Biliopancreatic Endoscopy in Altered Anatomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
3. Results
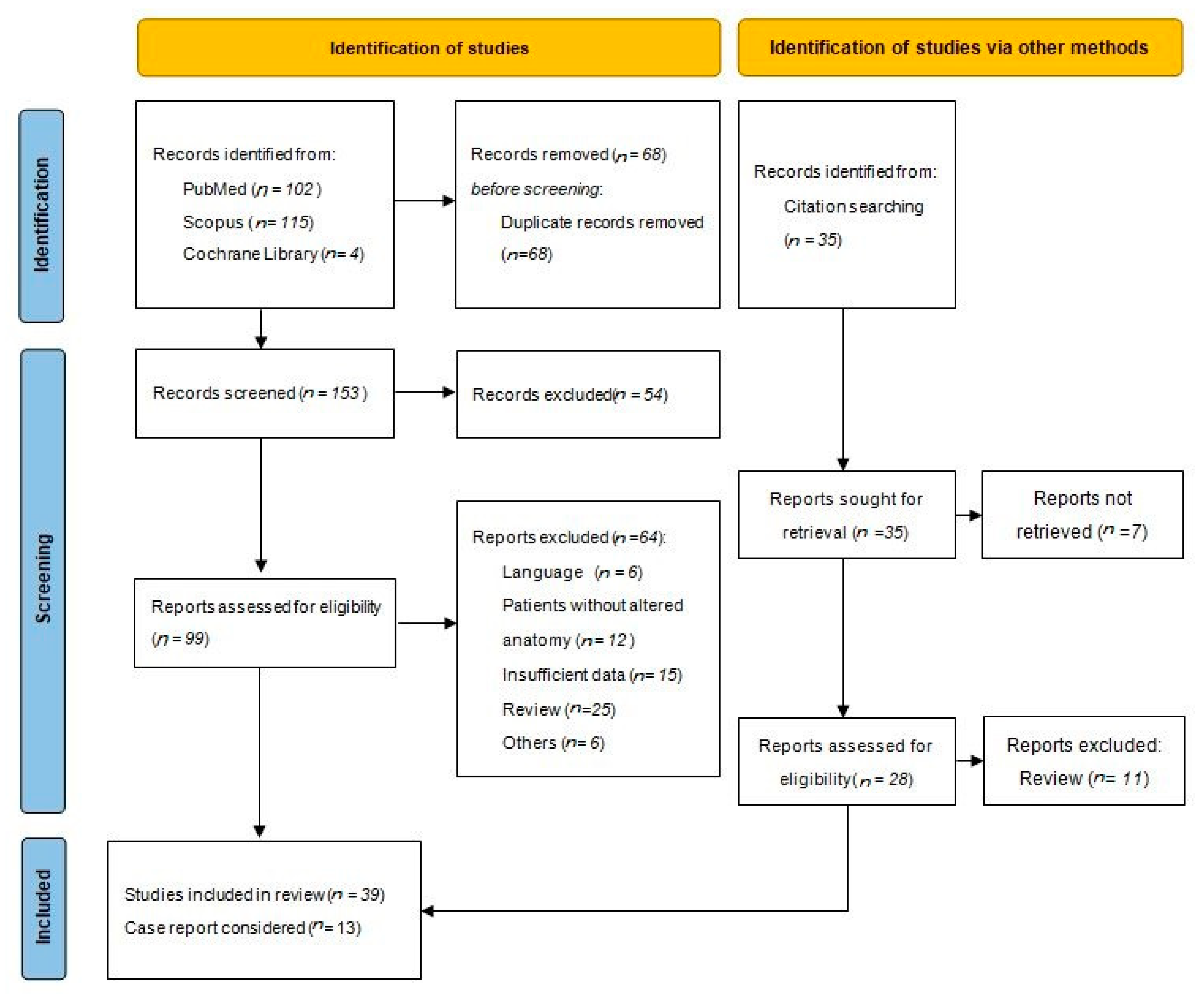
3.1. Literature Search
3.2. Surgical Anatomical Variant
3.3. Diagnostic Endoscopic Ultrasound in Altered Anatomy
3.4. Biliopancreatic Interventional Endoscopy in Altered Anatomy
3.4.1. Endoscopic Retrograde Cholangiopancreatography (ERCP)
3.4.2. EUS-Guided Procedures
3.4.3. Alternative Access
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. CA Cancer J. Clin. 2014, 64, 364. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Buchwald, H.; Oien, D.M. Metabolic/bariatric surgery worldwide 2011. Obes. Surg. 2013, 23, 427–436. [Google Scholar] [CrossRef]
- Santry, H.P.; Gillen, D.L.; Lauderdale, D.S. Trends in bariatric surgical procedures. J. Am. Med. Assoc. 2005, 294, 1909–1917. [Google Scholar] [CrossRef]
- Nagem, R.G.; Lázaro-da-Silva, A.; de Oliveira, R.M.; Morato, V.G. Gallstone-related complications after roux-en-Y gastric bypass: A prospective study. Hepatobiliary Pancreat. Dis. Int. 2012, 11, 630–635. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Bowman, E.; Greenberg, J.; Garren, M.; Guda, N.; Rajca, B.; Benson, M.; Pfau, P.; Soni, A.; Walker, A.; Gopal, D. Laparoscopic-assisted ERCP and EUS in patients with prior roux-en-Y gastric bypass surgery: A dual-center case series experience. Surg. Endosc. 2016, 30, 4647–4652. [Google Scholar] [CrossRef]
- Wilson, J.A.; Hoffman, B.; Hawes, R.H.; Romagnuolo, J. EUS in patients with surgically altered upper GI anatomy. Gastrointest. Endosc. 2010, 72, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Chahal, P.; Baron, T.H.; Topazian, M.D.; Petersen, B.T.; Levy, M.J.; Gostout, C.J. Endoscopic retrograde cholangiopancreatography in post-Whipple patients. Endoscopy 2006, 38, 1241–1245. [Google Scholar] [CrossRef]
- Gostout, C.J.; Bender, C.E. Cholangiopancreatography, sphincterotomy, and common duct stone removal via roux-en-Y limb enteroscopy. Gastroenterology 1988, 95, 156–163. [Google Scholar] [CrossRef]
- Elton, E.; Hanson, B.L.; Qaseem, T.; Howell, D.A. Diagnostic and therapeuticERCP using an enteroscope and a pediatric colonoscopeinlong-limb surgical bypass patients. Gastrointest. Endosc. 1998, 47, 62–67. [Google Scholar] [CrossRef]
- Hintze, R.E.; Adler, A.; Veltzke, W.; Abou-Rebyeh, H. Endoscopic access to the papilla of Vater for endoscopic retrograde cholangiopancreatography in patients with Billroth II or Roux-en-Y gastrojejunostomy. Endoscopy 1997, 29, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.E.; Cass, O.W.; Freeman, M.L. ERCP in patients with long-limb Roux-en-Y gastrojejunostomy and intact papilla. Gastrointest. Endosc. 2002, 56, 225–232. [Google Scholar] [CrossRef]
- Imazu, H.; Kanazawa, K.; Ikeda, K.; Kakutani, H.; Sumiyama, K.; Ang, T.L.; Omar, S.; Tajiri, H. Initial evaluation of a novel multibending backward-oblique viewing duodenoscope in endoscopic retrograde cholangiopancreatography. Endoscopy 2012, 44, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Adler, D.G. Initial report of a variable stiffness duodenoscope for use during endoscopic retrograde cholangiopancreatography. J. Clin. Gastroenterol. 2011, 45, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.C.; Moon, J.H.; Choi, H.J.; Ko, B.M.; Hong, S.J.; Cheon, Y.K.; Cho, Y.D.; Lee, J.S.; Lee, M.S.; Shim, C.S. The utility of a multibending endoscope for selective cannulation during ERCP in patients with a Billroth II gastrectomy (with video). Gastrointest. Endosc. 2009, 69, 931–934. [Google Scholar] [CrossRef]
- Moreels, T.G. ERCP in the patient with surgically altered anatomy. Curr. Gastroenterol. Rep. 2013, 15, 343. [Google Scholar] [CrossRef]
- Yamauchi, H.; Kida, M.; Okuwaki, K.; Miyazawa, S.; Iwai, T.; Takezawa, M.; Kikuchi, H.; Watanabe, M.; Imaizumi, H.; Koizumi, W. Short-type single balloon enteroscope for endoscopic retrograde cholangiopancreatography with altered gastrointestinal anatomy. World J. Gastroenterol. 2013, 19, 1728–1735. [Google Scholar] [CrossRef]
