Anticholinergic Burden of Geriatric Ward Inpatients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of Study, Participants and Measurements
2.2. Anticholinergic Cognitive Burden Scale
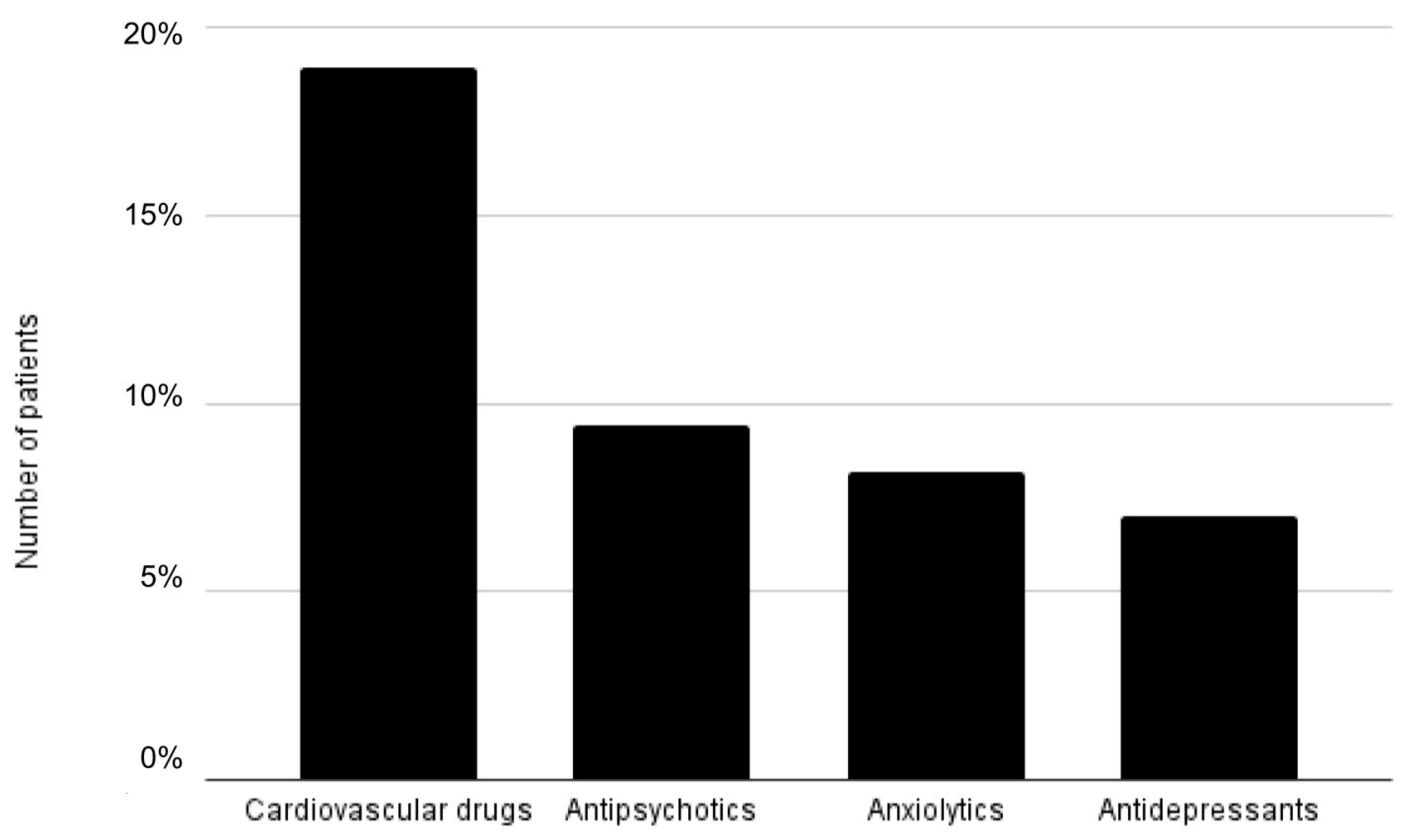
2.3. Anticholinergic Medications
2.4. Statistical Analysis
2.5. Ethics
3. Results
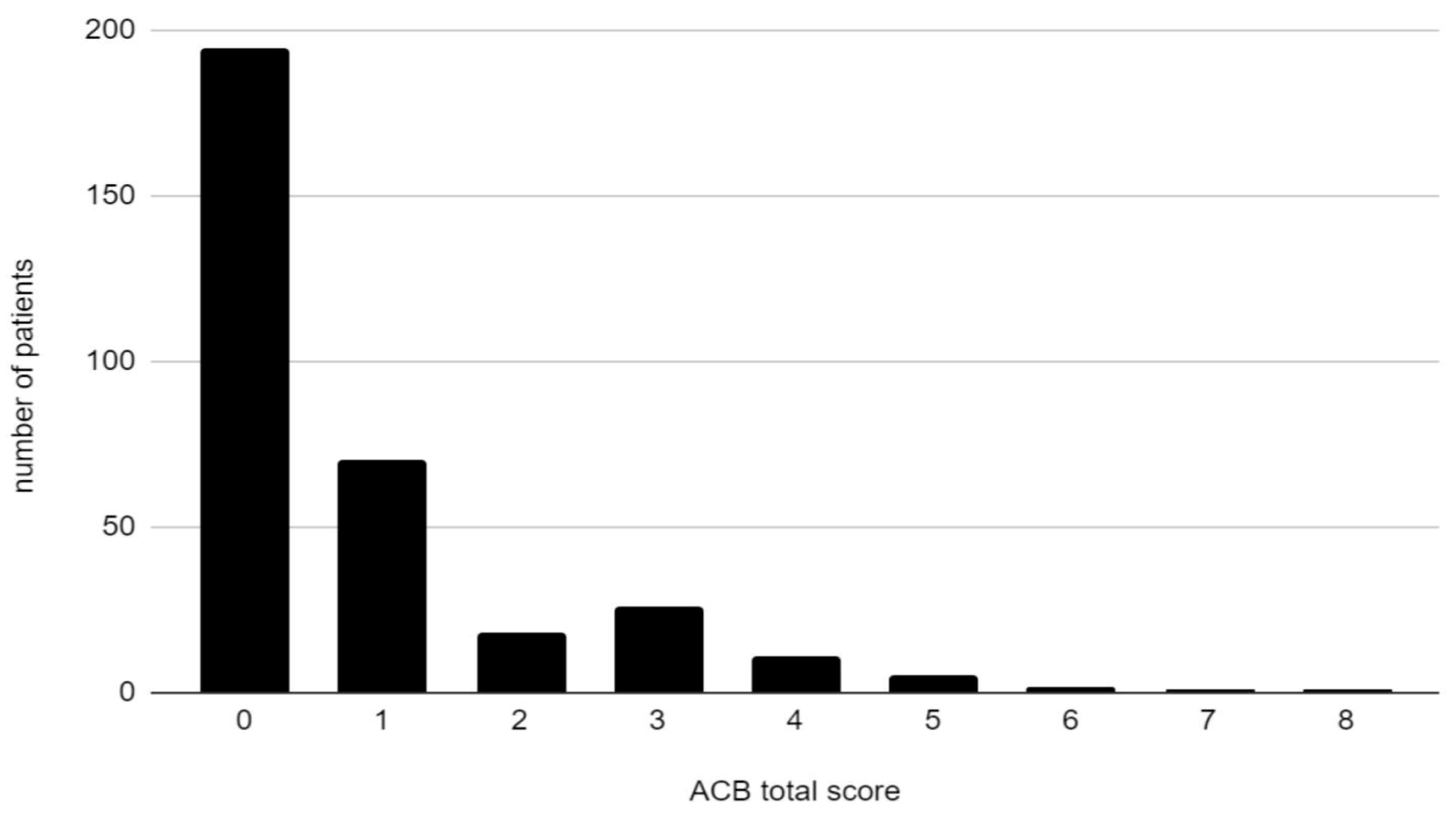
3.1. Prevalence of Anticholinergic Burden
3.2. Association of Anticholinergic Burden with Geriatric Disabilities
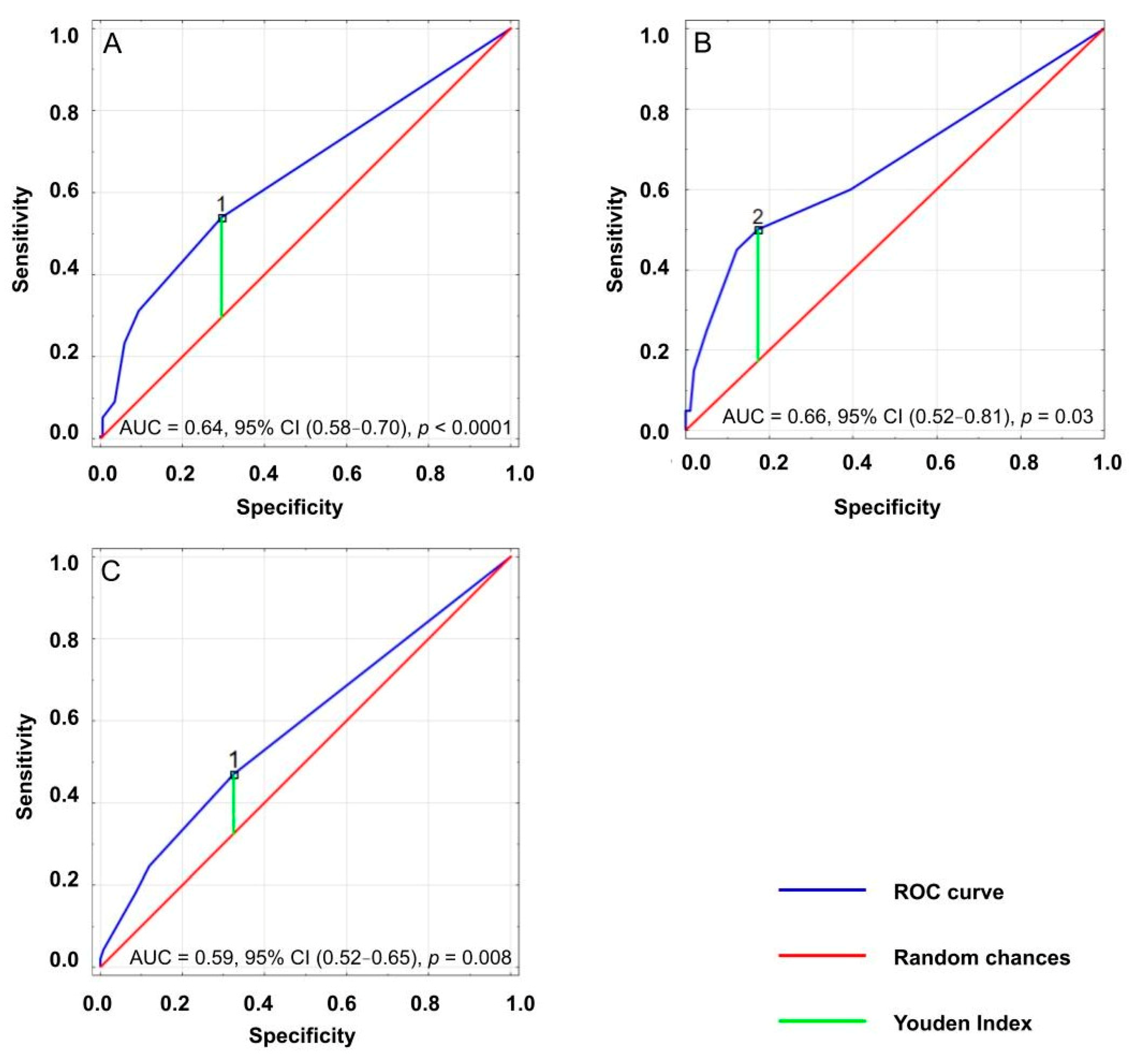
3.3. Cut-Off Point of ACB Score Significantly Associated with Geriatric Problems
