Extramammary Paget’s Disease of the Vulva: Report of Two Cases
Abstract
:1. Introduction
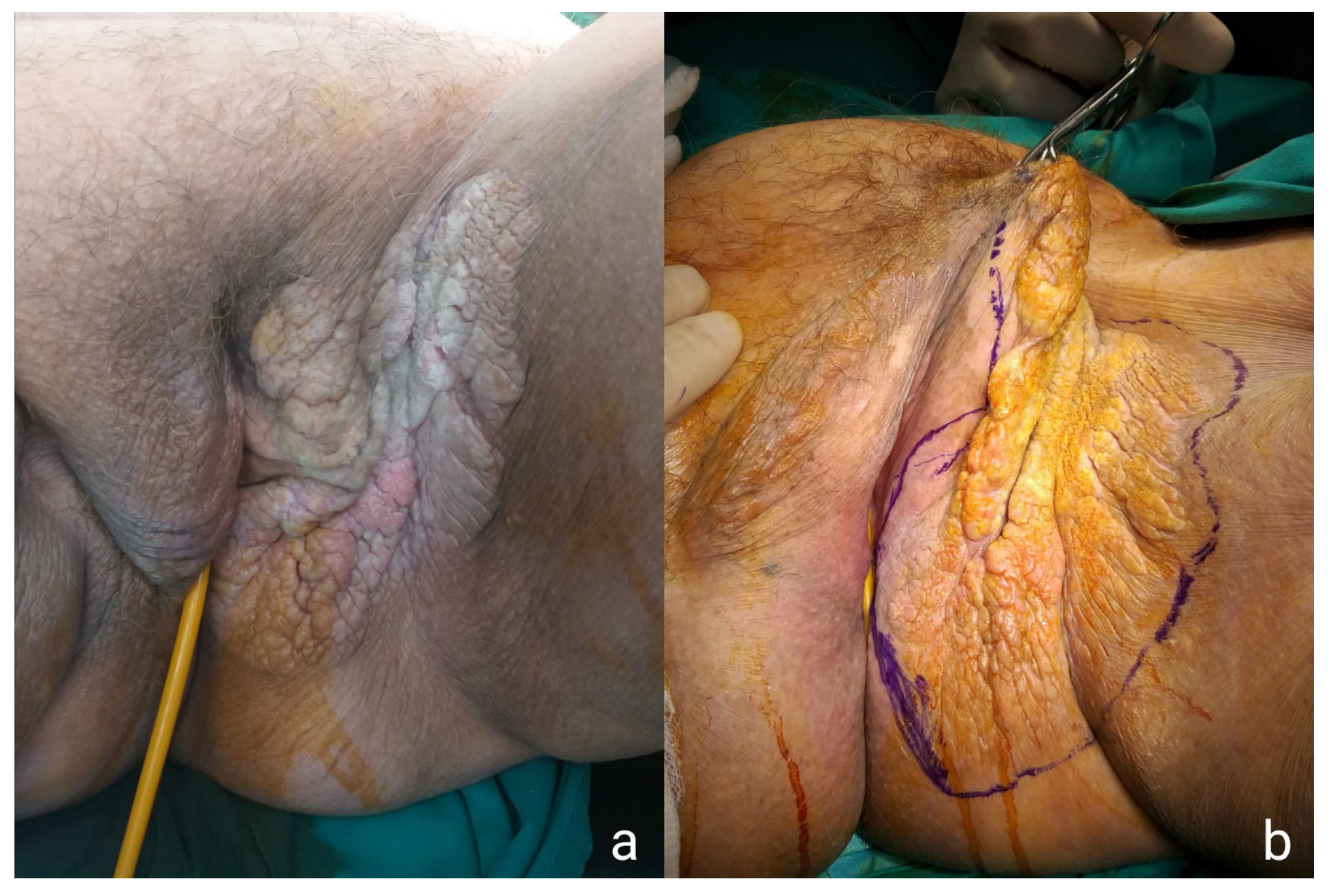
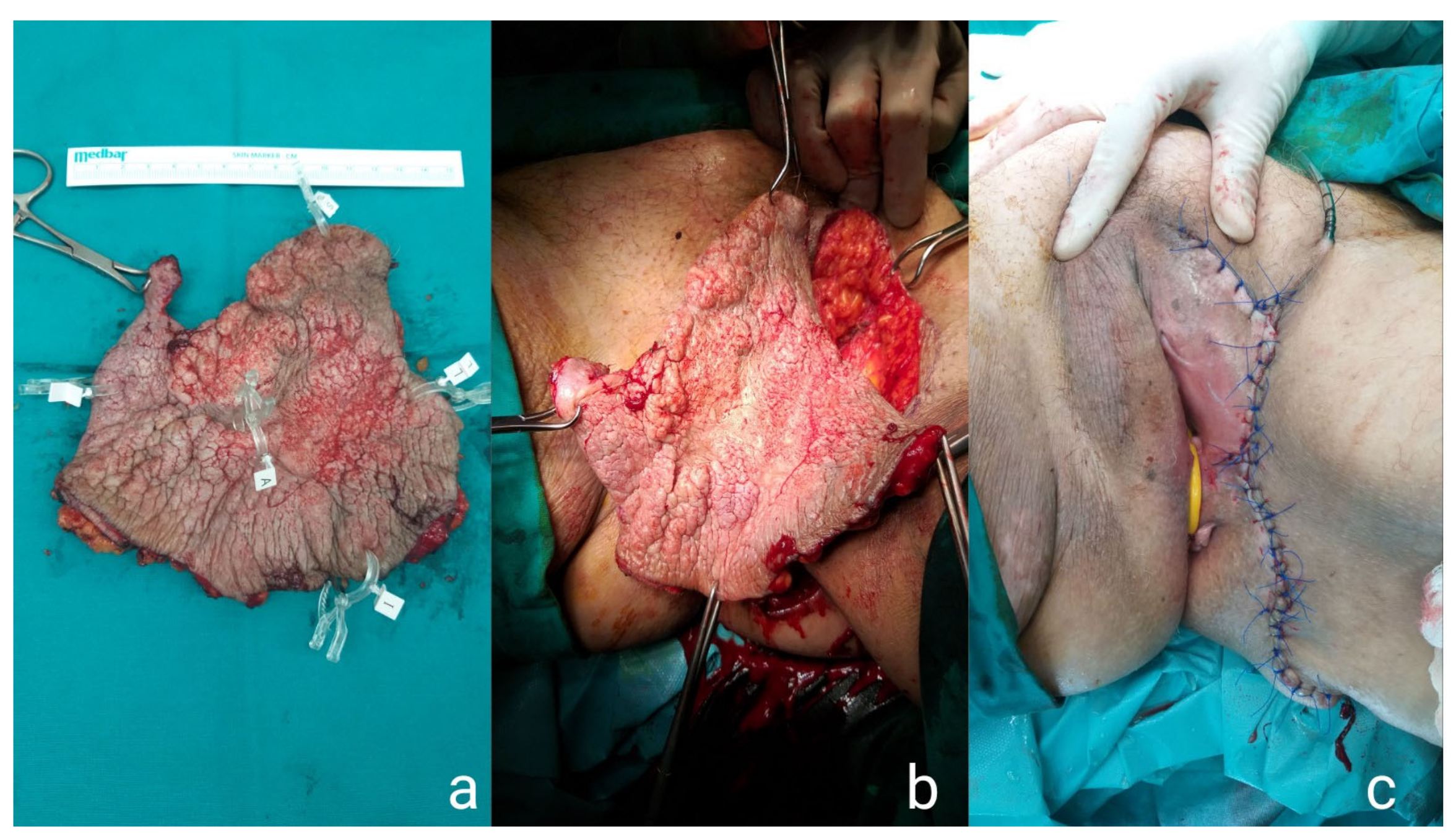
2. Case Presentations
2.1. Case Presentation 1
2.2. Case Presentation 2
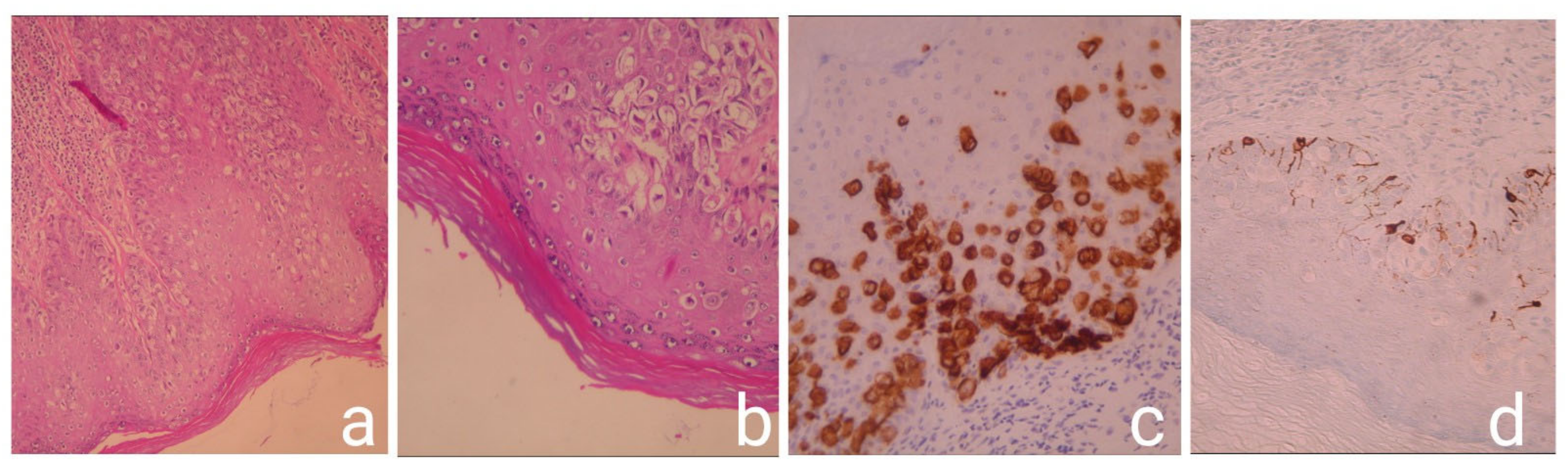
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Paget, J. On disease of the mammary areola preceding cancer of the mammary gland. St Bartholomew’s Hosp. Rep. 1874, 10, 87–89. [Google Scholar] [CrossRef]
- Jimenez, R.E.; Hieken, T.J.; Peters, M.S.; Visscher, D.W. Paget disease of the breast. In The Breast: Comprehensive Management of Benign and Malignant Diseases; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 169–176. [Google Scholar] [CrossRef]
- Jacobaeus, H. Paget’s disease und sein verhültniss zum milchdrüsenkarzinom. Virchows Arch. 1904, 178, 124–142. [Google Scholar] [CrossRef]
- Marcoval, J.; Penín, R.M.; Vidal, A.; Bermejo, J. Extramammary Paget Disease. Enfermedad de Paget extramamaria. Actas Dermo-Sifiliográficas 2020, 111, 306–312. [Google Scholar] [CrossRef]
- Shabihkhani, M.; Patil, P.; Amador, B.M.; Plaza, J.A.; Osunkoya, A.O.; Lombardo, K.A.; Epstein, J.I.; Matoso, A. Extramammary Paget Disease of the Scrotum: A Contemporary Clinicopathologic Analysis of 20 Cases in the United States. Appl. Immunohistochem. Mol. Morphol. 2019, 28, 524–531. [Google Scholar] [CrossRef]
- Phyo, A.K.; Mun, K.-S.; Kwan, K.C.; Ann, C.C.; Kuppusamy, S. Genitourinary extramammary Paget’s disease: Review and outcome in a multidisciplinary setting. Int. J. Clin. Exp. Pathol. 2020, 13, 2369–2376. [Google Scholar] [PubMed]