- Fugazza, A.; Anderloni, A.; Paduano, D.; Badalamenti, M.; Maselli, R.; Carrara, S.; Gabbiadini, R.; Colombo, M.; Spadaccini, M.; Cappello, A.; et al. Underwater cap-assisted endoscopic retrograde cholangiopancreatography in patients with surgically altered anatomy: A pilot study. Endoscopy 2021, 53, 927–931. [Google Scholar] [CrossRef]
- Haapamäki, C.; Udd, M.; Kylänpää, L. Benign biliary strictures treated with fully covered metallic stents in patients with surgically altered anatomy using double balloon enteroscopy. J. Laparoendosc. Adv. Surg. Tech. 2015, 25, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Kuwatani, M.; Kawahata, S. Peroral ultra-slim endoscopy-guided biliary drainage and stone extraction for postoperative upper gastrointestinal stenosis with a naïve papilla (with videos). J. Hepatobiliary Pancreat. Sci. 2015, 22, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, E.; Thosani, N.; Catalano, M. Dual-operator technique by use of digital cholangioscope through colonoscope-assisted ERCP in a patient with altered anatomy. Video GIE 2016, 1, 76–77. [Google Scholar] [CrossRef] [PubMed]
- Samarasena, J.B.; Huang, J.Y.; Chin, M.; Chang, K.J.; Lee, J.G. Altered anatomy ERCP with spiral overtube-assisted stent placement. Gastrointest. Endosc. 2016, 84, 738. [Google Scholar] [CrossRef] [PubMed]
- Tonozuka, R.; Itoi, T.; Sofuni, A.; Tsuchiya, T.; Ishii, K.; Tanaka, R.; Honjo, M.; Mukai, S.; Yamamoto, K.; Fujita, M.; et al. Novel peroral direct digital cholangioscopy-assisted lithotripsy using a monorail technique through the overtube in patients with surgically altered anatomy (with video). Dig. Endosc. 2019, 31, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.T.; Friedland, S.; Watson, R.R.; Hwang, J.H. Use of a rigidizing overtube for altered-anatomy ERCP. Video GIE 2020, 5, 664–666. [Google Scholar] [CrossRef]
- Rizzo, G.E.M.; Di Carlo, G.; Rizzo, G.; Ferro, G.; Corbo, G.; Sciumè, C. Mirizzi syndrome in a patient with partial gastrectomy with Billroth II anastomosis: A case report. Int. J. Surg. Case Rep. 2020, 77, 549–553. [Google Scholar] [CrossRef]
- Mazzeo, C.; Badessi, G.; Pallio, S.; Viscosi, F.; Cucinotta, E. Laparoscopic assisted ERCP in patient with Roux-en-Y gastric bypass. A case report. Int. J. Surg. Case Rep. 2021, 81, 105837. [Google Scholar] [CrossRef]
- Aabakken, L.; Bretthauer, M.; Line, P. Double-balloon enteroscopyforendoscopic retrograde cholangiography in patients with a roux-en-Y anastomosis. Endoscopy 2007, 39, 1068–1071. [Google Scholar] [CrossRef]
- Ishihara, Y.; Matsumoto, K.; Kato, H.; Tsutsumi, K.; Tomoda, T.; Matsumi, A.; Miyamoto, K.; Yamazaki, T.; Saragai, Y.; Fujii, Y.; et al. Treatment outcomes, including risk factors of stone recurrence, for hepatolithiasis using balloon-assisted endoscopy in patients with hepaticojejunostomy (with video). Surg. Endosc. 2021, 35, 1895–1902. [Google Scholar] [CrossRef]
- Kogure, H.; Sato, T.; Nakai, Y.; Ishigaki, K.; Hakuta, R.; Saito, K.; Takahara, N.; Hamada, T.; Mizuno, S.; Yamada, A.; et al. Endoscopic management of pancreatic diseases in patients with surgically altered anatomy: Clinical outcomes of combination of double-balloon endoscopy- and endoscopic ultrasound-guided interventions. Dig. Endosc. 2021, 33, 441–450. [Google Scholar] [CrossRef]
- Sato, T.; Kogure, H.; Nakai, Y.; Ishigaki, K.; Hakuta, R.; Saito, K.; Takahara, N.; Hamada, T.; Mizuno, S.; Yamada, A.; et al. Double-balloon endoscopy-assisted treatment of hepaticojejunostomy anastomotic strictures and predictive factors for treatment success. Surg. Endosc. 2020, 34, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Mori, Y.; Nakashima, Y.; Ohtsuka, T.; Nakamura, S.; Gotoh, Y.; Date, K.; Sadakari, Y.; Nakata, K.; Miyasaka, Y.; et al. Endoscopic retrograde cholangiopancreatography in patients with surgically altered gastrointestinal anatomy: A retrospective study. Int. Surg. 2019, 103, 184–190. [Google Scholar] [CrossRef]
- Yamada, A.; Kogure, H.; Nakai, Y.; Takahara, N.; Mizuno, S.; Tada, M.; Koike, K. Performance of a new short-type double-balloon endoscope with advanced force transmission and adaptive bending for pancreaticobiliary intervention in patients with surgically altered anatomy: A propensity-matched analysis. Dig. Endosc. 2019, 31, 86–93. [Google Scholar] [CrossRef]
