Effect of Genistein Supplementation on Exercise-Induced Inflammation and Oxidative Stress in Mice Liver and Skeletal Muscle
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Protocol
2.3. Sampling and Measurements
2.3.1. Sample Processing
2.3.2. Measurement of Plasma Biochemical Parameters
2.3.3. Measurement of Oxidative Stress Markers
2.3.4. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.3.5. Data Analysis
3. Results
3.1. Effect of Single-Dose GE Administration on Endurance Running Capacity
3.2. Effect of Exhaustive Exercise and GE Administration on Blood Biomarkers
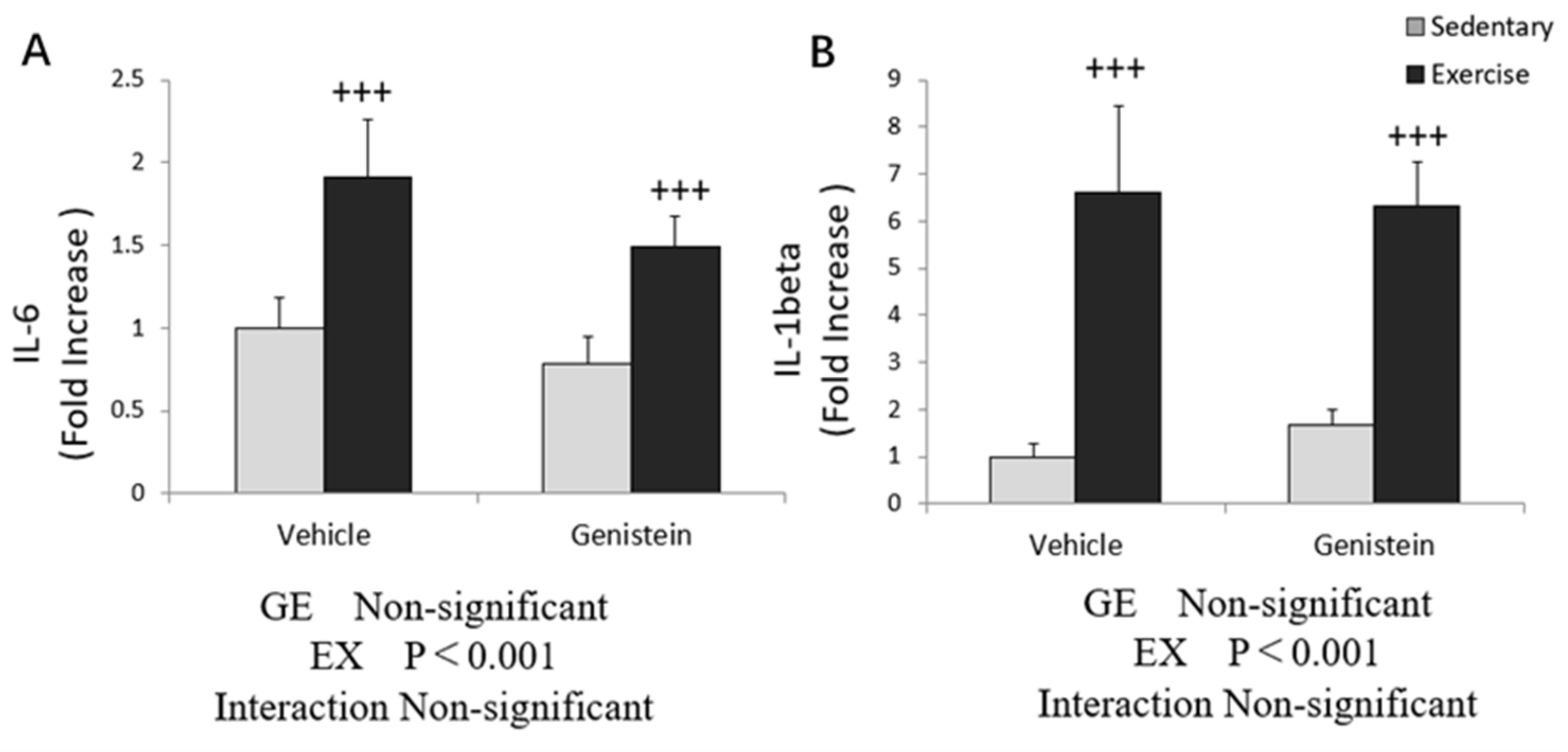
3.3. Effects of Exhaustive Exercise and GE Administration on Inflammation-Related Gene Expression Levels of Skeletal Muscle
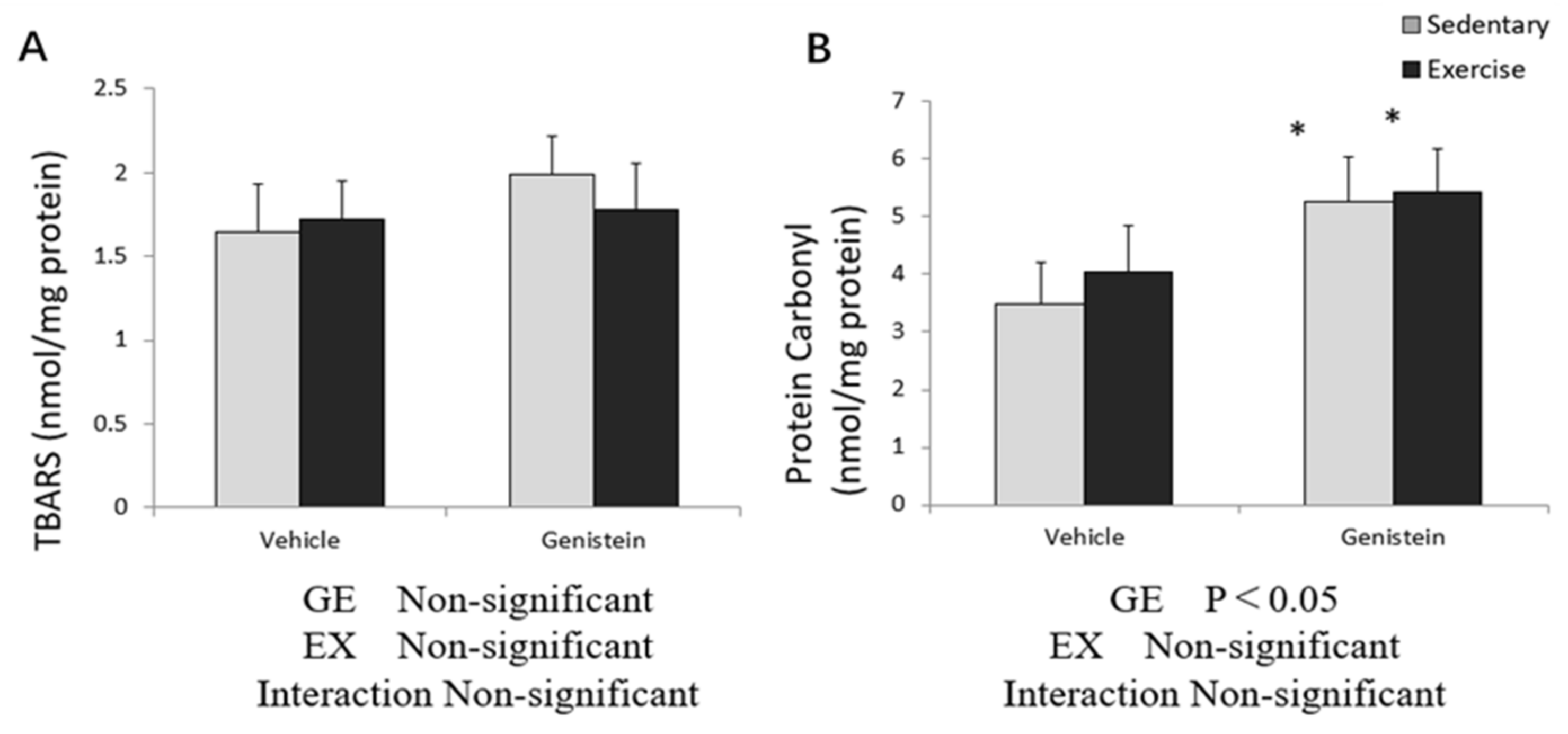
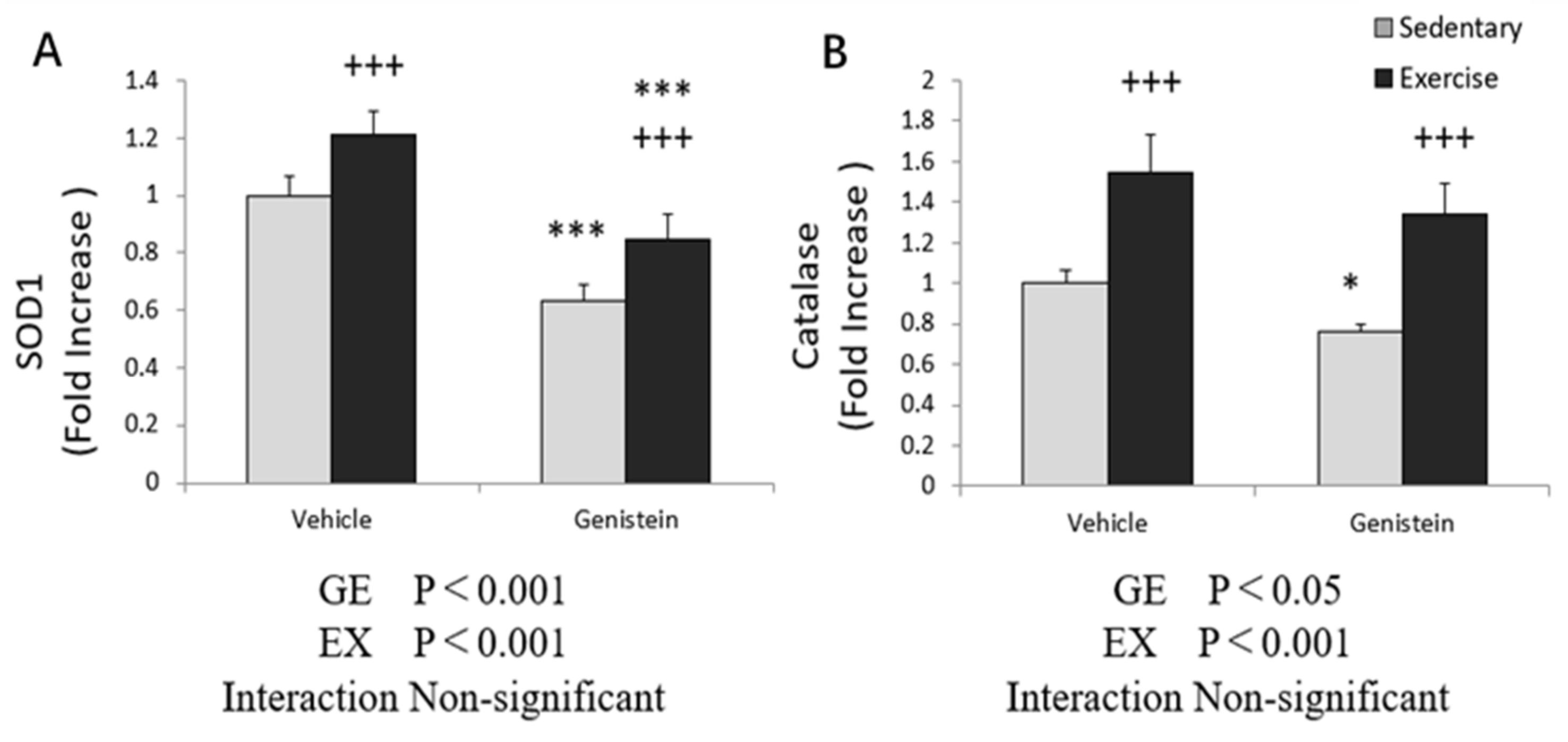
3.4. Markers of Oxidative Stress in Skeletal Muscles
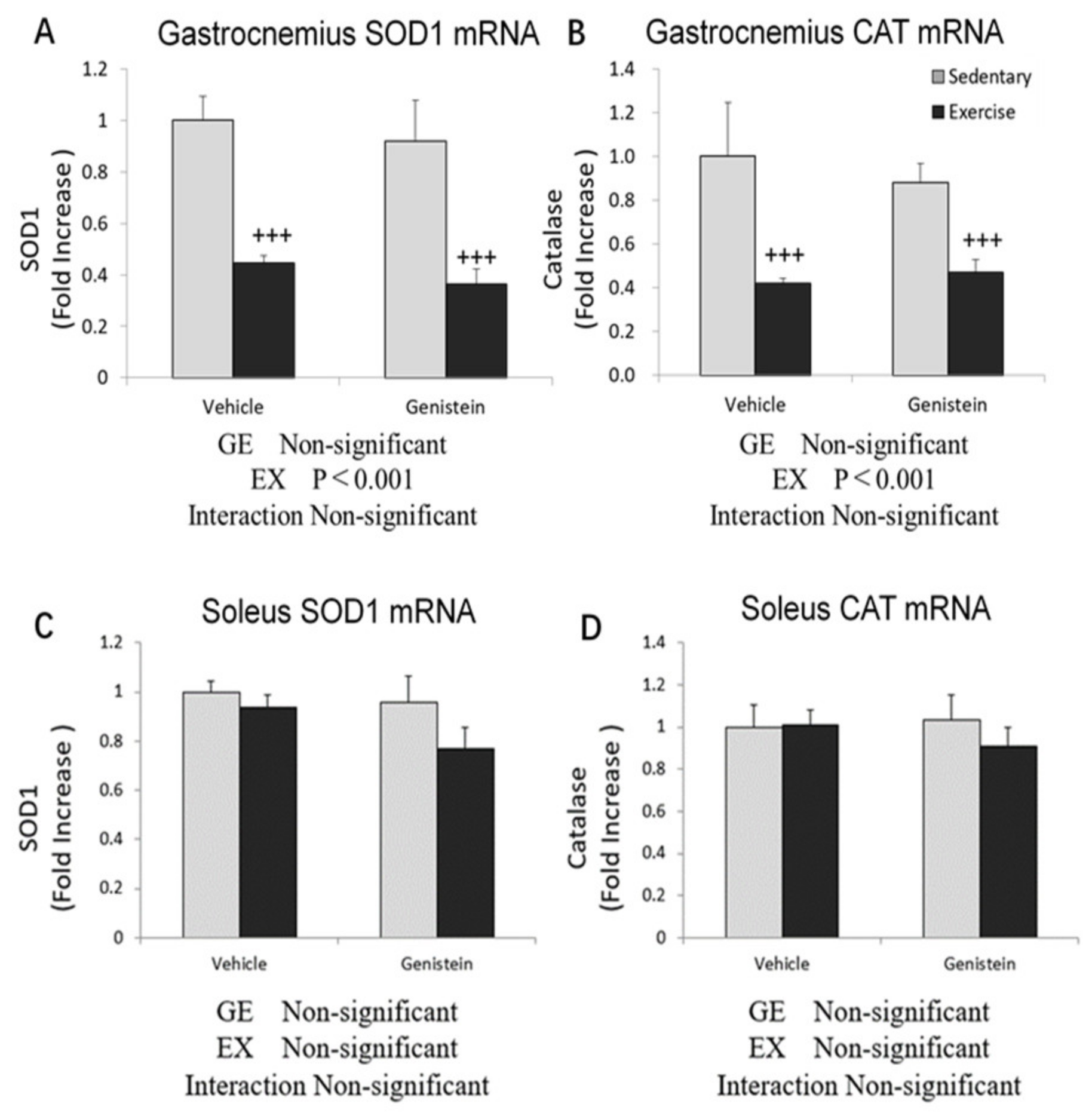
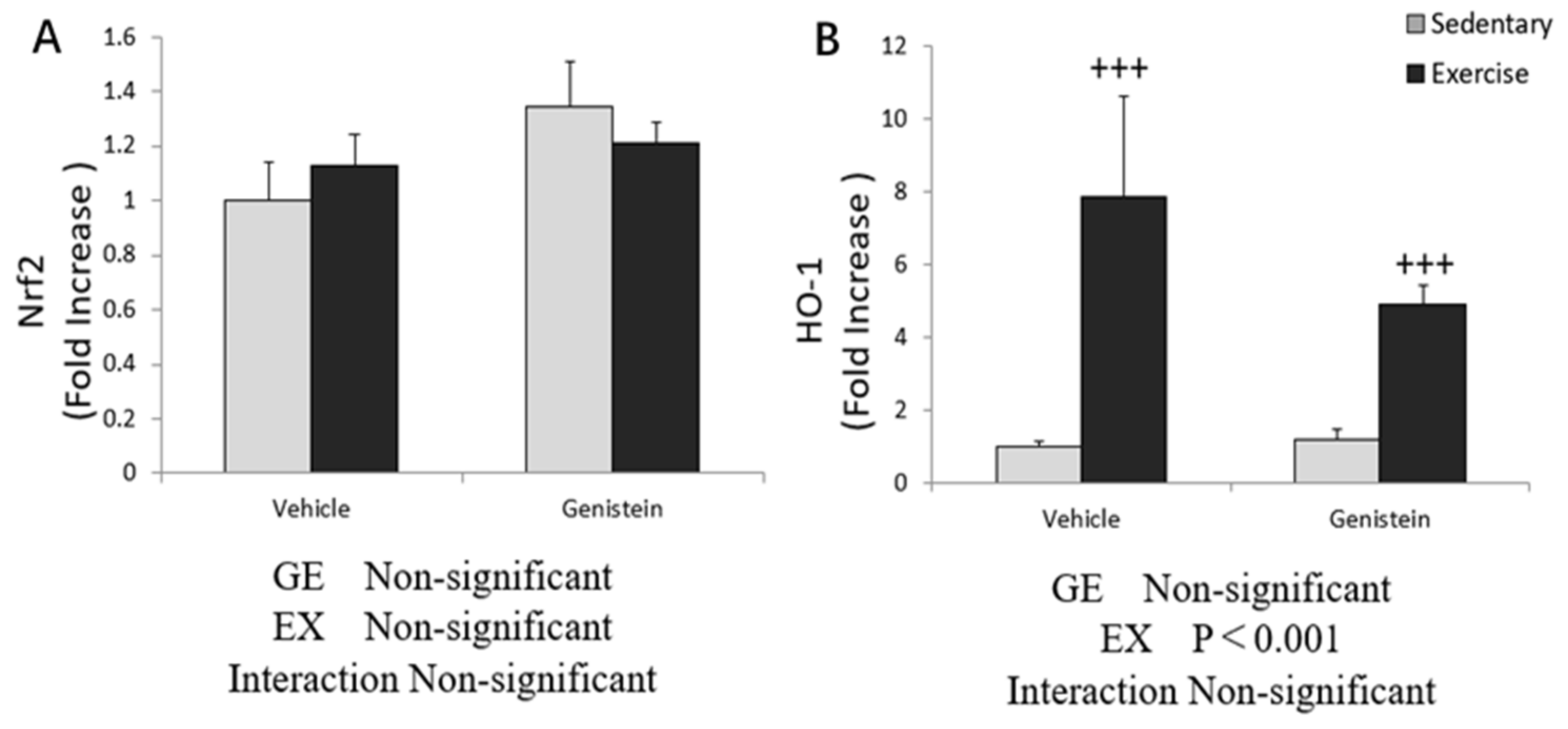
3.5. Effects of Exhaustive Exercise and GE Administration on Antioxidant Capacity-Related Gene Expression Levels of Skeletal Muscle
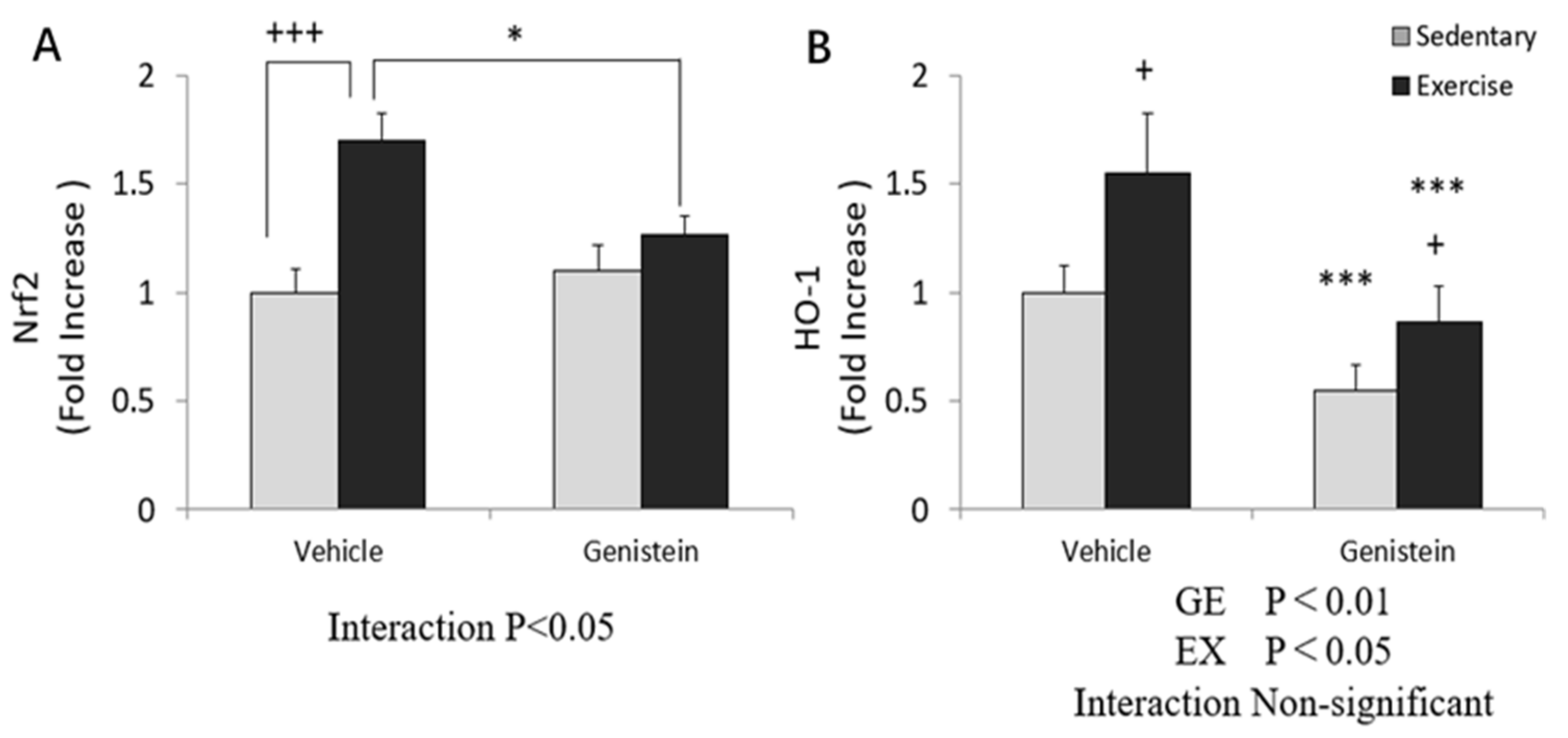
3.6. Effect of Exhaustive Exercise and GE Administration on Nrf2 and HO-1 Expression Level of Skeletal Muscle
3.7. Effects of Exhaustive Exercise and GE Administration on Inflammation-Related Gene Expression Levels of Liver
3.8. Markers of Oxidative Stress in Liver
3.9. Effects of Exhaustive Exercise and GE Administration on Antioxidant Capacity-Related Gene Expression Levels of Liver