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gerretsen, P.; Pollock, B.G. Drugs with anticholinergic properties: A current perspective on use and safety. Expert Opin. Drug Saf. 2011, 10, 751–765. [Google Scholar] [CrossRef]
- Richardson, K.; Fox, C.; Maidment, I.; Steel, N.; Loke, Y.K.; Arthur, A.; Myint, P.K.; Grossi, C.M.; Mattishent, K.; Bennett, K.; et al. Anticholinergic drugs and risk of dementia: Case-control study. BMJ 2018, 361, k1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lie, J.D.; Tu, K.N.; Shen, D.D.; Wong, B.M. Pharmacological Treatment of Insomnia. Pharmacy Ther. 2015, 40, 759–771. [Google Scholar]
- Corsonello, A.; Pedone, C.; Incalzi, R. Age-Related Pharmacokinetic and Pharmacodynamic Changes and Related Risk of Adverse Drug Reactions. Curr Med. Chem. 2010, 17, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.E.; Peterson, C. Aging Decreases Oxidative Metabolism and the Release and Synthesis of Acetylcholine. J. Neurochem. 1981, 37, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Migirov, A.; Datta, A.R. Physiology, Anticholinergic Reaction. [Updated 2 October 2020]. In StatPearls; [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: www.ncbi.nlm.nih.gov/books/NBK546589/ (accessed on 19 August 2021).