- Adams, S.J.; Kanthan, R. Paget’s disease of the male breast in the 21st century: A systematic review. Breast 2016, 29, 14–23. [Google Scholar] [CrossRef]
- Kanitakis, J. Mammary and extramammary Paget’s disease. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 581–590. [Google Scholar] [CrossRef]
- Nasioudis, D.; Bhadra, M.; Ko, E. Extramammary Paget disease of the vulva: Management and prognosis. Gynecol. Oncol. 2019, 157, 146–150. [Google Scholar] [CrossRef]
- Filho, L.L.L.; Lopes, I.M.R.S.; Lopes, L.R.S.; Enokihara, M.M.S.E.S.; Michalany, A.O.; Matsunaga, N. Mammary and extramammary Paget’s disease. An. Bras. Dermatol. 2015, 90, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Flores, A.; Eraña, I.; Cuevas, J. “Extramammary-Type” Paget Disease of the Breast. Am. J. Dermatopathol. 2018, 40, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.R.; Long, B.J.; Weaver, A.L.; McGree, M.E.; Bakkum-Gamez, J.N.; Brewer, J.D.; Cliby, W.A. Evidence-Based Screening Recommendations for Occult Cancers in the Setting of Newly Diagnosed Extramammary Paget Disease. Mayo Clin. Proc. 2018, 93, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Shaco-Levy, R.; Bean, S.M.; Vollmer, R.T.; Jewell, E.; Jones, E.L.; Valdes, C.L.; Bentley, R.C.; Selim, M.A.; Robboy, S.J. Paget disease of the vulva: A study of 56 cases. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 149, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Hatta, N.; Yamada, M.; Hirano, T.; Fujimoto, A.; Morita, R. Extramammary Paget’s disease: Treatment, prognostic factors and outcome in 76 patients. Br. J. Dermatol. 2007, 158, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.R.; Hurst, E.A. Extramammary Paget Disease: A Review of the Literature—Part I: History, Epidemiology, Pathogenesis, Presentation, Histopathology, and Diagnostic Work-up. Dermatol. Surg. 2020, 46, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Snast, I.; Sharon, E.; Kaftory, R.; Noyman, Y.; Oren-Shabtai, M.; Lapidoth, M.; Hodak, E.; Mimouni, D.; Mazor, S.; Levi, A. Nonsurgical Treatments for Extramammary Paget Disease: A Systematic Review and Meta-Analysis. Dermatology 2020, 236, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Asel, M.; LeBoeuf, N.R. Extramammary Paget’s Disease. Hematol. Oncol. Clin. North Am. 2018, 33, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Hata, H.; Abe, R.; Hoshina, D.; Saito, N.; Homma, E.; Aoyagi, S.; Shimizu, H. MUC5AC expression correlates with invasiveness and progression of extramammary Paget’s disease. J. Eur. Acad. Dermatol. Venereol. 2013, 28, 727–732. [Google Scholar] [CrossRef]
- Simonds, R.M.; Segal, R.J.; Sharma, A. Extramammary Paget’s disease: A review of the literature. Int. J. Dermatol. 2018, 58, 871–879. [Google Scholar] [CrossRef]
- Lam, C.; Funaro, D. Extramammary Paget’s Disease: Summary of Current Knowledge. Dermatol. Clin. 2010, 28, 807–826. [Google Scholar] [CrossRef]