3.10. Effects of Exhaustive Exercise and GE Administration on Nrf2 and HO-1 Expression Levels of Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Tominaga, T.; Ruhee, R.T.; Ma, S. Characterization and modulation of systemic inflammatory response to exhaustive exercise in relation to oxidative stress. Antioxidants 2020, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Penedo, F.J.; Dahn, J.R. Exercise and well-being: A review of mental and physical health benefits associated with physical activity. Curr. Opin. Psychiatry 2005, 18, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Chronic Inflammation as an Immunological Abnormality and Effectiveness of Exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Totsuka, M.; Nakaji, S.; Yamada, M.; Kudoh, S.; Liu, Q.; Sugawara, K.; Yamaya, K.; Sato, K. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J. Appl. Physiol. 1999, 87, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Chung, H.Y.; Goto, S. Exercise and hormesis: Oxidative stress-related adaptation for successful aging. Biogerontology 2005, 6, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Malaguti, M.; Angeloni, C.; Hrelia, S. Polyphenols in exercise performance and prevention of exercise-induced muscle damage. Oxid. Med. Cell Longev. 2013, 2013, 825928. [Google Scholar] [CrossRef]
- Tidball, J.G. Inflammatory cell response to acute muscle injury. Med. Sci. Sports Exerc. 1995, 27, 1022–1032. [Google Scholar] [CrossRef]