- 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr Soc. 2019, 67, 674–694. [Google Scholar] [CrossRef] [PubMed]
- Boustani, M.; Campbell, N.; Munger, S.; Maidment, I.; Fox, C. Impact of anticholinergics on the aging brain: A review and practical application. Aging Health 2008, 4, 311–320. [Google Scholar] [CrossRef]
- Egberts, A.; van der Craats, S.T.; van Wijk, M.D.; Alkilabe, S.; van den Bemt, P.M.L.A.; Mattace-Raso, F.U.S. Anticholinergic drug exposure is associated with delirium and postdischarge institutionalization in acutely ill hospitalized older patients. Pharmacol. Res. Perspect. 2017, 5, e00310. [Google Scholar] [CrossRef] [PubMed]
- Green, A.R.; Reifler, L.M.; Bayliss, E.A.; Weffald, L.A.; Boyd, C.M. Drugs Contributing to Anticholinergic Burden and Risk of Fall or Fall-Related Injury among Older Adults with Mild Cognitive Impairment, Dementia and Multiple Chronic Conditions: A Retrospective Cohort Study. Drugs Aging 2019, 36, 289–297. [Google Scholar] [CrossRef]
- Campbell, N.L.; Boustani, M.A.; Lane, K.A.; Gao, S.; Hendrie, H.; Khan, B.A.; Murrell, J.R.; Unverzagt, F.W.; Hake, A.; Smith-Gamble, V.; et al. Use of anticholinergics and the risk of cognitive impairment in an African American population. Neurology 2010, 75, 152–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasina, L.; Djade, C.D.; Lucca, U.; Nobili, A.; Tettamanti, M.; Franchi, C.; Salerno, F.; Corrao, S.; Marengoni, A.; Iorio, A.; et al. Association of Anticholinergic Burden with Cognitive and Functional Status in a Cohort of Hospitalized Elderly: Comparison of the Anticholinergic Cognitive Burden Scale and Anticholinergic Risk Scale. Drugs Aging 2013, 30, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano-Ortega, G.; Johnston, K.M.; Cheung, A.; Wagg, A.; Campbell, N.L.; Dmochowski, R.R.; Ng, D.B. A review of published anticholinergic scales and measures and their applicability in database analyses. Arch. Gerontol. Geriat. 2020, 87, 103885. [Google Scholar] [CrossRef]
- Grodzicki, T.; Kocemba, J.; Skalska, A. Geriatria z Elementami Gerontologii Ogólnej. Podręcznik dla Lekarzy i Studentów, 1st ed.; ViaMedica: Gdańsk, Poland, 2006; p. 74. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Neuls, P.D.; Clark, T.L.; Van Heuklon, N.C.; Proctor, J.E.; Kilker, B.J.; Bieber, M.E.; Donlan, A.V.; Carr-Jules, S.A.; Neidel, W.H.; Newton, R.A. Usefulness of the Berg Balance Scale to predict falls in the elderly. J. Geriatr Phys. Ther. 2011, 34, 3–10. [Google Scholar] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Clifford, R.J., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Aging Brain Care. Anticholinergic Cognitive Burden Scale [Internet]. 2012. Available online: www.agingbraincare.org/tools/abc-anticholinergic-cognitive-burden-scale (accessed on 26 August 2019).
