Extracorporeal Membrane Oxygenation for Secondary Organizing Pneumonia after Severe SARS-CoV-2 Infection: A Case Report
Abstract
:1. Background
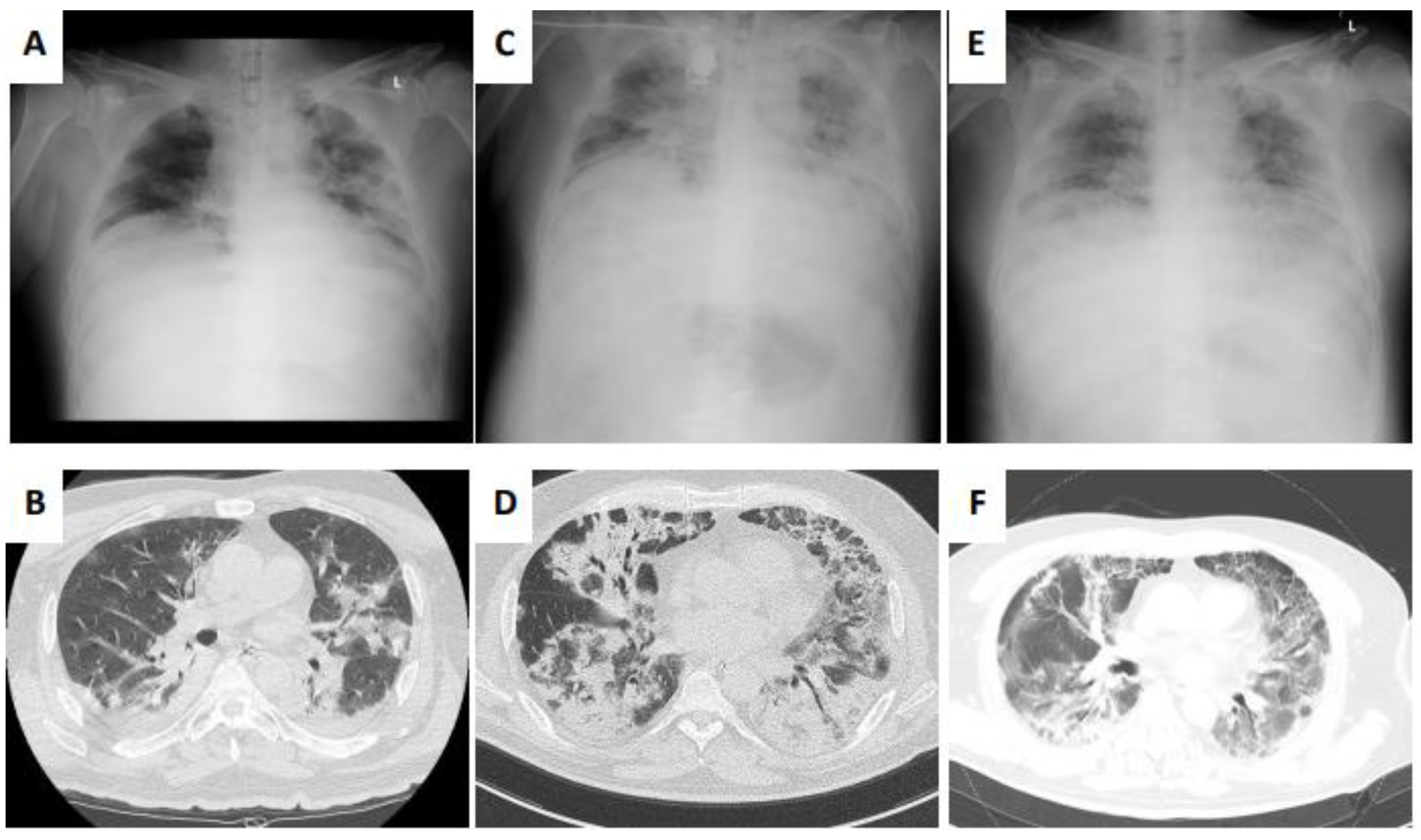
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Barbaro, R.P.; MacLaren, G.; Boonstra, P.S.; Iwashyna, T.J.; Slutsky, A.S.; Fan, E.; Bartlett, R.H.; Tonna, J.E.; Hyslop, R.; Fanning, J.J.; et al. Extracorporeal Life Support Organization. Extracorporeal membrane oxygenation support in COVID-19: An international cohort study of the Extracorporeal Life Support Organization registry. Lancet 2020, 396, 1071–1078. [Google Scholar] [CrossRef]
- Cordier, J.F. Cryptogenic organising pneumonia. Eur. Respir. J. 2006, 28, 422–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hani, C.; Trieu, N.H.; Saab, I.; Dangeard, S.; Bennani, S.; Chassagnon, G.; Revel, M.P. COVID-19 pneumonia: A review of typical CT findings and differential diagnosis. Diagn. Interv. Imaging 2020, 101, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Lou, J.; Xi, D.; Bai, Y.; Ma, G.; Zhao, B.; Liu, D.; Bao, G.; Lei, Z.; Wang, M. Chest computed tomography findings of coronavirus disease 2019 (COVID-19) pneumonia. Eur. Radiol. 2020, 30, 5489–5498. [Google Scholar] [CrossRef] [PubMed]
- Copin, M.C.; Parmentier, E.; Duburcq, T.; Poissy, J.; Mathieu, D. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020, 46, 1124–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Han, X.; Jiang, N.; Cao, Y.; Alwalid, O.; Gu, J.; Fan, Y.; Zheng, C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect. Dis. 2020, 20, 425–434. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [PubMed]
- Munshi, L.; Walkey, A.; Goligher, E.; Pham, T.; Uleryk, E.M.; Fan, E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: A systematic review and meta-analysis. Lancet Respir. Med. 2019, 7, 163–172. [Google Scholar] [CrossRef]
- Shekar, K.; Badulak, J.; Peek, G.; Boeken, U.; Dalton, H.J.; Arora, L.; Zakhary, B.; Ramanathan, K.; Starr, J.; Akkanti, B.; et al. Extracorporeal Life Support Organization Coronavirus Disease 2019 Interim Guidelines: A Consensus Document from an International Group of Interdisciplinary Extracorporeal Membrane Oxygenation Providers. ASAIO J. 2020, 66, 707–721. [Google Scholar] [CrossRef]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef]
- Polak, S.B.; Van Gool, I.C.; Cohen, D.; von der Thüsen, J.H.; van Paassen, J. A systematic review of pathological findings in COVID-19: A pathophysiological timeline and possible mechanisms of disease progression. Mod. Pathol. 2020, 33, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, M.B.; DeSouza, S.A.; Moreira, A.L.; Stover, D.E.; Heelan, R.T.; Iyriboz, T.A.; Taur, Y.; Travis, W.D. A comparison of the pathological, clinical and radiographical, features of cryptogenic organising pneumonia, acute fibrinous and organising pneumonia and granulomatous organising pneumonia. J. Clin. Pathol. 2015, 68, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Kory, P.; Kanne, J.P. SARS-CoV-2 organising pneumonia: ‘Has there been a widespread failure to identify and treat this prevalent condition in COVID-19?’. BMJ Open Respir. Res. 2020, 7, e000724. [Google Scholar] [CrossRef] [PubMed]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [PubMed]