- Kawanishi, N.; Mizokami, T.; Niihara, H.; Yada, K.; Suzuki, K. Neutrophil Depletion Attenuates Muscle Injury after Exhaustive Exercise. Med. Sci. Sports Exerc. 2016, 48, 1917–1924. [Google Scholar] [CrossRef]
- Incir, S.; Bolayirli, I.M.; Inan, O.; Aydın, M.S.; Bilgin, I.A.; Sayan, I.; Esrefoglu, M.; Seven, A. The effects of genistein supplementation on fructose induced insulin resistance, oxidative stress and inflammation. Life Sci. 2016, 158, 57–62. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Sulforaphane Protects Cells against Lipopolysaccharide-Stimulated Inflammation in Murine Macrophages. Antioxidants 2019, 8, 577. [Google Scholar] [CrossRef]
- Ma, S.; Yada, K.; Lee, H.; Fukuda, Y.; Iida, A.; Suzuki, K. Taheebo polyphenols attenuate free fatty acid-induced inflammation in murine and human macrophage cell lines as inhibitor of cyclooxygenase-2. Front. Nutr. 2017, 4, 63. [Google Scholar] [CrossRef]
- Kwon, S.H.; Kang, M.J.; Huh, J.S.; Ha, K.W.; Lee, J.R.; Lee, S.K.; Lee, B.S.; Han, I.H.; Lee, M.S.; Lee, M.W.; et al. Comparison of oral bioavailability of genistein and genistin in rats. Int. J. Pharm. 2007, 337, 148–154. [Google Scholar] [CrossRef]
- Uruno, A.; Motohashi, H. The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide 2011, 25, 153–160. [Google Scholar] [CrossRef]
- Lignitto, L.; LeBoeuf, S.E.; Homer, H.; Jiang, S.; Askenazi, M.; Karakousi, T.R.; Pass, H.I.; Bhutkar, A.J.; Tsirigos, A.; Ueberheide, B.; et al. Nrf2 Activation Promotes Lung Cancer Metastasis by Inhibiting the Degradation of Bach1. Cell 2019, 178, 316–329. [Google Scholar] [CrossRef]