- Cho, S.; Kamalaporn, P.; Kandel, G.; Kortan, P.; Marcon, N.; May, G. ‘Short’ double-balloon enteroscope for endoscopic retrograde cholangiopancreatography in patients with a surgically altered upper gastrointestinal tract. Can. J. Gastroenterol. 2011, 25, 615–619. [Google Scholar] [CrossRef]
- Hakuta, R.; Kogure, H.; Nakai, Y.; Hamada, T.; Sato, T.; Suzuki, Y.; Inokuma, A.; Kanai, S.; Nakamura, T.; Noguchi, K.; et al. Feasibility of balloon endoscope-assisted endoscopic retrograde cholangiopancreatography for the elderly. Endosc. Int. Open 2020, 8, E1202–E1211. [Google Scholar] [CrossRef]
- Shimatani, M.; Takaoka, M.; Ikeura, T.; Mitsuyama, T.; Okazaki, K. Evaluation of endoscopic retrograde cholangiopancreatography using a newly developed short-type single-balloon endoscope in patients with altered gastrointestinal anatomy. Dig. Endosc. 2014, 26, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, Y.; Sullivan, C.T.; Gelrud, A. Single balloon enteroscopy (SBE) assisted therapeutic endoscopic retrograde cholangiopancreatography (ERCP) in patients with roux-en-Y anastomosis. Dig. Dis. Sci. 2014, 59, 465–470. [Google Scholar] [CrossRef]
- Wang, A.Y.; Sauer, B.G.; Behm, B.W.; Ramanath, M.; Cox, D.G.; Ellen, K.L.; Shami, V.M.; Kahaleh, M. Single-balloon enteroscopy effectively enables diagnostic and therapeutic retrograde cholangiography in patients with surgically altered anatomy. Gastrointest. Endosc. 2010, 71, 641–649. [Google Scholar] [CrossRef]
- Yane, K.; Katanuma, A.; Maguchi, H.; Takahashi, K.; Kin, T.; Ikarashi, S.; Sano, I.; Yamazaki, H.; Kitagawa, K.; Yokoyama, K.; et al. Short-type single-balloon enteroscope-assisted ERCP in postsurgical altered anatomy: Potential factors affecting procedural failure. Endoscopy 2017, 49, 69–74. [Google Scholar] [CrossRef]
- Lenze, F.; Meister, T.; Matern, P.; Heinzow, H.S.; Domschke, W.; Ullerich, H. Single-balloon enteroscopy-assisted endoscopic retrograde cholangiopancreaticography in patients with surgically altered anatomy: Higher failure rate in malignant biliary obstruction-a prospective single center cohort analysis. Scand. J. Gastroenterol. 2014, 49, 766–771. [Google Scholar] [CrossRef]
- Ali, M.F.; Modayil, R.; Gurram, K.C.; Brathwaite, C.E.M.; Friedel, D.; Stavropoulos, S.N. Spiral enteroscopy-assisted ERCP in bariatric-length Roux-en-Y anatomy: A large single-center series and review of the literature (with video). Gastrointest. Endosc. 2018, 87, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Zouhairi, M.E.; Watson, J.B.; Desai, S.V.; Swartz, D.K.; Castillo-Roth, A.; Haque, M.; Jowell, P.S.; Branch, M.S.; Burbridge, R.A. Rotational assisted endoscopic retrograde cholangiopancreatography in patients with reconstructive gastrointestinal surgical anatomy. World J. Gastrointest. Endosc. 2015, 7, 278–282. [Google Scholar] [CrossRef]
- Lennon, A.M.; Kapoor, S.; Khashab, M.; Corless, E.; Amateau, S.; Dunbar, K.; Chandrasekhara, V.; Singh, V.; Okolo, P.I. Spiral assisted ERCP is equivalent to single balloon assisted ERCP in patients with Roux-en-Y anatomy. Dig. Dis. Sci. 2012, 57, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Anvari, S.; Lee, Y.; Patro, N.; Soon, M.S.; Doumouras, A.G.; Hong, D. Double-balloon enteroscopy for diagnostic and therapeutic ERCP in patients with surgically altered gastrointestinal anatomy: A systematic review and meta-analysis. Surg. Endosc. 2021, 35, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Brozzi, L.; Petrone, M.C.; Poley, J.W.; Carrara, S.; Barresi, L.; Fabbri, C.; Rimbas, M.; De Angelis, C.; Arcidiacono, P.G.; Signoretti, M.; et al. Outcomes of biliopancreatic EUS in patients with surgically altered upper gastrointestinal anatomy: A multicenter study. Endosc. Int. Open 2020, 8, E869–E876. [Google Scholar] [CrossRef] [PubMed]
- Mukai, S.; Itoi, T.; Sofuni, A.; Tsuchiya, T.; Tanaka, R.; Tonozuka, R.; Honjo, M.; Fujita, M.; Yamamoto, K.; Nagakawa, Y. EUS-guided antegrade intervention for benign biliary diseases in patients with surgically altered anatomy (with videos). Gastrointest. Endosc. 2019, 89, 399–407. [Google Scholar] [CrossRef]