- Angus, D.C.; Derde, L.; Al-Beidh, F.; Annane, D.; Arabi, Y.; Beane, A.; van Bentum-Puijk, W.; Berry, L.; Bhimani, Z.; Bonten, M.; et al. Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA 2020, 324, 1317–1329. [Google Scholar] [PubMed]
- Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.T.; Toeg, H.; De Paulis, R.; Atluri, P. Establishment and management of mechanical circulatory support during the COVID-19 pandemic. Circulation 2020, 142, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Belhadjer, Z.; Meot, M.; Bajolle, F.; Khraiche, D.; Legendre, A.; Abakka, S.; Auriau, J.; Grimaud, M.; Oualha, M.; Beghetti, M.; et al. 25 Syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation 2020, 142, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.P.; Stammers, A.H.; St, L.J.; Hayanga, J.; Firstenberg, M.S.; Mongero, L.B.; Tesdahl, E.A.; Rajagopal, K.; Cheema, F.H.; Coley, T.; et al. Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in coronavirus disease 2019: Experience with 32 patients. ASAIO J. 2020, 66, 722–730. [Google Scholar] [CrossRef]
- Le Breton, C.; Besset, S.; Freita-Ramos, S.; Amouretti, M.; Billiet, P.A.; Dao, M.; Dumont, L.M.; Federici, L.; Gaborieau, B.; Longrois, D.; et al. Extracorporeal membrane oxygenation for refractory COVID-19 acute respiratory distress syndrome. J. Crit. Care 2020, 60, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Ziehr, D.R.; Alladina, J.; Petri, C.R.; Maley, J.H.; Moskowitz, A.; Medoff, B.D.; Hibbert, K.A.; Thompson, B.T.; Hardin, C.C. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: A cohort study. Am. J. Respir. Crit. Care Med. 2020, 201, 1560–1564. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hu, M.; Yu, Y.; Zhang, X.; Fang, M.; Lian, Y.; Peng, Y.; Wu, L.; Wu, Y.; Yi, J.; et al. Extracorporeal membrane oxygenation for SARS-CoV-2 acute respiratory distress syndrome: A retrospective study from Hubei, China. Front. Med. 2020, 7, 611460. [Google Scholar] [CrossRef] [PubMed]
- Shaefi, S.; Brenner, S.K.; Gupta, S.; O’Gara, B.P.; Krajewski, M.L.; Charytan, D.M.; Chaudhry, S.; Mirza, S.H.; Peev, V.; Anderson, M.; et al. Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med. 2021, 47, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Dechert, R.E.; Park, P.K.; Bartlett, R.H. Evaluation of the oxygenation index in adult respiratory failure. J. Trauma Acute Care Surg. 2014, 76, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Ambros, A.; Soler, J.A.; Martinez, D.; Ferrando, C.; Solano, R.; Mosteiro, F.; Blanco, J.; Martin-Rodriguez, C.; Fernandez, M.M.; et