- Done, A.J.; Traustadóttir, T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016, 10, 191–199. [Google Scholar] [CrossRef]
- Yang, L.; Shen, L.; Li, Y.; Li, Y.; Yu, S.; Wang, S. Hyperoside attenuates dextran sulfate sodium-induced colitis in mice possibly via activation of the Nrf2 signalling pathway. J Inflamm. 2017, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Supko, J.G.; Malspeis, L. Plasma pharmacokinetics of genistein in mice. Int. J. Oncol. 1995, 7, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Gomar, F.; Pareja-Galeano, H.; Perez-Quilis, C.; Santos-Lozano, A.; Fiuza-Luces, C.; Garatachea, N.; Lippi, G.; Lucia, A. Effects of allopurinol on exercise-induced muscle damage: New therapeutic approaches? Cell Stress Chaperones 2015, 20, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S.; Dhawan, M.; Singh Sandhu, J. Four Weeks of Supplementation with Isolated Soy Protein Attenuates Exercise-Induced Muscle Damage and Enhances Muscle Recovery in Well Trained Athletes: A Randomized Trial. Asian J. Sports Med. 2016, 7, e33528. [Google Scholar] [CrossRef] [PubMed]
- Rahman Mazumder, M.A.; Hongsprabhas, P. Genistein as antioxidant and antibrowning agents in in vivo and in vitro: A review. Biomed. Pharmacother. 