Colchicine to Prevent Sympathetic Denervation after an Acute Myocardial Infarction: The COLD-MI Trial Protocol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design
- -
- Sympathetic denervation at 6 months after an AMI;
- -
- Heart rate variability at 1 and 6 months after an AMI.
2.2. Trial Population
- -
- Adult patients;
- -
- Presenting with a clinical episode of STEMI (chest pain and ECG abnormalities (12 leads) consistent with the diagnosis of AMI: elevation of the ST segment on at least 2 contiguous branches, or appearance of a recent left branch block);
- -
- Chest pain lasting less than 12 h;
- -
- Coronary occlusion on initial angiography (TIMI flow of the causal artery of the infarction at 1 or 0);
- -
- Eligible for a revascularization procedure by percutaneous coronary angioplasty.
- -
- Any history of myocardial infarction prior to the current episode;
- -
- Severe hepatic or renal dysfunction (known GFR ≤30mL/min);
- -
- Chronic treatment with Colchicine (mainly Familial Mediterranean Fever);
- -
- Chronic treatment with a potent CYP3A4 inhibitor or P-glycoprotein inhibitor in patients with kidney or liver failure;
- -
- Any history of severe reaction or known severe intolerance to colchicine;
- -
- Association with macrolides (except spiramycin) or with pristinamycin;
- -
- Hemodynamic instability (need for amines for more than 24 h, assistance circulatory system) at the time of inclusion;
- -
- Disorders of conscience compromising informed consent;
- -
- Swallowing disorders and major digestive disorders (chronic diarrhoea, inflammatory bowel disease, etc..), gastrointestinal tract disease such as uncontrolled ulcerative colitis or Crohn’s disease, with immunosuppression;
- -
- Bone marrow aplasia, active chronic inflammatory disease, chronic active infection;
- -
- Active cancer, bone marrow aplasia;
- -
- Chronic active infection or recent sepsis (7 days);
- -
- Chronic treatment (for more than 6 months) with corticosteroids or NSAIDs or repeated high doses of less than 7 days, corresponding to a corticoid dose of >1mg/kg/d for more than 3 consecutive days or ibuprofen >600 mg/d for more than 3 days) or treatment with potent CYP3A4 inhibitors such as cyclosporin, verapamil, inhibitors of proteases boosted by ritonavir and telaprevir;
- -
- Contraindication to an examination of the study (scintigraphy, …);
- -
- Legal protection measure (guardianship, curatorship);
- -
- Participation in another clinical trial.
2.3. End Points
2.3.1. Biological Assessments
- -
- Variations in the levels of Nerve Growth Factor, C-reactive Protein (CRP) and Sst2 in plasma between hospitalisation, 1- and 6-months follow-up;
- -
- Assessment of infarct size with area under the curve of the creatine kinase and troponin during the hospitalisation (day 1 to day 5).
2.3.2. Imaging Assessment
- -
- SPECT imaging at 6 months follow-up:
- ○
- heart/mediastinum ratio (quantitative index in cardiac imaging 123 I-MIBG SPECT),
- ○
- isotopic LVEF;
- -
- TTE at 1 and 6 months:
- ○
- Left ventricular ejection fraction (LVEF) in percent;
- ○
- Global longitudinal strain imaging in percent.
2.3.3. Rhythmic Evaluation
- -
- ECG parameters (QRS duration, corrected QT) at 1- and 6-months follow-up;
- -
- HRV parameters on a 24 hour-recording Holter at 1- and 6-months follow-up. In a 24h Holter monitoring, HRV parameters are measured. According to the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology [10], we decided to assess both time and frequency domain measures:
- ○
- Time-domain measures are represented by the Standard Deviation of NN intervals (SDNN), the Root mean square of successive RR interval differences (RMSSD) and the Baseline width of the RR interval histogram (TINN). All these measures are expressed in milliseconds.
- ○
- Frequency-domain measures divide the heart rate into 4 bands. We use the Very Low (VLF), Low (LF) and High (HF) Frequency bands, expressed in absolute power (ms squared divided by cycles per second);
- -
- Number of premature ventricular contraction (PVC) at 1- and 6-months follow-up;
- -
- Number of bursts (2 or 3 PVC) at 1- and 6-months follow-up;
- -
- Number of episodes of supraventricular or ventricular tachycardia (defined by >3 PVC) at 1- and 6-months follow-up.
2.3.4. Exploratory Clinical Evaluation
- -
- Number of adverse events;
- -
- Number of all-cause hospitalizations, hospitalization for heart failure or death.