- Ito, T.; Kaku-Ito, Y.; Furue, M. The diagnosis and management of extramammary Paget’s disease. Expert Rev. Anticancer. Ther. 2018, 18, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Terrier, J.-E.; Tiffet, O.; Raynaud, N.; Cinotti, E. In Vivo Reflectance Confocal Microscopy Combined With the “Spaghetti” Technique: A New Procedure for Defining Surgical Margins of Genital Paget Disease. Dermatol. Surg. 2015, 41, 862–864. [Google Scholar] [CrossRef] [PubMed]
- Gentileschi, S.; Servillo, M.; Garganese, G.; Fragomeni, S.M.; De Bonis, F.; Scambia, G.; Salgarello, M. Surgical therapy of vulvar cancer: How to choose the correct reconstruction? J. Gynecol. Oncol. 2016, 27, e60. [Google Scholar] [CrossRef] [Green Version]
- Watring, W.G.; Roberts, J.A.; Lagasse, L.D.; Berman, M.L.; Ballon, S.C.; Moore, J.G.; Schlesinger, R.E. Treatment of recurrent Paget’s disease of the vulva with topical bleomycin. Cancer 1978, 41, 10–11. [Google Scholar] [CrossRef]
- Mazzilli, S.; Dattola, A.; Criscuolo, A.A.; Cosio, T.; Bianchi, L.; Campione, E.; Di Prete, M.; Botti, E. Usefulness of Topical Imiquimod 3.75% in Cytokeratin 7 Positive Extramammary Paget Disease of the Vulva: Towards Personalized Therapy. Dermatol. Pract. Concept. 2021, 11, e2021011. [Google Scholar] [CrossRef] [PubMed]
- Karam, A.; Berek, J.S.; Stenson, A.; Rao, J.; Dorigo, O. HER-2/neu targeting for recurrent vulvar Paget’s disease: A case report and literature review. Gynecol. Oncol. 