2016, 82, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Bakhiet, R.M.; Hart, V.; Holtzman, G. Isoflavones improve plasma homocysteine status and antioxidant defense system in healthy young men at rest but do not ameliorate oxidative stress induced by 80% VO2pk exercise. Ann. Nutr. Metab. 2005, 49, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Ibrahim, S.; El-Amir, A.; El-Rafei, A.M.; Allam, N.K.; Abdellatif, A. Genistein Loaded Nanofibers Protect Spinal Cord Tissue Following Experimental Injury in Rats. Biomedicines 2018, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Z.; Pang, X.; Yang, J.; Yu, H.; Zhang, Y.; Zhou, H.; Zhao, J. MiR-34a/sirtuin-1/foxo3a is involved in genistein protecting against ox-LDL-induced oxidative damage in HUVECs. Toxicol. Lett. 2017, 277, 115–122. [Google Scholar] [CrossRef]
- Chen, W.; Lin, Y.C.; Ma, X.Y.; Jiang, Z.Y.; Lan, S.P. High concentrations of genistein exhibit pro-oxidant effects in primary muscle cells through mechanisms involving 5-lipoxygenase-mediated production of reactive oxygen species. Food Chem. Toxicol. 2014, 67, 72–79. [Google Scholar] [CrossRef]
- Chen, W.; Ma, X.; Lin, Y.; Xiong, Y.; Zheng, C.; Hu, Y.; Yu, D.; Jiang, Z. Dietary supplementation with a high dose of daidzein enhances the antioxidant capacity in swine muscle but experts pro-oxidant function in liver and fat tissues. J. Anim. Sci. Biotechnol. 2016, 7, 43. [Google Scholar] [CrossRef]
- Kruk, I.; Aboul-Enein, H.Y.; Michalska, T.; Lichszteld, K.; Kładna, A. Scavenging of reactive oxygen species by the plant phenols genistein and oleuropein. Luminescence 2005, 20, 81–89. [Google Scholar] [CrossRef]