- Grimes, K.L.; Maciel, V.H.; Mata, W.; Arevalo, G.; Singh, K.; Arregui, M.E. Complications of laparoscopic transgastric ERCP in patients with roux-en-Y gastric bypass. Surg. Endosc. 2015, 29, 1753–1759. [Google Scholar] [CrossRef]
- Bove, V.; Tringali, A.; Familiari, P.; Gigante, G.; Boškoski, I.; Perri, V.; Mutignani, M.; Costamagna, G. ERCP in patients with prior billroth II gastrectomy: Report of 30 years’ experience. Endoscopy 2015, 47, 611–616. [Google Scholar] [CrossRef]
- Iwashita, T.; Yasuda, I.; Doi, S.; Uemura, S.; Mabuchi, M.; Okuno, M.; Mukai, T.; Itoi, T.; Moriwaki, H. Endoscopic ultrasound-guided antegrade treatments for biliary disorders in patients with surgically altered anatomy. Dig. Dis. Sci. 2013, 58, 2417–2422. [Google Scholar] [CrossRef]
- Lee, T.H.; Hwang, J.C.; Choi, H.J.; Moon, J.H.; Cho, Y.D.; Yoo, B.M.; Park, S.H.; Kim, J.H.; Kim, S.J. One-step transpapillary balloon dilation under cap-fitted endoscopy without a preceding sphincterotomy for the removal of bile duct stones in billroth II gastrectomy. GutLiver 2012, 6, 113–117. [Google Scholar] [CrossRef][Green Version]
- James, T.W.; Fan, Y.C.; Baron, T.H. EUS-Guided Hepaticoenterostomy as a portal to allow definitive antegrade treatment of benign biliary diseases in patients with surgically altered anatomy. Gastrointest. Endosc. 2018, 88, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Bures, C.; Seika, P.; Veltzke-Schliecker, W.; Adler, A.; Kröll, D.; Zorron, R. Intragastric single-port surgery (IGS) accesses the gastric remnant and allows ERCP for common bile duct stones after RYGB: A simple solution for a difficult problem. Surg. Obes. Relat. Dis. 2019, 15, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Wagh, M.S.; Draganov, P.V. Prospective evaluation of spiral overtube-assisted ERCP in patients with surgically altered anatomy. Gastrointest. Endosc. 2012, 76, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Law, R.; Song, L.M.K.W.; Petersen, B.T.; Baron, T.H. Single-session ERCP in patients with previous Roux-en-Y gastric bypass using percutaneous-assisted transprosthetic endoscopic therapy: A case series. Endoscopy 2013, 45, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hisa, T.; Momoi, T.; Shimizu, T.; Furutake, M.; Takamatsu, M.; Ohkubo, H. Endoscopic ultrasound-guided antegrade stone removal in a patient with pancreatic stones and anastomotic stricture after end-to-side pancreaticojejunostomy. Pancreatology 2013, 13, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Hisada, Y.; Hijioka, S.; Ohba, A.; Nagashio, Y.; Kanai, Y.; Okusaka, T.; Saito, Y. Novel endoscopic ultrasound-guided hepaticoduodenostomy using a forward-viewing echoendoscope for altered anatomy. Endoscopy 2021, 53, E340–E342. [Google Scholar] [CrossRef]
- Anderloni, A.; Fugazza, A.; Maroni, L.; Troncone, E.; Milani, O.; Cappello, A.; Alkandari, A.; Repici, A. Endoscopic ultrasound-guided gallbladder drainage by transduodenal lumen-apposing metal stent in a patient with Roux-en-Y reconstruction. Ann. Gastroenterol. 2019, 32, 522–524. [Google Scholar] [CrossRef]
- Novikov, A.; Kumta, N.A.; Samstein, B.; Kahaleh, M. Endoscopic ultrasound-guided transhepatic biliary drainage in altered anatomy: A two-step approach. Endoscopy 2016, 48 (Suppl. S1). [Google Scholar] [CrossRef]
- Perez-Miranda, M.; Sanchez-Ocaña, R.; De La Serna Higuera, C.; Diez-Redondo, P.; Nuñez, H.; Vallecillo, M.A. Transenteric anastomosis with lumen-apposing metal stent as a conduit for iterative endotherapy of malignant biliary obstruction in altered anatomy. Gastrointest. Endosc. 2014, 80, 339. [Google Scholar] [CrossRef]
- Mangiavillano, B.; Carrara, S.; Eusebi, L.H.; Auriemma, F.; Bianchetti, M.; Repici, A. Water-filled technique for therapeutic pancreato-biliary EUS in patients with surgically altered anatomy. Endosc. Int. Open 2021, 9, E487–E489. [Google Scholar] [CrossRef]
