, J.; Komrokji, R.S.; Cavo, M.; Martino, B.; Niederwieser, D.; Reiter, A.; Scott, B.L.; Baer, M.R.; Hoffman, R.; Odenike, O.; et al. Imetelstat is effective treatment for patients with intermediate-2 or high-risk myelofibrosis who have relapsed on or are refractory to Janus Kinase inhibitor therapy: Results of a phase 2 randomized study of two dose levels. Blood 2018, 132, 685. [Google Scholar] [CrossRef]
- Nakatake, M.; Monte-Mor, B.; Debili, N.; Casadevall, N.; Ribrag, V.; Solary, E.; Vainchenker, W.; Plo, I. JAK2(V617F) negatively regulates p53 stabilization by enhancing MDM2 via la expression in myeloproliferative neoplasms. Oncogene 2012, 31, 1323–1333. [Google Scholar] [CrossRef]
- Al-Ali, H.K.; Delgado, R.G.; Lange, A.; Pluta, A.; McLornan, D.P.; Vachhani, P.; Laurent Damaj, G.; Jost, P.J.; Rejto, L.; Hus, M.; et al. KRT-232, A first-in-class, murine double minute 2 inhibitor, for myelofibrosis relapsed or refractory to janus-associated kinase inhibitor treatment. Hemasphere 2020, 4, 215. [Google Scholar]
- Niebel, D.; Kirfel, J.; Janzen, V.; Höller, T.; Majores, M.; Gütgemann, I. Lysinespecific demethylase 1 (LSD1) in hematopoietic and lymphoid neoplasms. Blood 2014, 124, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Pettit, K.; Yacoub, A.; Gerds, A.; Bradley, T.; Tatarczuch, M.; Curtin, N.; Shortt, J.; Rossetti, J.; Burbury, K.; Ewing, J.; et al. A phase 2 study of bomedemstat (IMG-7289), a lysine-specific demethylase-1 (LSD1) inhibitor, for the treatment of myelofibrosis (MF). Hemasphere 2020, 4, 1080. [Google Scholar]
- Kremyanskaya, M.; Ginzburg, Y.; Kuykendall, A.T.; Yacoub, A.; Yang, J.; Gupta, S.K.; Valone, F.; Khanna, S.; Verstovsek, S.; Hoffman, R. PTG-300 Eliminates the Need for Therapeutic Phlebotomy in Both Low and High-Risk Polycythemia Vera Patients. Blood 2020, 136, 33–35. [Google Scholar] [CrossRef]
- Červinek, L. Ropeginterferon alfa-2 b for the therapy of polycythemia vera. Vnitr. Lek. 2020, 66, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.E.; Wu, Y.Y.; Hsu, C.C.; Chen, Y.J.; Tsou, H.Y.; Li, C.P.; Lai, Y.H.; Lu, C.H.; Chen, P.T.; Chen, C.C. Real-world experience with Ropeginterferon-alpha 2b (Besremi) in Philadelphia-negative myeloproliferative neoplasms. J. Formos. Med. Assoc. 2021, 120, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Guerini, V.; Barbui, V.; Spinelli, O.; Salvi, A.; Dellacasa, C.; Carobbio, A.; Introna, M.; Barbui, T.; Golay, J.; Rambaldi, A. The histone deacetylase inhibitor ITF2357 selectively targets cells bearing mutated JAK2(V617F). Leukemia 2008, 22, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Rambaldi, A.; Dellacasa, C.M.; Finazzi, G.; Carobbio, A.; Ferrari, M.L.; Guglielmelli, P.; Gattoni, E.; Salmoiraghi, S.; Finazzi, M.C.; Di Tollo, S.; et al. A pilot study of the histone-deacetylase inhibitor givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br. J. Haematol. 2010, 150, 446–455. [Google Scholar] [CrossRef]
- Finazzi, G.; Vannucchi, A.M.; Martinelli, V.; Ruggeri, M.; Nobile, F.; Specchia, G.; Pogliani, E.M.; Olimpieri, O.M.; Fioritoni, G.; Musolino, C.; et al. A phase II study of givinostat in combination with hydroxycarbamide in patients with polycythaemia vera unresponsive to hydroxycarbamide monotherapy. Br. J. Haematol. 2013, 161, 688–694. [Google Scholar] [CrossRef] [PubMed]
| Treatment Strategy | Drug | Mechanism of Action | Phase—NCT Number (Trial) | Study Design | Status |
|---|---|---|---|---|---|