al. Age, PaO2/ FIO2, and plateau pressure score: A proposal for a simple outcome score in patients with the acute respiratory distress syndrome. Crit. Care Med. 2016, 44, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Tsushima, K.; Kurita, N.; Fujiwara, A.; Soeda, S.; Yamaguchi, A.; Sugiyama, S.; Togashi, Y.; Kono, Y.; Kasagi, S.; et al. Clinical characteristics classified by the serum KL-6 level in patients with organizing pneumonia. Sarcoidosis Vasc. Diffus. Lung Dis. 2013, 30, 43–51. [Google Scholar]
- Yamagishi, T.; Kodaka, N.; Watanabe, K.; Nakano, C.; Oshio, T.; Niitsuma, K.; Shimada, N.; Matsuse, H. A retrospective clinical research of relapsed organizing pneumonia. Ann. Thorac. Med. 2020, 15, 15–20. [Google Scholar] [PubMed]
- Horii, H.; Kamada, K.; Nakakubo, S.; Yamashita, Y.; Nakamura, J.; Nasuhara, Y.; Konno, S. Rapidly progressive organizing pneumonia associated with COVID-19. Respir. Med. Case Rep. 2020, 31, 101295. [Google Scholar] [CrossRef] [PubMed]
- Fouladseresht, H.; Doroudchi, M.; Rokhtabnak, N.; Abdolrahimzadehfard, H.; Roudgari, A.; Sabetian, G.; Paydar, S. Predictive monitoring and therapeutic immune biomarkers in the management of clinical complications of COVID-19. Cytokine Growth Factor Rev. 2020, 9, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Kerget, B.; Kerget, F.; Koçak, A.O.; Kızıltunç, A.; Araz, Ö.; Uçar, E.Y.; Akgün, M. Are Serum Interleukin 6 and Surfactant Protein D Levels Associated with the Clinical Course of COVID-19? Lung 2020, 198, 777–784. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, T.; Onitsuka, C.; Kawahara, T.; Fukuda, Y.; Homma, T.; Watanabe, T.; Ohsugi, K.; Ichikawa, Y.; Shono, A.; Kotani, T.; et al. Extracorporeal Membrane Oxygenation for Secondary Organizing Pneumonia after Severe SARS-CoV-2 Infection: A Case Report. Medicina 2021, 57, 1013. https://doi.org/10.3390/medicina57101013
Kimura T, Onitsuka C, Kawahara T, Fukuda Y, Homma T, Watanabe T, Ohsugi K, Ichikawa Y, Shono A, Kotani T, et al. Extracorporeal Membrane Oxygenation for Secondary Organizing Pneumonia after Severe SARS-CoV-2 Infection: A Case Report. Medicina. 2021; 57(10):1013. https://doi.org/10.3390/medicina57101013
Chicago/Turabian StyleKimura, Tomoyuki, Chisato Onitsuka, Tomoko Kawahara, Yosuke Fukuda, Tetsuya Homma, Taro Watanabe, Koichi Ohsugi, Yuki Ichikawa, Atsuko Shono, Toru Kotani, and et al. 2021. "Extracorporeal Membrane Oxygenation for Secondary Organizing Pneumonia after Severe SARS-CoV-2 Infection: A Case Report" Medicina 57, no. 10: 1013. https://doi.org/10.3390/medicina57101013
APA StyleKimura, T., Onitsuka, C., Kawahara, T., Fukuda, Y., Homma, T., Watanabe, T., Ohsugi, K., Ichikawa, Y., Shono, A., Kotani, T., & Sagara, H. (2021). Extracorporeal Membrane Oxygenation for Secondary Organizing Pneumonia after Severe SARS-CoV-2 Infection: A Case Report. Medicina, 57(10), 1013. https://doi.org/10.3390/medicina57101013