- Kedia, P.; Sharaiha, R.Z.; Kumta, N.A.; Kahaleh, M. Internal EUS-directed transgastric ERCP (EDGE): Game over. Gastroenterology 2014, 147, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Kedia, P.; Kumta, N.A.; Widmer, J.; Sundararajan, S.; Cerefice, M.; Gaidhane, M.; Sharaiha, R.; Kahaleh, M. Endoscopic ultrasound-directed transgastric ERCP (EDGE) for Roux-en-Y anatomy: A novel technique. Endoscopy 2015, 47, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Tyberg, A.; Nieto, J.; Salgado, S.; Weaver, K.; Kedia, P.; Sharaiha, R.Z.; Gaidhane, M.; Kahaleh, M. Endoscopic Ultrasound (EUS)-Directed Transgastric Endoscopic Retrograde Cholangiopancreatography or EUS: Mid-Term Analysis of an Emerging Procedure. Clin. Endosc. 2017, 50, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Ngamruengphong, S.; Nieto, J.; Kunda, R.; Kumbhari, V.; Chen, Y.I.; Bukhari, M.; El Zein, M.H.; Bueno, R.P.; Hajiyeva, G.; Ismail, A.; et al. Endoscopic ultrasound-guided creation of a transgastric fistula for the management of hepatobiliary disease in patients with Roux-en-Y gastric bypass. Endoscopy 2017, 49, 549–552. [Google Scholar] [CrossRef]
- James, T.W.; Baron, T.H. Endoscopic Ultrasound-Directed Transgastric ERCP (EDGE): A Single-Center US Experience with Follow-up Data on Fistula Closure. Obes. Surg. 2019, 29, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, M.; Kowalski, T.; Nieto, J.; Kunda, R.; Ahuja, N.K.; Irani, S.; Shah, A.; Loren, D.; Brewer, O.; Sanaei, O.; et al. An international, multicenter, comparative trial of EUS-guided gastrogastrostomy-assisted ERCP versus enteroscopy-assisted ERCP in patients with Roux-en-Y gastric bypass anatomy. Gastrointest. Endosc. 2018, 88, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Chiang, A.L.; Gaidhane, M.; Loren, D.E.; Kahaleh, M.; Schlachterman, A.; Millman, J.; Tyberg, A.; Nieto, J.; Kedia, P.; Tarnasky, P.R.; et al. 338 impact of EUS-directed Transgastric ERCP (EDGE procedure) access route on technical success and adverse events: A multicenter experience. Gastrointest. Endosc. 2018, 87, AB70–AB71. [Google Scholar] [CrossRef]
- Kedia, P.; Tarnasky, P.R.; Nieto, J.; Steele, S.L.; Siddiqui, A.; Xu, M.M.; Tyberg, A.; Gaidhane, M.; Kahaleh, M. EUS-directed Transgastric ERCP (EDGE) Versus Laparoscopy-assisted ERCP (LA-ERCP) for Roux-en-Y Gastric Bypass (RYGB) Anatomy: A Multicenter Early Comparative Experience of Clinical Outcomes. J. Clin. Gastroenterol. 2019, 53, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, W.; Krafft, M.R.; Abdelqader, A.; Nasr, J. Su1167 EUS-directed Transgastric ERCP with 20 MM lumen-apposing metal stents in patients with roux-en-Y gastric bypass, are we closer to perfection? Gastrointest. Endosc. 2019, 89, AB297. [Google Scholar] [CrossRef]
- Runge, T.M.; Chang, A.; Kowalski, T.E.; James, T.W.; Baron, T.; Nieto, J.; Diehl, D.L.; Krafft, M.R.; Nasr, J.Y.; Kumar, V.; et al. EUS-Directed Transgastric ERCP (EDGE): A Retrospective, Multicenter Study. Endoscopy 2020, 53, 611–618. [Google Scholar] [CrossRef]
- Khan, M.A.; Kedia, P.; Tyberg, A.; Shrestha, S.; Ismail, M.K.; Gaidhane, M.; Tarnasky, P.R.; Kahaleh, M. Comparison of EUS directed Transgastric endoscopic retrograde Cholangiopancreatography in patients with roux en-Y bypass: A meta-analysis. Gastrointest. Endosc. 2018, 87, AB452–AB453. [Google Scholar] [CrossRef]
- Khara, H.S.; Parvataneni, S.; Park, S.; Choi, J.; Kothari, T.H.; Kothari, S.T. Review of ERCP techniques in roux-en-Y gastric bypass patients: Highlight on the novel EUS-directed transgastric ERCP (EGDE) technique. Curr. Gastroenterol. Rep. 2021, 23, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Thompson, C.C.; Ryou, M. Gastric access temporary for endoscopy (GATE): A proposed algorithm for EUS-directed transgastric ERCP in gastric bypass patients. Surg. Endosc. 2019, 33, 2024–2033. [Google Scholar] [CrossRef] [PubMed]
- Krafft, M.R.; Hsueh, W.; James, T.W.; Runge, T.M.; Baron, T.H.; Khashab, M.A.; Irani, S.S.; Nasr, J.Y. The EDGI new take on EDGE: EUS-directed transgastric intervention (EDGI), other than ERCP, for Roux-en-Y gastric bypass anatomy: A multicenter study. Endosc. Int. Open. 2019, 7, E1231–E1240. [Google Scholar] [CrossRef]