- Tian, H.; Zhou, G.; Zhu, Z. Evaluation of Cardioprotective Effects of Genistein against Diabetes-induced Cardiac Dysfunction in Rats. Trop. J. Pharm. Res. 2015, 14, 2015–2022. [Google Scholar] [CrossRef][Green Version]
- Singh, P.; Sharma, S.; Rath, S.K. Genistein induces deleterious effects during its acute exposure in Swiss mice. BioMed Res. Int. 2014, 2014, 619617. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Protective Effects of Sulforaphane on Exercise-Induced Organ Damage via Inducing Antioxidant Defense Responses. Antioxidants 2020, 9, 136. [Google Scholar] [CrossRef]
- Miao, Z.Y.; Xia, X.; Che, L.; Song, Y.T. Genistein attenuates brain damage induced by transient cerebral ischemia through up-regulation of Nrf2 expression in ovariectomized rats. Neurol. Res. 2018, 40, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.C.; Wang, C.C.; Lu, J.W.; Lee, C.H.; Chen, S.C.; Ho, Y.J.; Peng, Y.J. Chondroprotective Effects of Genistein against Osteoarthritis Induced Joint Inflammation. Nutrients 2019, 11, 1180. [Google Scholar] [CrossRef]
- Suraweera, T.L.; Rupasinghe, H.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE Pathway by Dietary Flavonoids: A Friend or Foe for Cancer Management? Antioxidants 2020, 9, 973. [Google Scholar] [CrossRef]
- Paredes-Gonzalez, X.; Fuentes, F.; Jeffery, S.; Saw, C.L.; Shu, L.; Su, Z.Y.; Kong, A.N. Induction of NRF2-mediated gene expression by dietary phytochemical flavones apigenin and luteolin. Biopharm. Drug. Dispos. 2015, 36, 440–451. [Google Scholar] [CrossRef]