- Cebron Lipovec, N.; Jazbar, J.; Kos, M. Anticholinergic Burden in Children, Adults and Older Adults in Slovenia: A Nationwide Database Study. Sci Rep. 2020, 10, 9337. [Google Scholar] [CrossRef] [PubMed]
- Pfistermeister, B.; Tümena, T.; Gaßmann, K.G.; Maas, R.; Fromm, M.F. Anticholinergic burden and cognitive function in a large German cohort of hospitalized geriatric patients. PLoS ONE 2017, 10, e0171353. [Google Scholar] [CrossRef]
- West, T.; Pruchnicki, M.C.; Porter, K.; Emptage, R. Evaluation of anticholinergic burden of medications in older adults. J. Am. Pharm Assn. 2013, 53, 496–504. [Google Scholar] [CrossRef]
- Vaughan, R.; Flynn, R.; Greene, N. Anticholinergic burden of patients with dementia attending a Psychiatry of Later Life service. Irish J. Psychol Med. 2019, 1–6. [Google Scholar] [CrossRef]
- Pieper, N.; Grossi, C.; Chan, W.Y.; Loke, Y.K.; Savva, G.; Haroulis, C.; Steel, N.; Fox, C.; Maidment, I.; Arthur, A.; et al. Anticholinergic drugs and incident dementia, mild cognitive impairment and cognitive decline: A meta-analysis. Age Ageing 2020, 49, 939–947. [Google Scholar] [CrossRef]
- Boccardi, V.; Baroni, M.; Paolacci, L. Anticholinergic burden and functional status in older people with cognitive impairment: Results from the ReGAl project. J. Nutr. Health Aging 2017, 21, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Marcum, Z.A.; Wirtz, H.S.; Pettinger, M.; LaCroix, A.Z.; Carnahan, R.; Cauley, J.A.; Bea, J.W.; Gray, S.L. Anticholinergic medication use and falls in postmenopausal women: Findings from the women’s health initiative cohort study. J. Gerontol. 2016, 16, 76. [Google Scholar] [CrossRef] [Green Version]
- Coupland, C.A.C.; Hill, T.; Dening, T.; Morriss, R.; Moore, M.; Hippisley-Cox, J. Anticholinergic Drug Exposure and the Risk of Dementia. JAMA Internal Med. 2019, 179, 1084. [Google Scholar] [CrossRef]
- Naharci, M.I.; Tasci, I. Frailty status and increased risk for falls: The role of anticholinergic burden. Arch. Gerontol Geriat. 2020, 90, 104136. [Google Scholar] [CrossRef]
- Suehs, B.T.; Caplan, E.O.; Hayden, J.; Ng, D.B.; Gaddy, R.R. The Relationship Between Anticholinergic Exposure and Falls, Fractures, and Mortality in Patients with Overactive Bladder. Drugs Aging 2019, 36, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Lockery, J.E.; Broder, J.C.; Ryan, J.; Stewart, A.C.; Woods, R.L.; Chong, T.T.-J.; Cloud, G.C.; Murray, A.; Rigby, J.D.; Shah, R.; et al. A Cohort Study of Anticholinergic Medication Burden and Incident Dementia and Stroke in Older Adults. J. General Intern. Med. 2021, 36, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Canadian Institute for Health Information. Seniors and the Health Care System: What Is the Impact of Multiple Chronic Conditions? [Internet]. 2011. Available online: www.secure.cihi.ca/free_products/air-chronic_disease_aib_en.pdf (accessed on 19 August 2021).
- Rochon, P.A.; Gurwitz, J.H. The prescribing cascade revisited. Lancet 2017, 389, 1778–1780. [Google Scholar] [CrossRef]
- Anthierens, S.; Tansens, A.; Petrovic, M.; Christiaens, T. Qualitative insights into general practitioners views on polypharmacy. BMC Fam Pract. 2010, 11, 65. [Google Scholar] [CrossRef] [Green Version]
- Yeh, Y.C.; Liu, C.L.; Peng, L.N.; Lin, M.H.; Chen, L.K. Potential benefits of reducing medication-related anticholinergic burden for demented older adults: A prospective cohort study. Geriatr. Gerontol. Int. 2013, 13, 694–700. [Google Scholar] [CrossRef]
- Kersten, H.; Molden, E.; Tolo, I.K.; Skovlund, E.; Engedal, K.; Wyller, T.B. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: A randomized controlled trial. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Jaïdi, Y.; Guilloteau, A.; Nonnonhou, V.; Bertholon, L.A.; Badr, S.; Morrone, I.; Novella, J.L.; Mahmoudi, R. Threshold for a Reduction in Anticholinergic Burden to Decrease Behavioral and Psychological Symptoms of Dementia. J. Am. Med. Dir. Assoc. 2019, 20, 159–164.e3. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total Cohort n = 329 |