- Donatelli, G.; Cereatti, F.; Daho, F. EUS-guided anastomosis complication in a patient with roux-en-Y gastric bypass: Dehiscence of the surgical anastomosis during endoscopic mucosal resection across EUS-guided jejunum-gastric anastomosis with lumen apposing metal stent. Obes. Surg. 2021, 31, 3384–3385. [Google Scholar] [CrossRef]
- Law, R.; Grimm, I.S.; Baron, T.H. Modified percutaneous assisted transprosthetic endoscopic therapy for transgastric ERCP in a gastric bypass patient. Endoscopy 2016, 48 (Suppl. S1), E16–E17. [Google Scholar] [CrossRef]
- Attam, R.; Leslie, D.; Freeman, M.; Ikramuddin, S.; Andrade, R. EUS-assisted, fluoroscopically guided gastrostomy tube placement in patients with Roux-en-Y gastric bypass: A novel technique for access to the gastric remnant. Gastrointest. Endosc. 2011, 74, 677–682. [Google Scholar] [CrossRef]
- Siddiki, H.; Baron, T.H. The sand dollar sign: A reliable EUS image to identify the excluded stomach during EUS-guided gastrogastrostomy. Gastrointest. Endosc. 2018, 88, 398–399. [Google Scholar] [CrossRef]
- Peters, M.; Papasavas, P.K.; Caushaj, P.F.; Kania, R.J.; Gagné, D.J. Laparoscopic transgastric endoscopic retrograde cholangiopancreatography for benign common bile duct stricture after Roux-en-Y gastric bypass. Surg. Endosc. 2002, 16, 1106. [Google Scholar] [CrossRef]
- Banerjee, N.; Parepally, M.; Byrne, T.K.; Pullatt, R.C.; Coté, G.A.; Elmunzer, B.J. Systematic review of transgastric ERCP in Roux-en-Y gastric bypass patients. Surg. Obes. Relat. Dis. 2017, 13, 1236–1242. [Google Scholar] [CrossRef]
| First Name, Year | Article Type | N. of Patient | Type of Surgery | Indication | Endoscopic Technique | Technical Success | E-AEs Reported |
|---|---|---|---|---|---|---|---|
| Brozzi [45], 2021 | Retrospective study | 242 | 86 (35.5%) Billroth II; 77 (31.8%) PD; 23 (9.5%) Billroth I; 19 (7.9%) distal esophagectomy; 15 (6.2%) total gastrectomy; 14 (5.8%) sleeve gastrectomy; 8 (3.3%) Roux-en-Y. | Suspected solid pancreatic lesions documented on cross-sectional imaging (46.7%), suspected cystic pancreatic lesions (18.2%), suspected choledocholithiasis (10.3%), post-pancreatic resection follow-up (6.2%), unexplained CBD dilation in 14 (5.8%), main pancreatic duct dilation (4.9%), suspected extrahepaticcholangiocarcinoma (2.1%), chronic pancreatitis (2.1%), pancreatic cancer screening (2.1%), idiopathic recurrent pancreatitis (1.6%). | Conventional EUS | Overall: 78.2% TA–success rate: 71.3% | 3 (1.24%) |
| Ishihara [29], 2021 | Retrospective study | 73 | PD with pancreaticojejunostomy and HJ: bowel reconstruction methods were Child in 46 (63%), Roux-en-Y in 22 (30%),and other in 5 (7%). | Intrahepatic bile duct stones | sDBE-ERCP | 92% | 6.8% |
| Kogure [30], 2021 | Retrospective study | 40 | Surgical reconstructions: 26 Billroth-II, 13 Roux-en-Y, and 1 Imanaga | 18 pancreatojejunostomy anastomotic stricture (PJAS), four pancreatic duct stone (PDS), 4 pancreatic fistula (PF), 11 PJAS with PDS, 3 PJAS with PF. | sDBE-ERCP and EUS-PD | sDBE-ERCP 70.7% (29/41) EUS-PD: 100% (9/9) | 12.2% |
| Sato [31], 2020 | Retrospective study | 102 | Roux-en-Y 80 (78.4%), Billroth-II 22 (21.6%) | HJ anastomotic strictures | sDBE-ERCP | 89.2% | 17.6% |
| Mukai [46], 2019 | Retrospective study | 48 | 9 gastrectomy with Roux-en-Y. 2 gastrectomy with Billroth-II. 17 HJ with Roux-en-Y. 3 hepaticoduodenostomy. 6 PD with Whipple | Benign biliary diseases: common bile duct stones [n = 11], intrahepatic bile duct stones [n = 5], anastomotic strictures [n = 21] | EUS-guided antegrade intervention | 91.9% | 8.1% |
| Fujimoto [32], 2018 | Retrospective study | 102 | Gastrectomy + R-Y (38/102); Gastrectomy + B-II (24/102); PD (23/102); HJ+R-Y (17/102) | CBD stones, anastomotic stricture of HJ, IHBD stones, chronic pancreatitis and pancreatic stone, cholangitis, stenosis of afferent loop | sDBE-ERCP or a regular gastroendoscope | 80% | 1.96% |