2008, 111, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti, M.; Mazzeo, R.; De Scordilli, M.; Del Fabro, A.; Vitale, M.G.; Bortot, L.; Nicoloso, M.S.; Corsetti, S.; Bonotto, M.; Scalone, S.; et al. Human epidermal growth factor receptor-2 (HER2) is a potential therapeutic target in extramammary Paget’s disease of the vulva. Int. J. Gynecol. Cancer 2020, 30, 1672–1677. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosmidis, C.S.; Sevva, C.; Roulia, P.; Koulouris, C.; Varsamis, N.; Koimtzis, G.; Theodorou, V.; Mystakidou, C.M.; Georgakoudi, E.; Anthimidis, G. Extramammary Paget’s Disease of the Vulva: Report of Two Cases. Medicina 2021, 57, 1029. https://doi.org/10.3390/medicina57101029
Kosmidis CS, Sevva C, Roulia P, Koulouris C, Varsamis N, Koimtzis G, Theodorou V, Mystakidou CM, Georgakoudi E, Anthimidis G. Extramammary Paget’s Disease of the Vulva: Report of Two Cases. Medicina. 2021; 57(10):1029. https://doi.org/10.3390/medicina57101029
Chicago/Turabian StyleKosmidis, Christoforos S., Christina Sevva, Panagiota Roulia, Charilaos Koulouris, Nikolaos Varsamis, Georgios Koimtzis, Vasiliki Theodorou, Chrysi Maria Mystakidou, Eleni Georgakoudi, and Georgios Anthimidis. 2021. "Extramammary Paget’s Disease of the Vulva: Report of Two Cases" Medicina 57, no. 10: 1029. https://doi.org/10.3390/medicina57101029
APA StyleKosmidis, C. S., Sevva, C., Roulia, P., Koulouris, C., Varsamis, N., Koimtzis, G., Theodorou, V., Mystakidou, C. M., Georgakoudi, E., & Anthimidis, G. (2021). Extramammary Paget’s Disease of the Vulva: Report of Two Cases. Medicina, 57(10), 1029. https://doi.org/10.3390/medicina57101029