|---|---|
| ACB (score) | 0.85 ± 1.38 |
| ACB score of at least 1 (%) | 40.73 |
| ACB score of 0 (%) | 59.27 |
| ACB score of 1 (%) | 21.28 |
| ACB score of 2 (%) | 5.47 |
| ACB score of at least 3 (%) | 13.98 |
| Medications with an ACB score ≥2 (%) | 13.68 |
| Sex, women (%) | 72.04 |
| Age (years) | 79.61± 6.82 |
| Total number of drugs | 8.59 ± 6.54 |
| BMI (kg/m2) | 27.96 ± 5.67 |
| Systolic blood pressure (mmHg) | 140.87 ± 21.66 |
| Diastolic blood pressure (mmHg) | 81.44 ± 12.42 |
| Independently ambulating (%) | 57.85 |
| Ambulatory with assistance (%) | 34.77 |
| Nonambulatory (%) | 7.38 |
| Barthel Index (score) | 73.64 ± 26.93 |
| IADL Index (score) | 18.92 ± 6.52 |
| Berg Balance Scale (score) | 33.26 ± 16.66 |
| MMSE (score) | 22.05 ± 6.97 |
| Dementia (%) | 47.68 |
| Previous stroke (%) | 8.23 |
| Diabetes (%) | 30.89 |
| Variable | Patients with Dementia n = 154 | Patients without Dementia n = 169 | p Value |
|---|---|---|---|
| ACB (score) | 1.26 ± 1.60 | 0.50 ± 1.04 | <0.001 |
| ACB score = 0 (%) | 46.10 | 70.41 | <0.001 |
| ACB score = 1 (%) | 22.73 | 20.12 | 0.397 |
| ACB score = 2 (%) | 7.79 | 3.55 | 0.149 |
| ACB score ≥ 3 (%) | 23.38 | 5.92 | <0.001 |
| Any medication with an ACB score ≥2 (%) | 22.73 | 5.92 | <0.001 |
| Variable | Patients with a Risk of Falls n = 187 | Patient with Low Risk of Falls n = 126 | p Value |
|---|---|---|---|
| ACB (score) | 1.06 ± 1.56 | 0.57 ± 1.05 | 0.004 |
| ACB score = 0 (%) | 52.94 | 67.46 | 0.010 |
| ACB score = 1 (%) | 22.46 | 20.63 | 0.744 |
| ACB score = 2 (%) | 6.42 | 3.17 | 0.407 |
| ACB score ≥ 3 (%) | 18.18 | 8.73 | 0.011 |
| Any medications with ACB score ≥2 (%) | 17.65 | 8.73 | 0.026 |
| Variable | Patients with Severe Disability n = 20 | Patients with Moderately Severe Functional Impairment n = 142 | Patients with Good Functional Capacity n = 160 | p Value |
|---|---|---|---|---|
| ACB (score) | 2.10 ± 2.30 | 0.94 ± 1.33 | 0.62 ± 1.20 | <0.001 |
| ACB score = 0 (%) | 40.00 | 51.41 | 68.75 | 0.002 |
| ACB score = 1 (%) | 10.00 | 26.06 | 18.75 | 0.123 |
| ACB score = 2 (%) | 5.00 | 8.45 | 1.88 | 0.054 |
| ACB score ≥ 3 (%) | 45.00 | 14.08 | 10.63 | <0.001 |
| Any medication with an ACB score ≥2 (%) | 40.00 | 14.08 | 10.63 | 0.001 |
| Outcomes | Cut-Off Value | Sensitivity | Specificity | Youden Index | AUC | p Value |
|---|---|---|---|---|---|---|
| Dementia | 1 | 0.54 | 0.30 | 0.24 | 0.64 | <0.001 |
| Severe disability | 2 | 0.50 | 0.17 | 0.33 | 0.66 | 0.026 |
| Risk of falls | 1 | 0.47 | 0.33 | 0.15 | 0.59 | 0.008 |
| Outcomes | OR | 95% CI | p Value |
|---|---|---|---|
| Dementia | 2.86 | 1.80–4.52 | <0.001 |
| Severe disability | 4.81 | 1.90–12.18 | <0.001 |
| Risk of falls | 1.88 | 1.17–3.02 | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilczyński, K.; Gorczyca, M.; Gołębiowska, J.; Szewieczek, J. Anticholinergic Burden of Geriatric Ward Inpatients. Medicina 2021, 57, 1115. https://doi.org/10.3390/medicina57101115
Wilczyński K, Gorczyca M, Gołębiowska J, Szewieczek J. Anticholinergic Burden of Geriatric Ward Inpatients. Medicina. 2021; 57(10):1115. https://doi.org/10.3390/medicina57101115
Chicago/Turabian StyleWilczyński, Krzysztof, Marta Gorczyca, Jagna Gołębiowska, and Jan Szewieczek. 2021. "Anticholinergic Burden of Geriatric Ward Inpatients" Medicina 57, no. 10: 1115. https://doi.org/10.3390/medicina57101115
APA StyleWilczyński, K., Gorczyca, M., Gołębiowska, J., & Szewieczek, J. (2021). Anticholinergic Burden of Geriatric Ward Inpatients. Medicina, 57(10), 1115. https://doi.org/10.3390/medicina57101115