| Yamada [33], 2019 | Prospective collected data–Propensity score matched patients | 326 | Gastrectomy with B-II, gastrectomy with R-Y, PD with B-II, PD with R-Y, HJ with R-Y, and liver transplantation with HJ | Biliary strictures, anastomosis stenoses, choledocholithiasis, intrahepatic stones, obstructive jaundice, bile duct leaks, pancreatic duct leaks, chronic pancreatitis with pancreatic duct strictures, intraductal pancreatic stones | sDBE-ERCP and cDBE-ERCP | Short-type DBE: 150 (92%) cDBE: 145 (89%) | 5.52% |
| Bowman [7], 2016 | Retrospective study | 16 | RYGB | Choledocholithiasis, CBD stenosis, recurrent acute pancreatitis, stone IOC, gallstone pancreatitis | LA-ERCP: 11 cases Combined LA-EUS plus LA-ERCP: 5 | 100% | 0% |
| Grimes [47], 2015 * | Retrospective study | 38 | RYGB | Chronic abdominal pain, including SOD, pancreatic duct stenosis, chronic pancreatitis, choledocolithiasis | LA ERCP | 95% | 13% |
| Bove [48], 2015 | Retrospective study | 713 | Gastrectomy with B-II reconstruction | Common bile duct stones (51.2%) and obstructive jaundice (24.8%) | c-ERCP or gastroscope | 93.8% | 4.3% |
| Shimatani [36], 2014 | Prospective study | 26 | 4 RYGB,8 R-Y HJ, 3 BII, 3 PD, 6 ppPD, 2 other reconstructions. | NA | sSBE-ERCP | 84.6% | 3.8% |
| Tomizawa [37], 2014 | Retrospective study | 14 | Roux-en-Y reconstruction after Whipple procedure (n = 4), HJ (n = 9) and partial gastrectomy (n =1). | Obstructive jaundice (n = 10), cholangitis (n = 7), post-PTC internalization (n = 3) and biliary stent extraction/exchange(n =2) | SBE-ERCP | 73% | 0% |
| Lenze [40], 2014 | Prospective study | 26 | 9 Billroth II with Roux-en-Y; 9 biliodigestive anastomosis with Roux-en-Y; 5 total gastrectomy with Roux-en-Y; 2 pp-Whipple PD; 1 Whipple PD | Obstructive cholestasis: 15 choledocolithiasis: 10 obstruction of pancreatic duct: 1 | SBE-ERCP | 57.7% | NA |
| Iwashita [49], 2013 | Retrospective Study | 7 | Total gastrectomy: 3 Subtotal gastrectomy: 2 Pancreaticoduodenectomy: 2 | 5 Choledocholithiasis,1 malignant biliary obstruction,1 bilioenteric anastomosis stricture | EUS-guided antegrade treatments | 100% | 28% (2/7) |
| Lee [50], 2012 | Retrospective study | 13 | Billroth II gastrectomy | Choledocolithiasis | EPBD-ERCP with forward-viewing endoscope | 92.3% | 0% |
| Cho [34], 2011 | Retrospective study | 20 | 6 patients Billroth II, 7 Roux-en-Ywith HJ, 5 Roux-en-Y with GJ, 1Roux-en-Y with EJ, 1 Whipple’s operation with choledochojejunostomy | Choledocholithiasis, stricture, cholangitis, bile leakage | sDBE-ERCP | 24/25 (96%) | NA |
| Wilson [8], 2010 | Retrospective study | 188 | BI, BII, RYGB, Whipple, Puestow, Nissen fundoplication, esophagectomy | NR | EUS-TA | 139/188 (73.94%) | 0% |
| Wang [38], 2010 | Retrospective study | 13 | Whipple (n =3), hepaticojejunostomy (n =3), Billroth II (n =1), and Roux-en-Y (n =9) | Cholangitis, choledocholithiasis, biliary pancreatitis, Retained stent from OLT, CBD stricture | SBE-ERCP | 92.3% | 15.39% |
| Hakuta [35], 2020 | Retrospective study | 568 | Gastrectomy B-II, Gastrectomy R-Y, PD R-Y, PD B-II, Extrahepatic bile duct resection with R-Y | Bile duct stone, benign biliary stricture, malignant biliary obstruction, cholangitis, pancreatic intervention | sDBE-ERCP | 79.93% | 10.04% |
| Fugazza [19], 2020 | Prospective study | 6 | 3 (50%) distal Gastrectomy RY, 2(33.3%) with Whipple pylorus preserving and 1(16.7%) with bariatric Gastro-jejunal Bypass | Jaundice or cholangitis secondary to bile duct stones | uERCP° | 100% | 0% |
| Yane [39], 2017 | Retrospective study | 117 | BII gastrectomy 13 (11.1), PD 51 (43.6), Roux-en-Y gastrectomy 25 (21.4), HJ with Roux-en-Y 28 (23.9) | Bile duct stone 28 (23.9), bile duct stricture 16 (13.7), stricture of choledo- or hepaticojejunal anastomosis 51 (43.6), stricture of pancreaticojejunal anastomosis 14 (12.0), Others 8 (6.8) | sSBE-ERCP | 81.8% | 5.9% |
| James [51], 2018 | Retrospective study | 20 | 9 RYGB, 6 Roux-en-Y HJ, 2 Billroth II procedures, and 3 Whipple procedures. | Common bile duct stones (n = 8), benign postoperative strictures (n = 7), chronic pancreatitis (n = 3), inflammatory stricture (n = 1), and treatment of a bile leak (n = 1) | EUS-guided hepaticoenterostomy | 90% | 15 5% |