- Valdameri, G.; Trombetta-Lima, M.; Worfel, P.R.; Pires, A.R.; Martinez, G.R.; Noleto, G.R.; Cadena, S.M.; Sogayar, M.C.; Winnischofer, S.M.; Rocha, M.E. Involvement of catalase in the apoptotic mechanism induced by apigenin in HepG2 human hepatoma cells. Chem. Biol. Interact. 2011, 193, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, X.; Yang, J.; Sun, X.; Hu, C.; Yan, Z.; Xu, X.; Lu, W.; Wang, X.; Cao, P. Chemoprevention of dietary digitoflavone on colitis-associated colon tumorigenesis through inducing Nrf2 signaling pathway and inhibition of inflammation. Mol. Cancer 2014, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Drummond, G.S.; Baum, J.; Greenberg, M.; Lewis, D.; Abraham, N.G. HO-1 overexpression and underexpression: Clinical implications. Arch. Biochem. 2019, 673, 108073. [Google Scholar] [CrossRef]




| Target Gene | Forward | Reverse |
|---|---|---|
| 18S | CGGCTACCACATCCAAGGA | AGCTGGAATTACCGCGGC |
| IL-6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| IL-1β | GGGCCTCAAAGGAAAGAATC | TTGCTTGGGATCCACACTCT |
| SOD1 | GAGACCTGGGCAATGTGACT | GTTTACTGCGCAATCCCAAT |
| CAT | CAGAGAGCGGATTCCTGAGAGA | CTTTGCCTTGGAGTATCTGGTGAT |
| Nrf2 | GAGTCGCTTGCCCTGGATATC | TCATGGCTGCCTCCAGAGAA |
| HO-1 | CACGCATATACCCGCTACCT | CCAGAGTGTTCATTCGAGCA |
| Biomarker | Con | GE | Ex | GE + Ex |
|---|---|---|---|---|
| Glucose (mg/dL) | 234.4 ± 11.7 | 217.9 ± 12.3 | 172.1 ± 23.8 +++ | 139.5 ± 17.6 +++ |