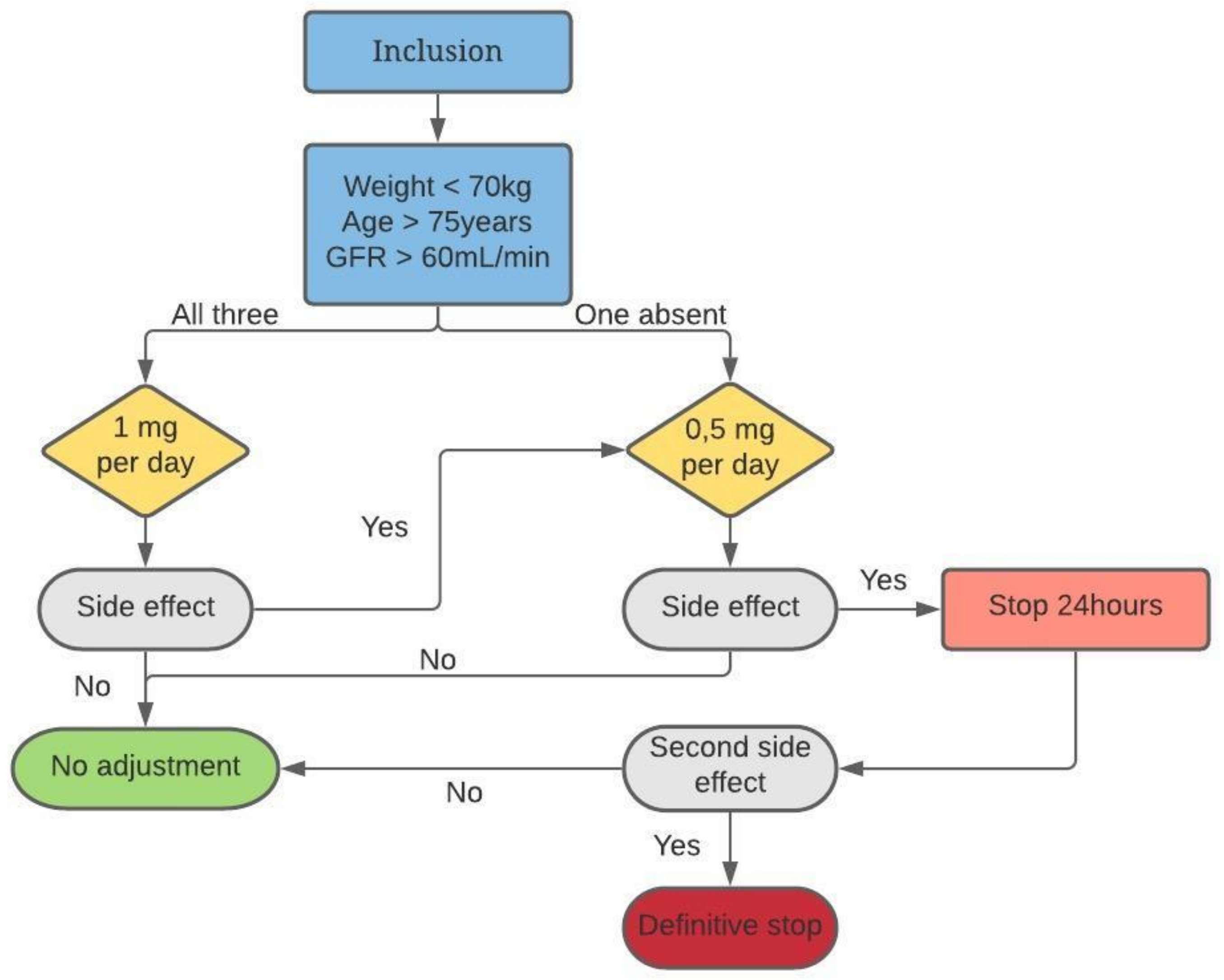
3. Intervention
4. Data Collection and Analysis
5. Discussion and Conclusions
Funding
Conflicts of Interest
References
- Yellon, D.M.; Hausenloy, D.J. Myocardial Reperfusion Injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef]
- Huet, F.; Akodad, M.; Fauconnier, J.; Lacampagne, A.; Roubille, F. Anti-inflammatory drugs as promising cardiovascular treatments. Expert. Rev. Cardiovasc. Ther. 2017, 15, 109–125. [Google Scholar] [CrossRef]
- Boogers, M.J.; Borleffs, C.J.; Henneman, M.M.; van Bommel, R.J.; van Ramshorst, J.; Boersma, E.; Dibbets-Schneider, P.; Stokkel, M.P.; van der Wall, E.E.; Schalij, M.J.; et al. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. J. Am. Coll. Cardiol. 2010, 55, 2769–2777. [Google Scholar] [CrossRef] [Green Version]
- Gardner, R.T.; Ripplinger, C.M.; Myles, R.C.; Habecker, B.A. Molecular Mechanisms of Sympathetic Remodeling and Arrhythmias. Circ. Arrhythm. Electrophysiol. 2016, 9, e001359. [Google Scholar] [CrossRef] [Green Version]
- Gimelli, A.; Masci, P.G.; Liga, R.; Grigoratos, C.; Pasanisi, E.M.; Lombardi, M.; Marzullo, P. Regional heterogeneity in cardiac sympathetic innervation in acute myocardial infarction: Relationship with myocardial oedema on magnetic resonance. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1692–1694. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Li, Y.-G. Cardiac Sympathetic Nerve Sprouting and Susceptibility to Ventricular Arrhythmias after Myocardial Infarction. Cardiol. Res. Pract. 2015, 2015, 698368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedon, C.; Huet, F.; Ben Bouallegue, F.; Vernhet, H.; Macia, J.C.; Cung, T.T.; Leclercq, F.; Cade, S.; Cransac, F.; Lattuca, B.; et al. Area at risk can be assessed by iodine-123-meta-iodobenzylguanidine single-photon emission computed tomography after myocardial infarction: A prospective study. Nucl. Med. Commun. 2018, 39, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Vauchot, F.; Ben Bouallègue, F.; Hedon, C.; Piot, C.; Roubille, F.; Mariano-Goulart, D. Assessment of the area at risk after acute myocardial infarction using 123I-MIBG SPECT: Comparison with the angiographic APPROACH-score. J. Nucl. Cardiol. 2018, 25, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.F.; Senior, R.; Cerqueira, M.D.; Wong, N.D.; Thomas, G.S.; Lopez, V.A.; Agostini, D.; Weiland, F.; Chandna, H.; Narula, J.; et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J. Am. Coll. Cardiol. 2010, 55, 2212–2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Electrophysiology Task Force of the European Society of Cardiology the North American Society of Pacing. Heart Rate Var. Circ. 1996, 93, 1043–1065.
- Ravelli, R.B.; Gigant, B.; Curmi, P.A.; Jourdain, I.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef]