| Bures [52], 2019 | Prospective Study | 8 | RYGB | Choledocholithiasis | LA-ERCP with intragastric single-port surgery | 100% | 0% |
| Ali [41], 2018 | Retrospective Study | 31 | 28 in RYGB and 7 “long- limb- RY” surgical reconstructions: 4 in patients with RY-HJ and 3 in patients with gastrectomies and RY reconstructions | Choledocholithiasis 14 (40%); malignant obstruction 6 (17%); SOD 5 (14%); stent placement 2 (6%); Stent extraction 2 (6%); biliary pancreatitis 2 (6%); type III choledochocele 1 (3%); bile leak 1 (3%); HJ stricture 1 (3%); ampullary stricture post prior sphincterotomy 1 (3%) | SE-ERCP | 86% | 0% |
| Zouhairi [42], 2015 | Retrospective study | 42 | 39 with gastric bypass Roux-en-Y, 2 with Billroth II gastrectomy, and 1 with hepaticojejunostomy associated with liver transplant | Choledocholithiasis: 13 (30.9%), biliary obstruction: 20 (47.6%), suspected sphincter of Oddi dysfunction: 4 (9.5%), abnormal liver enzymes: 1 (2.4%), ascending cholangitis: 2 (4.8%), and bile leak: 2 (4.8%) | SE-ERCP | 64.3% | 7.69% |
| Wagh [53], 2012 | Prospective study | 7 | Roux-en-Y HJ 2/7 (29%); RYGB 3/7 (43%); RYGB with HJ 1/7 (14%); BII gastrectomy with Braun enteroenterostomy 1/7 (14%) | Biliary obstruction 5/7 (72%); bile duct stone(s) 1/7 (14%); Pancreatic leak 1/7 (14%) | SE-ERCP | 69% | 0% |
| Law [54], 2013 | Retrospective study | 5 | RYGB | SOD (Type I [n =3], Type II [n =2]) | DAE-PATENT | 100% | 20% |
| First Name, Year | Article Type | N. of Patient | Type of Surgery | Endoscopic Technique | LAMS Diameter | Technical Success | Clinical Success | E-AEs Reported |
|---|---|---|---|---|---|---|---|---|
| Kedia [62], 2015 | Prospective study | 5 | RYGB | SS–EDGE 3 DS-EGDE 2 | 15 mm | 100% | 60% | stent dislodgement: 60% |
| Tyberg [63], 2016 | Prospective study | 16 | RYGB | SS–EDGE 4 DS-EGDE 6 | 15 mm | 100% | 91% | Stent migration(19%), 1 jejunal perforation |
| Ngamruengphong [64], 2017 | Retrospective study | 13 | RYGB | SS–EDGE 2 DS-EGDE 11 | 15 mm | 100% | 100% | Stent migration(33%) |
| James and Baron [65], 2018 | Retrospective Study | 19 | RYGB | SS–EDGE 4 DS-EGDE 15 | 15 mm | 100% | 100% | Stent malposition(6/19) |
| Bukhari [66], 2018 | Retrospective Study | 30 | RYGB | SS–EDGE 8 DS-EGDE 22 | 15 mm | 100% | 100% | LAMS migration(6.7%),bleeding(3.3%) |
| Chiang [67], 2018 | Oral Abstract–retrospective study | 66 | RYGB | SS–EDGE 43 DS-EGDE 23 | NR | 92.4% | NR | Bleeding (7.6%), LAMS malposition(4.5%), LAMS migration (4.5%), perforation (1.5%), pancreatitis(1.5%) |
| Kedia [68], 2018 | Retrospective study | 29 | RYGB | NR | 15 mm | 96.5% | 96.5% | Perforation (1),pancreatitis (2)stentdislodgement(3)bleeding (1). |
| Wang [73], 2019 | Retrospective study | 10 | RYGB | SS–GATE 7 DS-GATE 2 | 15 mm | 100% | 100% | Stent migration (20%), bleeding in one patient |
| Hsueh [69], 2019 | Oral Abstract –Retrospective study | 9 | RYGB | SS–EDGE 2 DS-EGDE 7 | 20 mm | 100% | 100% | None |
| Runge [70], 2020 | Retrospective study | 178 | RYGB | SS –EDGE 85 DS-EGDE 81 | NR | 98% | NR | Perforation(6), stentmigration(13),bleeding (2), Pneumoperitoneum(3),post ERCP pancreatitis(3), cholangitis (1) |
| Krafft [74], 2019 | Retrospective study | 14 | RYGB | SS –EDGI 5 DS-EGDI 2 | 20 mm (n =8) 15 mm(n =6) | 100% | 100% | Stentdislodgement(14.3%) |
| Khara [72], 2021 | Retrospective study | 76 | RYGB | 59 LA-ERCP 17 EDGE | 20 mm | Both 100% | Both 100% | 17% LA-ERCP 6% EDGE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarantino, I.; Rizzo, G.E.M. Biliopancreatic Endoscopy in Altered Anatomy. Medicina 2021, 57, 1014. https://doi.org/10.3390/medicina57101014
Tarantino I, Rizzo GEM. Biliopancreatic Endoscopy in Altered Anatomy. Medicina. 2021; 57(10):1014. https://doi.org/10.3390/medicina57101014
Chicago/Turabian StyleTarantino, Ilaria, and Giacomo Emanuele Maria Rizzo. 2021. "Biliopancreatic Endoscopy in Altered Anatomy" Medicina 57, no. 10: 1014. https://doi.org/10.3390/medicina57101014
APA StyleTarantino, I., & Rizzo, G. E. M. (2021). Biliopancreatic Endoscopy in Altered Anatomy. Medicina, 57(10), 1014. https://doi.org/10.3390/medicina57101014