| NEFA (μmol/L) | 2.2 ± 0.3 | 2.1 ± 0.3 | 3.0 ± 0.1 +++ | 3.2 ± 0.1 +++ |
| BUN (mg/dL) | 22.7 ± 1.5 | 23.9 ± 1.5 | 35.6 ± 1.7 +++ | 38.7 ± 2.5 +++ |
| CK (IU/L) | 950.6 ± 259.2 | 806.3 ± 258.0 | 5358.8 ± 830.4 +++ | 6585.4 ± 1331.1 +++ |
| LDH (IU/L) | 438.4 ± 56.3 | 406.9 ± 59.6 | 1690.5 ± 165.2 +++ | 1716.0 ± 253.3 +++ |
| ALT (IU/L) | 32.6 ± 2.0 | 33.4 ± 3.4 | 122.6 ± 19.3 +++ | 119.6 ± 20.1 +++ |
| AST (IU/L) | 120.8 ± 14.3 | 152.6 ± 28.2 | 500.6 ± 56.3 +++ | 485.6 ± 66.7 +++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Zhou, S.; Ma, S.; Suzuki, K. Effect of Genistein Supplementation on Exercise-Induced Inflammation and Oxidative Stress in Mice Liver and Skeletal Muscle. Medicina 2021, 57, 1028. https://doi.org/10.3390/medicina57101028
Wu C, Zhou S, Ma S, Suzuki K. Effect of Genistein Supplementation on Exercise-Induced Inflammation and Oxidative Stress in Mice Liver and Skeletal Muscle. Medicina. 2021; 57(10):1028. https://doi.org/10.3390/medicina57101028
Chicago/Turabian StyleWu, Cong, Siyi Zhou, Sihui Ma, and Katsuhiko Suzuki. 2021. "Effect of Genistein Supplementation on Exercise-Induced Inflammation and Oxidative Stress in Mice Liver and Skeletal Muscle" Medicina 57, no. 10: 1028. https://doi.org/10.3390/medicina57101028
APA StyleWu, C., Zhou, S., Ma, S., & Suzuki, K. (2021). Effect of Genistein Supplementation on Exercise-Induced Inflammation and Oxidative Stress in Mice Liver and Skeletal Muscle. Medicina, 57(10), 1028. https://doi.org/10.3390/medicina57101028