- Roubille, F.; Kritikou, E.; Busseuil, D.; Barrere-Lemaire, S.; Tardif, J.-C. Colchicine: An Old Wine in a New Bottle? AIAAMC 2013, 12, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Akodad, M.; Lattuca, B.; Nagot, N.; Georgescu, V.; Buisson, M.; Cristol, J.P.; Leclercq, F.; Macia, J.C.; Gervasoni, R.; Cung, T.T.; et al. COLIN trial: Value of colchicine in the treatment of patients with acute myocardial infarction and inflammatory response. Arch Cardiovasc. Dis. 2017, 110, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Akodad, M.; Fauconnier, J.; Sicard, P.; Huet, F.; Blandel, F.; Bourret, A.; de Santa Barbara, P.; Aguilhon, S.; LeGall, M.; Hugon, G.; et al. Interest of colchicine in the treatment of acute myocardial infarct responsible for heart failure in a mouse model. Int. J. Cardiol. 2017, 240, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Huet, F.; Fauconnier, J.; Legall, M.; Sicard, P.; Lozza, C.; Lacampagne, A.; Roubille, F. Low-dose colchicine prevents sympathetic denervation after myocardial ischemia-reperfusion: A new potential protective mechanism. Future Sci. OA 2020, 7, FSO656. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Roubille, F.; Tardif, J.-C. Colchicine for Secondary Cardiovascular Prevention in Coronary Disease. Circulation 2020, 142, 1901–1904. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Odemuyiwa, O.; Malik, M.; Farrell, T.; Bashir, Y.; Poloniecki, J.; Camm, J. Comparison of the predictive characteristics of heart rate variability index and left ventricular ejection fraction for all-cause mortality, arrhythmic events and sudden death after acute myocardial infarction. Am. J. Cardiol. 1991, 68, 434–439. [Google Scholar] [CrossRef]
- Gómez, A.M.; Kerfant, B.-G.; Vassort, G.; Pappano, A.J. Autonomic regulation of calcium and potassium channels is oppositely modulated by microtubules in cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2065–H2071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaseghi, M.; Lux, R.L.; Mahajan, A.; Shivkumar, K. Sympathetic stimulation increases dispersion of repolarization in humans with myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1838–H1846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roubille, F.; Tardif, J.-C. Cardioprotection--time to take into account clinical complexity: The case of antiplatelet agents: Editorial to «two classes of anti-platelet drugs reduce anatomical infarct size in monkey hearts» by Xi-Ming Yang et al. Cardiovasc. Drugs Ther. 2013, 27, 105–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.L.; Goldberg, R.J.; Anderson, F.A.; López-Sendón, J.; Montalescot, G.; Brieger, D.; Eagle, K.A.; Wyman, A.; Gore, J.M.; Global Registry of Acute Coronary Events Investigators. Beta-blocker use in ST-segment elevation myocardial infarction in the reperfusion era (GRACE). Am. J. Med. 2014, 127, 503–511. [Google Scholar] [CrossRef]
- Piccini, J.P.; Schulte, P.J.; Pieper, K.S.; Mehta, R.H.; White, H.D.; Van de Werf, F.; Ardissino, D.; Califf, R.M.; Granger, C.B.; Ohman, E.M.; et al. Antiarrhythmic drug therapy for sustained ventricular arrhythmias complicating acute myocardial infarction. Crit. Care Med. 2011, 39, 78–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouabdallaoui, N.; Tardif, J.-C.; Waters, D.D.; Pinto, F.J.; Maggioni, A.P.; Diaz, R.; Berry, C.; Koenig, W.; Lopez-Sendon, J.; Gamra, H.; et al. Time-to-treatment initiation of colchicine and cardiovascular outcomes after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur. Heart J. 2020, 41, 4092–4099. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Bresson, D.; Roubille, F.; Prieur, C.; Biere, L.; Ivanes, F.; Bouleti, C.; Dubreuil, O.; Rioufol, G.; Boutitie, F.; Sideris, G.; et al. Colchicine for Left Ventricular Infarct Size Reduction in Acute Myocardial Infarction: A Phase II, Multicenter, Randomized, Double-Blinded, Placebo-Controlled Study Protocol—The COVERT-MI Study. Cardiology 2021, 146, 151–160. [Google Scholar]
| Visit | Inclusion | Visit 1 | Visit 2 | Visit 3 | Data Collection | Visit 4 | Visit 5 | Visit 6 |
|---|---|---|---|---|---|---|---|---|
| Date | Day 0 | Day 1 | Day 2 | Day 3 | Day 4, 5 | Out of hospital | 1 month ± 1 week | 6 month ± 1 week |
| Informationa and written consent signature | X | |||||||
| Randomization | X | |||||||
| Clinical examination | X | X | X | X | ||||
| Review concomitant treatment | X | X | X | X | X | X | X | X |
| Blood sample (1) | X | X | X | X | X (3) | X | X | X |
| Specific analysis and biological collection (2) | X * | X * | X * | X * | X * | |||
| Urine pregnancy test (only women of childbearing potential) | X * | |||||||
| Transthoracic echocardiography | X | X | X | X | ||||
| Cardiac MRI | X | |||||||
| 123I-MIBG SPECT | X * | |||||||
| Holter ECG 24h | X | X | X | |||||
| Rest ECG | X | X | X | X | ||||
| AE collection | X * | X * | X * | X * | X * | X * | X * | |
| Study medication dispensing | X * | X * | X * | X * | X* | X * | ||
| Study medication return | X * | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huet, F.; Delbaere, Q.; Aguilhon, S.; Dupasquier, V.; Delseny, D.; Gervasoni, R.; Macia, J.-C.; Leclercq, F.; Jammoul, N.; Kahlouche, S.; et al. Colchicine to Prevent Sympathetic Denervation after an Acute Myocardial Infarction: The COLD-MI Trial Protocol. Medicina 2021, 57, 1047. https://doi.org/10.3390/medicina57101047
Huet F, Delbaere Q, Aguilhon S, Dupasquier V, Delseny D, Gervasoni R, Macia J-C, Leclercq F, Jammoul N, Kahlouche S, et al. Colchicine to Prevent Sympathetic Denervation after an Acute Myocardial Infarction: The COLD-MI Trial Protocol. Medicina. 2021; 57(10):1047. https://doi.org/10.3390/medicina57101047
Chicago/Turabian StyleHuet, Fabien, Quentin Delbaere, Sylvain Aguilhon, Valentin Dupasquier, Delphine Delseny, Richard Gervasoni, Jean-Christophe Macia, Florence Leclercq, Nidal Jammoul, Sandra Kahlouche, and et al. 2021. "Colchicine to Prevent Sympathetic Denervation after an Acute Myocardial Infarction: The COLD-MI Trial Protocol" Medicina 57, no. 10: 1047. https://doi.org/10.3390/medicina57101047
APA StyleHuet, F., Delbaere, Q., Aguilhon, S., Dupasquier, V., Delseny, D., Gervasoni, R., Macia, J.-C., Leclercq, F., Jammoul, N., Kahlouche, S., Soltani, S., Cardon, F., Dupuy, A.-M., Cristol, J.-P., Mariano-Goulart, D., Akodad, M., Nagot, N., & Roubille, F. (2021). Colchicine to Prevent Sympathetic Denervation after an Acute Myocardial Infarction: The COLD-MI Trial Protocol. Medicina, 57(10), 1047. https://doi.org/10.3390/medicina57101047