| Jak inhibitors | Momelotinib | JAK1-JAK2-ACVR1 inhibitor | 3—NCT01969838 (SIMPLIFY 1) | Momelotinib vs. Ruxolitinib in PMF or SMF | Completed |
| 3—NCT02101268 (SIMPLIFY 2) | Momelotinib vs. BAT in anemic or thrombocytopenic PMF or SMF | Completed | |||
| 3—NCT04173494 (MOMENTUM) | Momelotinib vs. Danazol in anemic PMF or SMF | Ongoing | |||
| Pacritinib | JAK2-FLT3-IRAK1-CSF1R inhibitor | 3—NCT01773187 (PERSIST 1) | Pacritinib vs. BAT in PMF or SMF | Completed | |
| 3—NCT02055781 (PERSIST 2) | Pacritinib vs. BAT in PMF or SMF and thrombocytopenia | Completed | |||
| 3—NCT03165734 (PACIFICA) | Pacritinib vs. BAT in PMF or SMF and severe thrombocytopenia | Ongoing | |||
| Fedratinib | JAK2-FLT3 inhibitor | 3—NCT01437787 (JAKARTA 1) | Fedratinib vs. placebo in PMF or SMF | Completed | |
| 2—NCT01523171 (JAKARTA 2) | Fedratinib in PMF or SMF previously treated with Ruxolitinib | Completed | |||
| 3—NCT03755518 (FREEDOM 1) | Fedratinib in PMF or SMF previously treated with Ruxolitinib | Ongoing | |||
| 3—NCT03952039 (FREEDOM 2) | Fedratinib vs. BAT in PMF or SMF previously treated with Ruxolitinib | Ongoing | |||
| Jaktinib | JAK1-JAK2-JAK3 inhibitor | 2—NCT03886415 | Jaktinib in PMF or SMF | Ongoing | |
| Combination therapies with ruxolitinib | Luspatercept | Activin ligand trap | 2—NCT03194542 | Luspatercept +/− Ruxolitinib in PMF | Ongoing |
| Parsaclisib | PI3Kδ inhibitor | 2—NCT02718300 | Parsaclisib + Ruxolitinib in PMF or SMF | Ongoing | |
| Navitoclax | BCL2/BCL-Xl inhibitor | 2—NCT03222609 (REFINE) | Navitoclax + Ruxolitinib in PMF or SMF | Ongoing | |
| Pelabresib | BET inhibitor | 2—NCT02158858 (MANIFEST) | Pelabresib +/− Ruxolitinib in PMF or SMF | Ongoing | |
| 3—NCT04603495 (MANIFEST 2) | Pelabresib + Ruxolitinib vs. Placebo + Ruxolitinib in PMF or SMF | Ongoing | |||
| Novel agents | Imetelstat | Telomerase inhibitor | 2—NCT02426086 (IMBARK) | Imetelstat in PMF previously treated with Ruxolitinib | Completed |
| KRT232 | MDM2 inhibitor | 2—NCT03662126 (BOREAS) | KRT232 in PMF or SMF previously treated with Ruxolitinib | Ongoing | |
| 3—NCT03662126 (BOREAS) | KRT232 vs. BAT in PMF or SMF previously treated with Ruxolitinib | Ongoing | |||
| Bomedemstat | LSD1 inhibitor | 2—NCT03136185 | Bomedemstat in PMF or SMF | Ongoing | |
| 2—NCT04254978 | Bomedemstat in ET | Ongoing | |||
| Rusfertide | Hepcidin mimetic | 2—NCT04057040 | Rusfertide in PV | Ongoing | |
| Besremi | Interferon-α | 3—NCT02218047 (CONTI-PV) | PEG-P-INF alpha-2b vs. BAT in PV | Completed | |
| Givinostat | Histone deacetylase inhibitor | 2—NCT0060307 | Givinostat in MPN | Completed | |
| 2—NCT00928707 | Givinostat + HU in MPN | Completed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penna, D. New Horizons in Myeloproliferative Neoplasms Treatment: A Review of Current and Future Therapeutic Options. Medicina 2021, 57, 1181. https://doi.org/10.3390/medicina57111181
Penna D. New Horizons in Myeloproliferative Neoplasms Treatment: A Review of Current and Future Therapeutic Options. Medicina. 2021; 57(11):1181. https://doi.org/10.3390/medicina57111181
Chicago/Turabian StylePenna, Domenico. 2021. "New Horizons in Myeloproliferative Neoplasms Treatment: A Review of Current and Future Therapeutic Options" Medicina 57, no. 11: 1181. https://doi.org/10.3390/medicina57111181
APA StylePenna, D. (2021). New Horizons in Myeloproliferative Neoplasms Treatment: A Review of Current and Future Therapeutic Options. Medicina, 57(11), 1181. https://doi.org/10.3390/medicina57111181





